Comparing the Efficiency of Valved Trocar Cannulas for Pars Plana Vitrectomy
Abstract
:1. Introduction
2. Materials and Methods
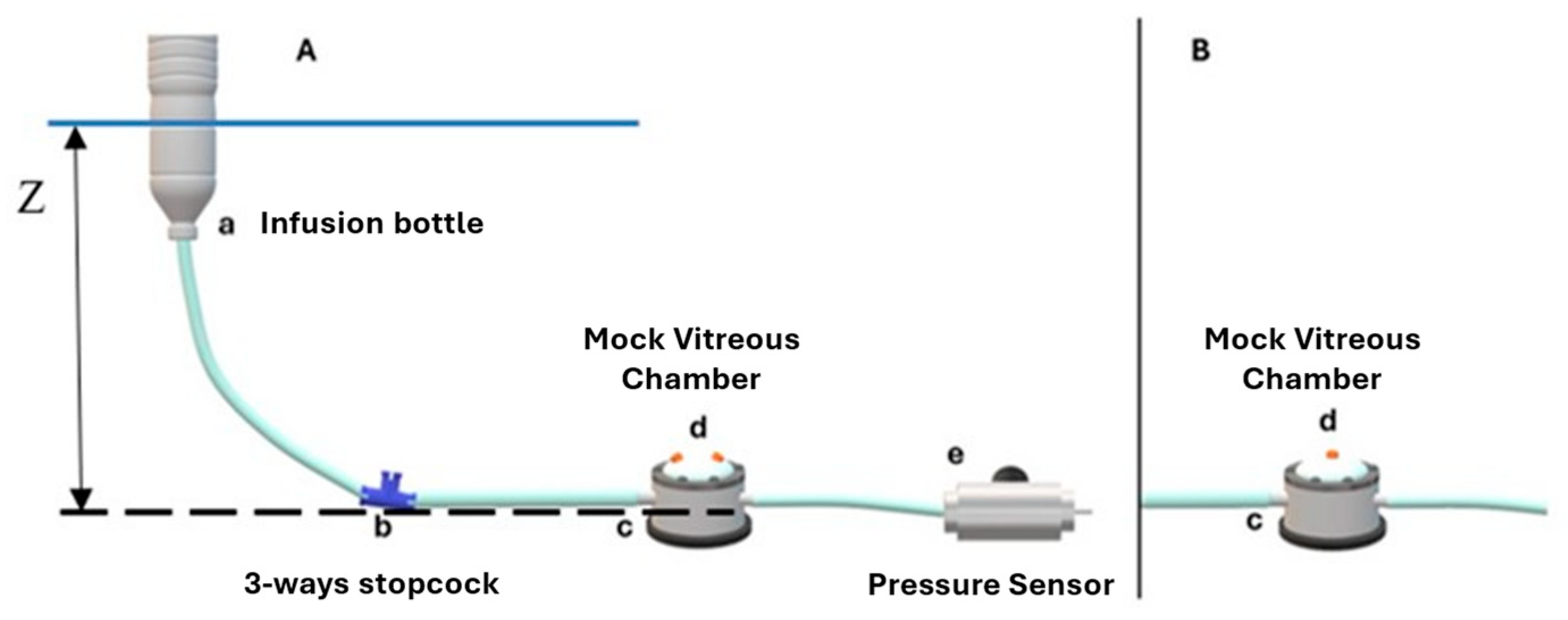
2.1. Experimental Setting
2.2. BSS Infusion Setup
2.3. Air Infusion Setup
3. Results
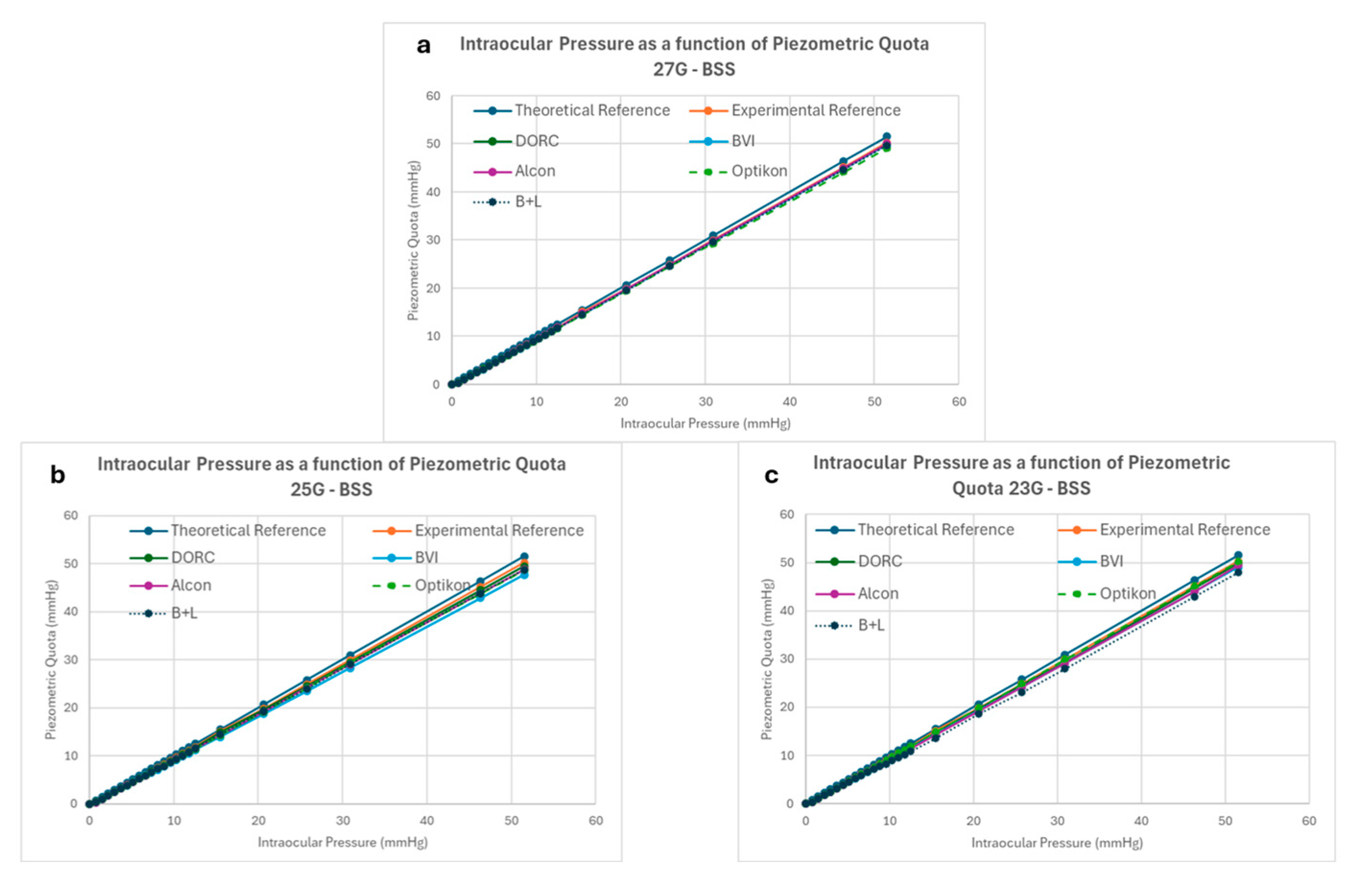
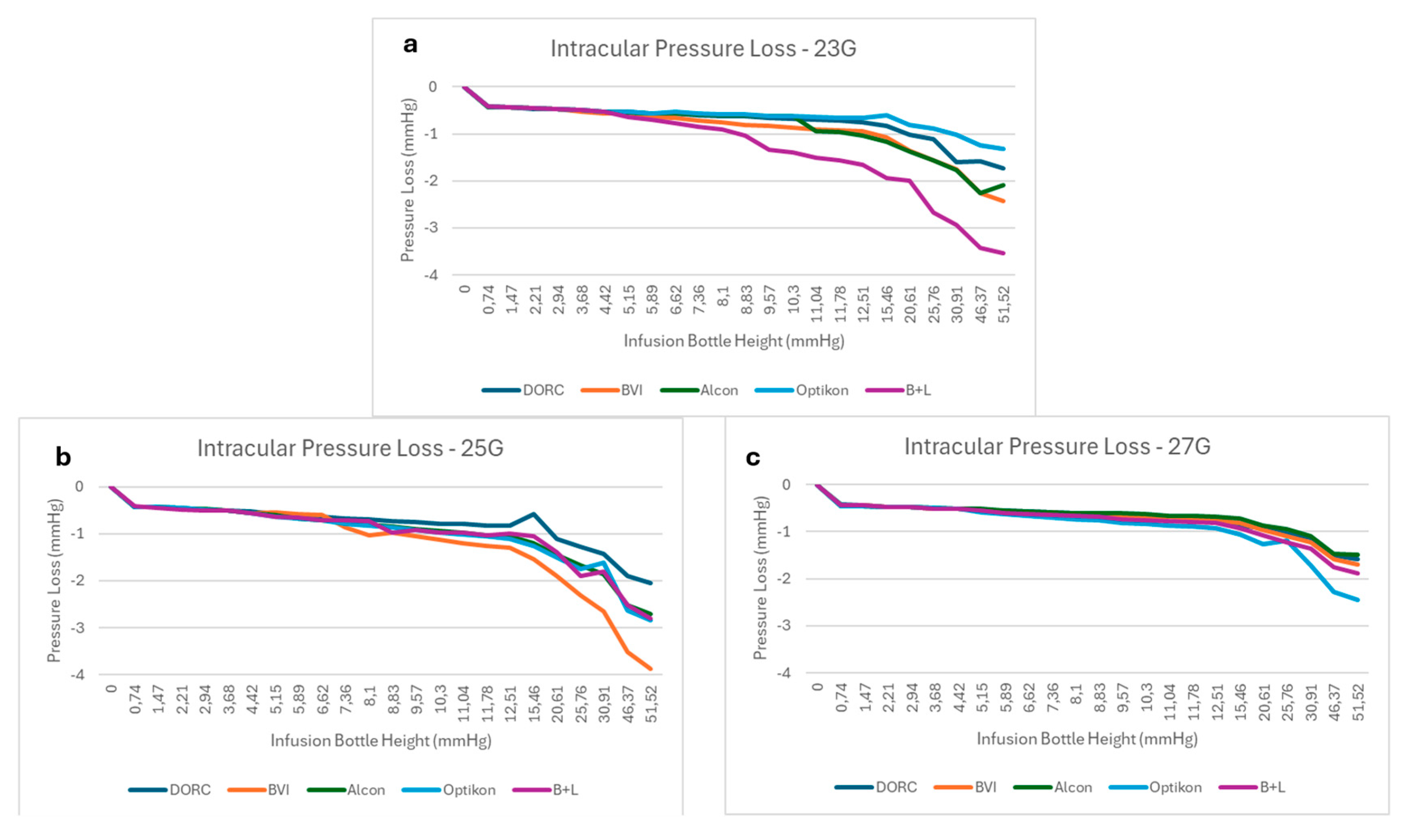
3.1. BSS Infusion Results
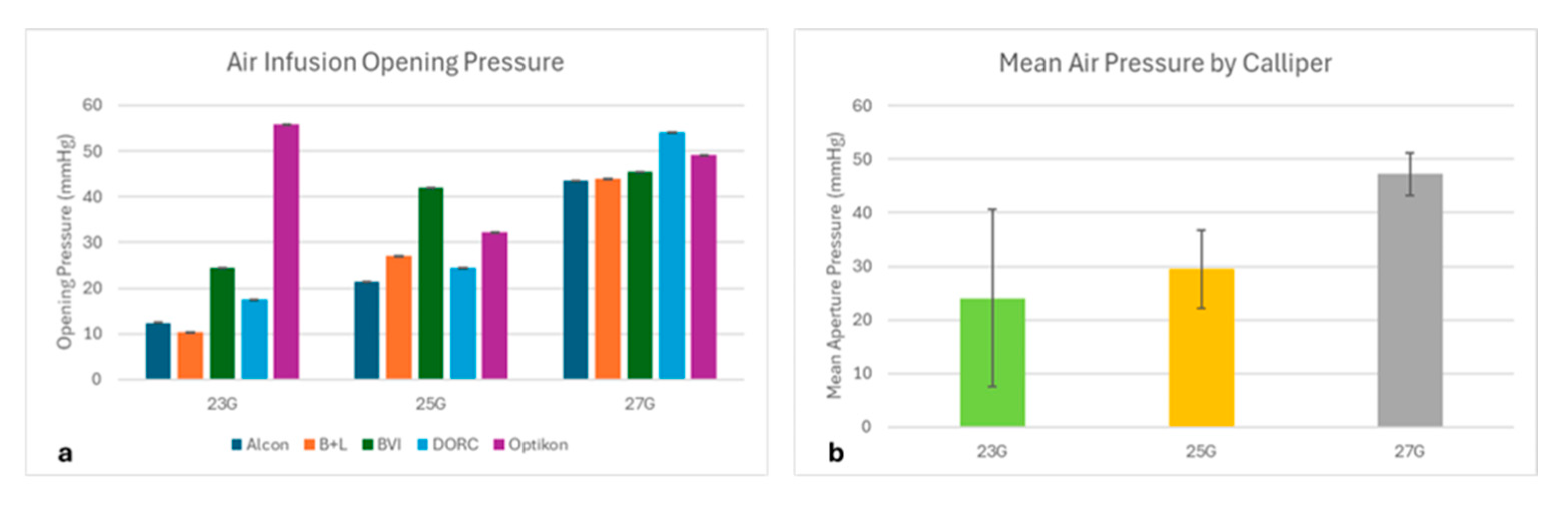
3.2. Air Infusion Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSS | Balanced Salt Solution |
| PPV | Pars Plana Vitrectomy |
| TLA | Three-letter acronym |
| LD | Linear dichroism |
References
- Steel, D.H.; Charles, S. Vitrectomy fluidics. Ophthalmologica 2011, 226 (Suppl. 1), 27–35. [Google Scholar] [CrossRef] [PubMed]
- Piper, J.G.; Han, D.P.; Abrams, G.W.; Mieler, W.F. Perioperative choroidal hemorrhage at pars plana vitrectomy. A case-control study. Ophthalmology 1993, 100, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Tarantola, R.M.; Folk, J.C.; Shah, S.S.; Boldt, H.C.; Abràmoff, M.D.; Russell, S.R.; Mahajan, V.B. Intraoperative choroidal detachment during 23-gauge vitrectomy. Retina 2011, 31, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Fujii, G.Y.; de Juan, E.; Humayun, M.S.; Pieramici, D.J.; Chang, T.S.; Ng, E.; Barnes, A.; Wu, S.L.; Sommerville, D.N. A new 25-gauge instrument system for transconjunctival sutureless vitrectomy surgery. Ophthalmology 2002, 109, 1807–1812; discussion 1813. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Wakabayashi, T.; Sato, T.; Ohji, M.; Tano, Y. A 27-gauge instrument system for transconjunctival sutureless microincision vitrectomy surgery. Ophthalmology 2010, 117, 93–102.e2. [Google Scholar] [CrossRef] [PubMed]
- Rossi, T.; Querzoli, G.; Malvasi, C.D.; Iossa, M.; Angelini, G.D.; Ripandelli, G. A new vitreous cutter blade engineered for constant flow vitrectomy. Retina 2014, 34, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, C. Transconjunctival sutureless 23-gauge vitrectomy. Retina 2005, 25, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Rossi, T.; Querzoli, G.; Angelini, G.; Malvasi, C.; Rossi, A.; Morini, M.; Esposito, G.; Micera, A.; di Luca, N.M.; Ripandelli, G. Hydraulic Resistance of Vitreous Cutters: The Impact of Blade Design and Cut Rate. Transl. Vis. Sci. Technol. 2016, 5, 1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abulon, D.J.K.; Charles, M.; Charles, D. Globe stability during simulated vitrectomy with valved and non-valved trocar cannulas. Clin. Ophthalmol. 2015, 9, 1745–1752. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oellers, P.; Stinnett, S.; Mruthyunjaya, P.; Hahn, P. Small-Gauge Valved Versus Nonvalved Cannula Pars Plana Vitrectomy for Retinal Detachment Repair. Retina 2016, 36, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Felcida, V.; Kumar, N.; Haynes, R.; Habal, S.; Tyagi, A.K. Retained silicone tip of diamond-dusted membrane scraper during vitrectomy in a valved cannula system. Eye 2015, 29, 574–576. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oellers, P.; Schneider, E.W.; Fekrat, S.; Mahmoud, T.H.; Mruthyunjaya, P.; Hahn, P. Retained Intraocular Perfluoro-n-octane After Valved Cannula Pars Plana Vitrectomy for Retinal Detachment. Ophthalmic Surg. Lasers Imaging Retin. 2015, 46, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Rossi, T.; Querzoli, G.; Angelini, G.; Rossi, A.; Malvasi, C.; Iossa, M.; Ripandelli, G. Ocular perfusion pressure during pars plana vitrectomy: A pilot study. Investig. Opthalmology Vis. Sci. 2014, 55, 8497–8505. [Google Scholar] [CrossRef] [PubMed]
- Rossi, T.; Querzoli, G.; Gelso, A.; Angelini, G.; Rossi, A.; Corazza, P.; Landi, L.; Telani, S.; Ripandelli, G. Ocular perfusion pressure control during pars plana vitrectomy: Testing a novel device. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 2325–2330. [Google Scholar] [CrossRef] [PubMed]
- Tabandeh, H.; Flynn, H.W., Jr. Suprachoroidal hemorrhage during pars plana vitrectomy. Curr. Opin. Ophthalmol. 2001, 12, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fang, D.; Peng, M.; Wei, Y.; Wang, L.; Fan, S.; Zhang, S. Systemic Approach to Prevent Inadvertent Perfusion in Eyes with Extensive Choroidal Detachment, Suprachoroidal Fluid, and Hypotony During Pars Plana Vitrectomy. Adv. Ther. 2019, 36, 257–264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kerrison, J.B.; Haller, J.A.; Elman, M.; Miller, N.R. Visual field loss following vitreous surgery. Arch. Ophthalmol. 1996, 114, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Fujii, G.Y.; de Juan, E., Jr.; Humayun, M.S.; Chang, T.S.; Pieramici, D.J.; Barnes, A.; Kent, D. Initial experience using the transconjunctival sutureless vitrectomy system for vitreoretinal surgery. Ophthalmology 2002, 109, 1814–1820. [Google Scholar] [CrossRef] [PubMed]





| 23G | 25G | 27G | |
|---|---|---|---|
| Alcon | 10.94 ± 1 | 5.42 ± 0.36 | 7.13 ± 0.55 |
| B+L | 3.02 ± 0.34 | 9.18 ± 0.35 | 5.25 ± 1.23 |
| BVI | 7.03 + 0.87 | 8.62 ± 0.52 | 10.02 ± 0.54 |
| DORC | 8.6 ± 0.78 | 10.25 ± 0.65 | 8.87 ± 0.9 |
| Optikon | 8.88 ± 0.71 | 16.15 ± 0.84 | 6.58 ± 0.59 |
| p value | <0.01 | <0.01 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, T.; Querzoli, G.; Angelini, G.B.; Pellizzaro, C.; Santoro, V.; Rosari, G.; Parravano, M.; Steel, D.H.; Romano, M.R. Comparing the Efficiency of Valved Trocar Cannulas for Pars Plana Vitrectomy. Bioengineering 2025, 12, 431. https://doi.org/10.3390/bioengineering12040431
Rossi T, Querzoli G, Angelini GB, Pellizzaro C, Santoro V, Rosari G, Parravano M, Steel DH, Romano MR. Comparing the Efficiency of Valved Trocar Cannulas for Pars Plana Vitrectomy. Bioengineering. 2025; 12(4):431. https://doi.org/10.3390/bioengineering12040431
Chicago/Turabian StyleRossi, Tommaso, Giorgio Querzoli, Giov Battista Angelini, Camilla Pellizzaro, Veronica Santoro, Giulia Rosari, Mariacristina Parravano, David H. Steel, and Mario R. Romano. 2025. "Comparing the Efficiency of Valved Trocar Cannulas for Pars Plana Vitrectomy" Bioengineering 12, no. 4: 431. https://doi.org/10.3390/bioengineering12040431
APA StyleRossi, T., Querzoli, G., Angelini, G. B., Pellizzaro, C., Santoro, V., Rosari, G., Parravano, M., Steel, D. H., & Romano, M. R. (2025). Comparing the Efficiency of Valved Trocar Cannulas for Pars Plana Vitrectomy. Bioengineering, 12(4), 431. https://doi.org/10.3390/bioengineering12040431







