Comparative Evaluation of Bovine- and Porcine-Deproteinized Grafts for Guided Bone Regeneration: An In Vivo Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Bone Grafts
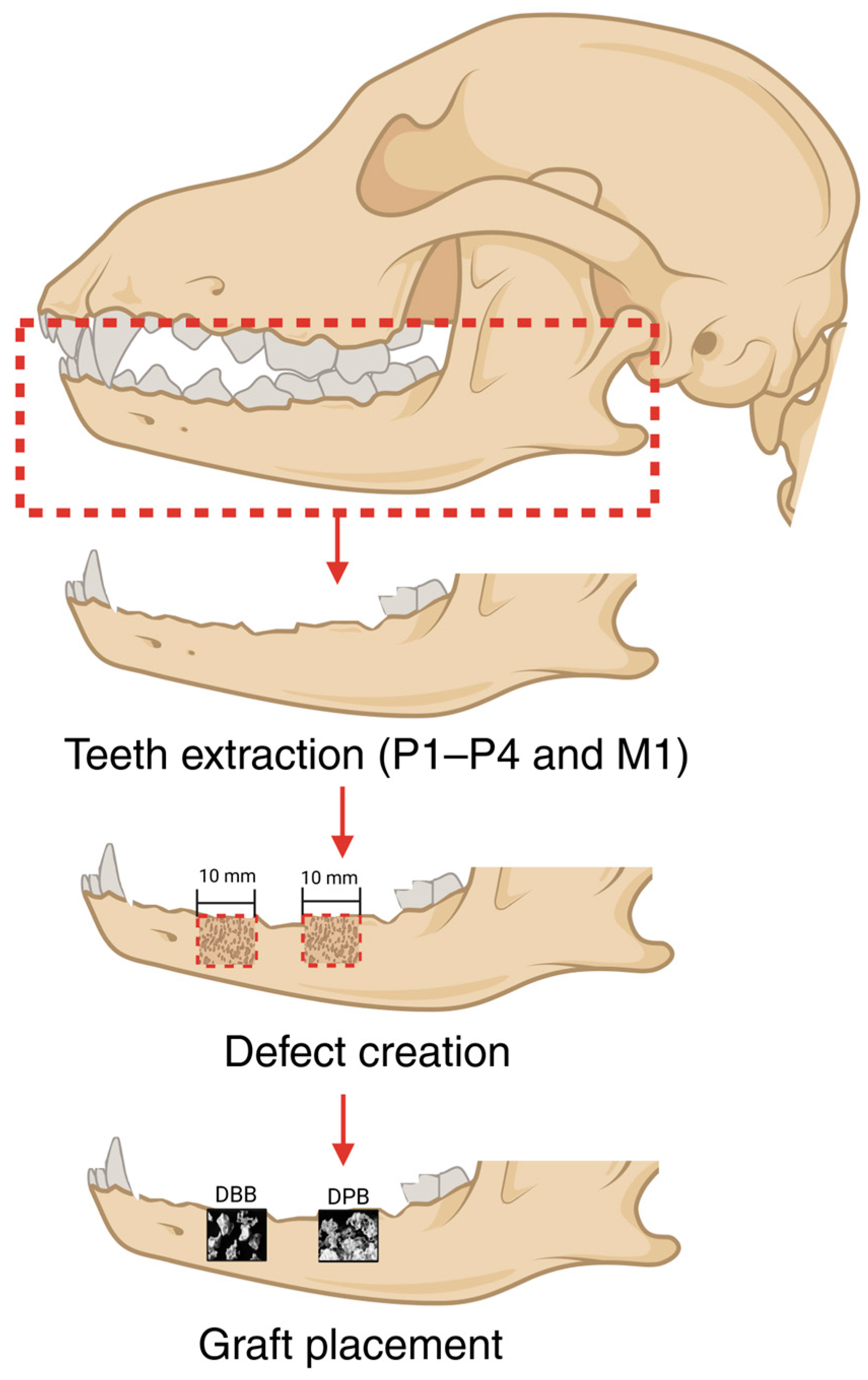
2.2. Surgical Procedure
2.3. Volumetric Reconstruction and Analysis
2.4. Histologic Preparation and Analysis
2.5. Statistical Analysis
3. Results
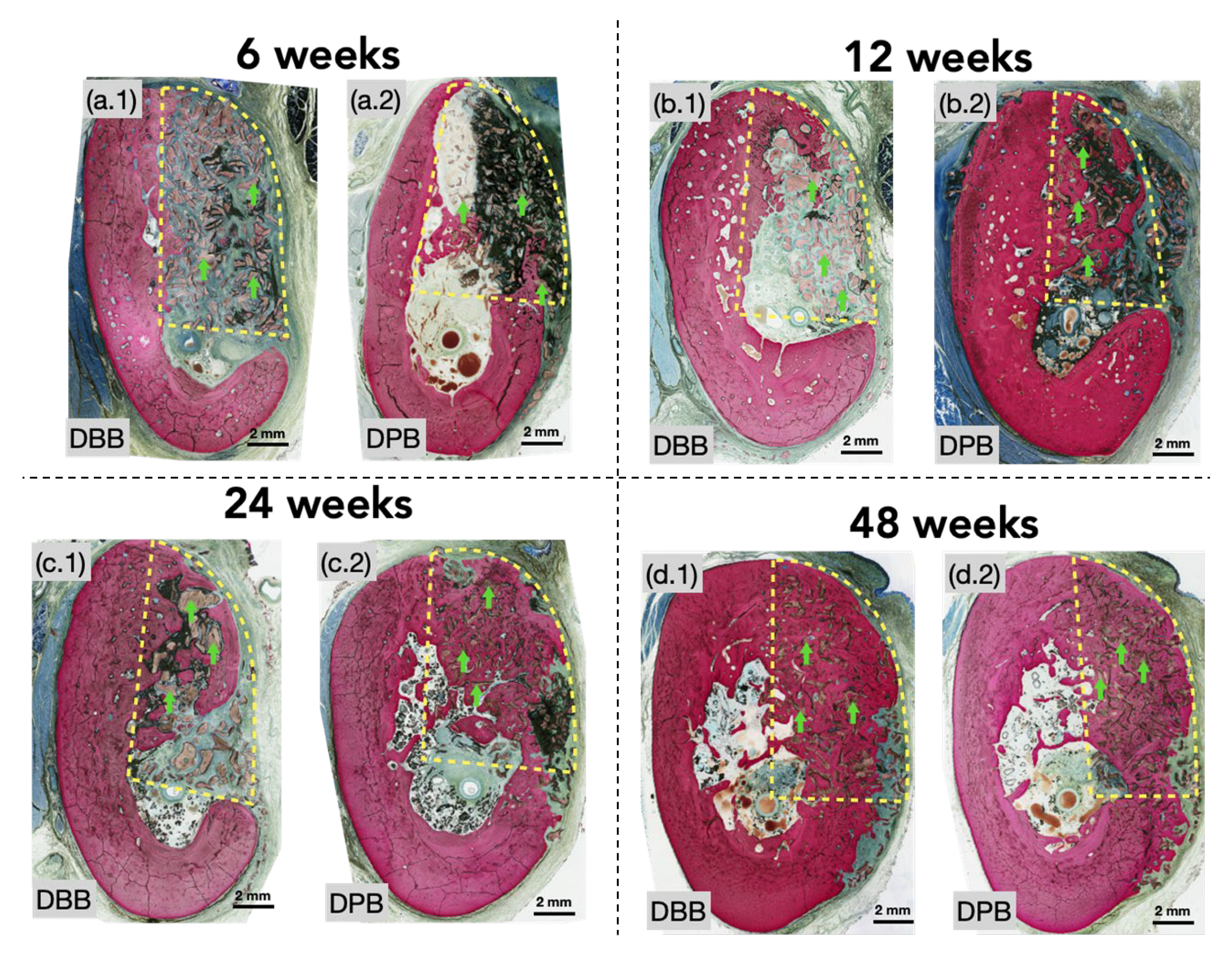
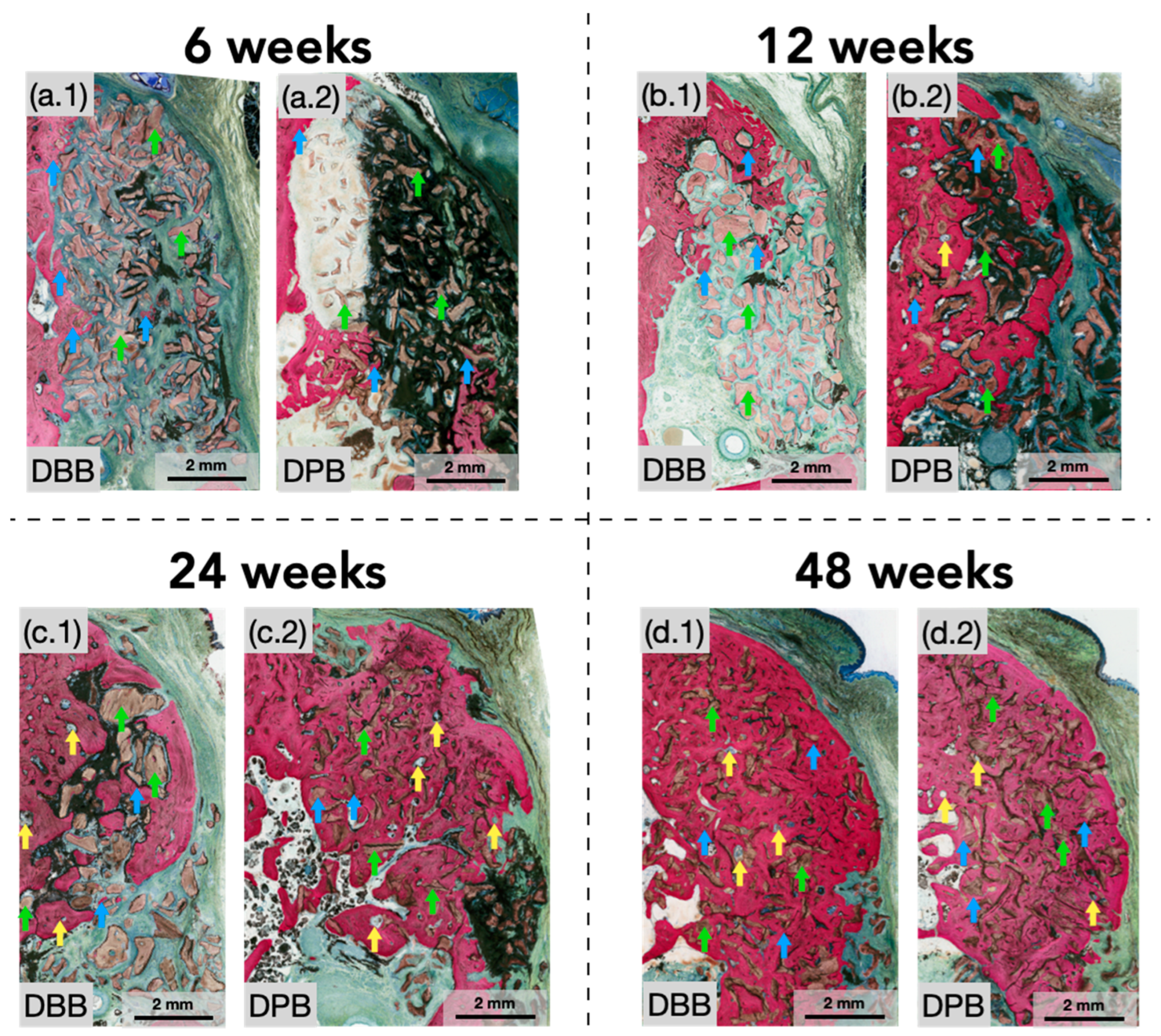
3.1. Qualitative Histological Evaluation
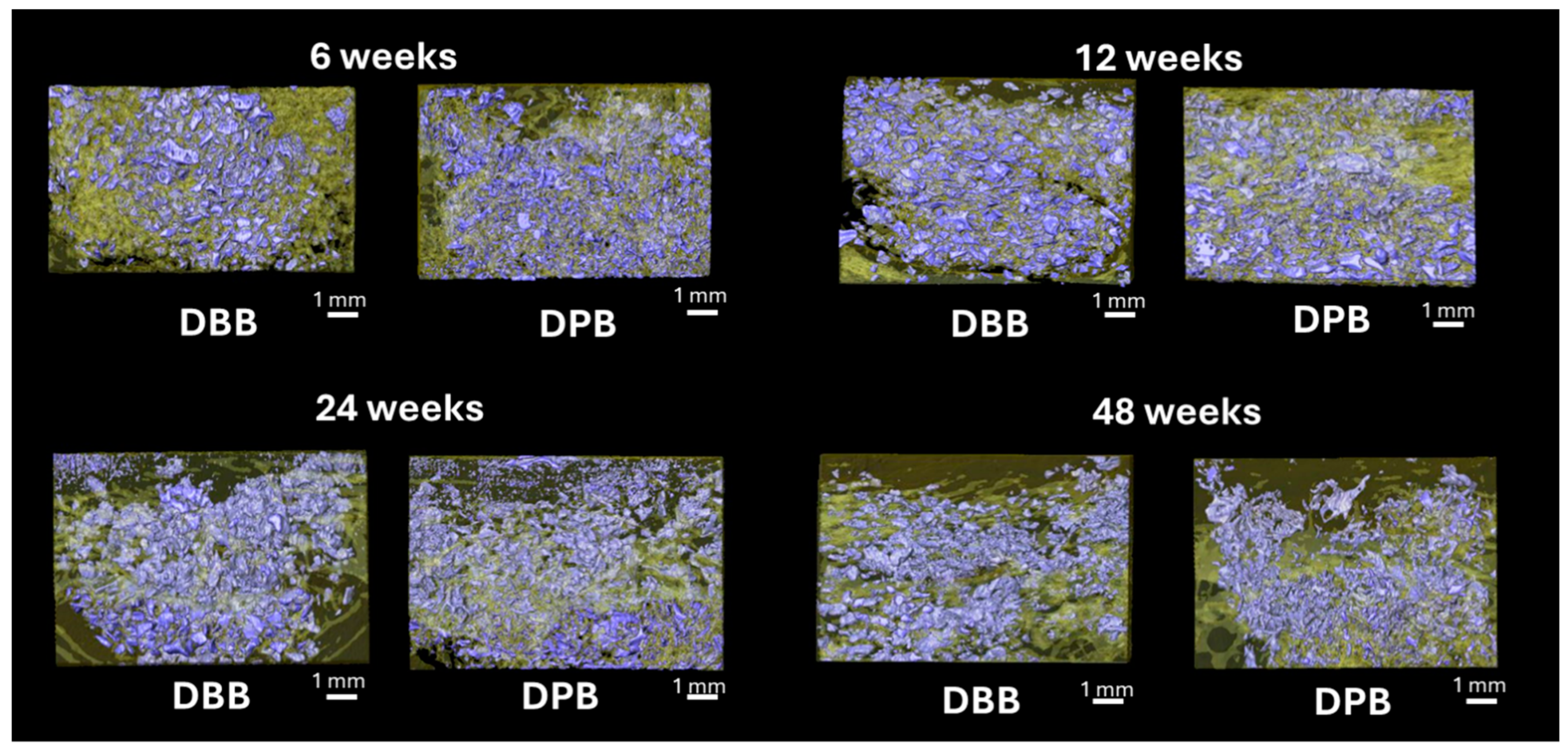
3.2. MicroCT and Volumetric Reconstruction Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chappuis, V.; Araújo, M.G.; Buser, D. Clinical relevance of dimensional bone and soft tissue alterations post-extraction in esthetic sites. Periodontology 2000 2017, 73, 73–83. [Google Scholar] [CrossRef]
- Batista, M.J.; Lawrence, H.P.; Rosário de Sousa, M.d.L. Impact of tooth loss related to number and position on oral health quality of life among adults. Health Qual. Life outcomes 2014, 12, 165. [Google Scholar] [CrossRef]
- Amler, M.H.; Johnson, P.L.; Salman, I. Histological and histochemical investigation of human alveolar socket healing in undisturbed extraction wounds. J. Am. Dent. Assoc. 1960, 61, 32–44. [Google Scholar] [CrossRef]
- Araújo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Atwood, D.A.; Coy, W.A. Clinical, cephalometric, and densitometric study of reduction of residual ridges. J. Prosthet. Dent. 1971, 26, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Hämmerle, C.H.; Araújo, M.G.; Simion, M. Evidence-based knowledge on the biology and treatment of extraction sockets. Clin. Oral Implant. Res. 2012, 23 (Suppl. S5), 80–82. [Google Scholar] [CrossRef] [PubMed]
- Busei, D.; Dula, K.; Belser, U.C.; Hirt, H.-P.; Berthold, H.; Dentistry, R. Localized ridge augmentation using guided bone regeneration. II. Surgical procedure in the mandible. Int. J. Periodontics Restor. Dent. 1995, 15, 11. [Google Scholar]
- Buser, D.; Dula, K.; Belser, U.; Hirt, H.-P.; Berthold, H.; Dentistry, R. Localized ridge augmentation using guided bone regeneration. I. Surgical procedure in the maxilla. J. Periodontics Restor. Dent. 1993, 13, 28. [Google Scholar]
- Zhou, X.; Zhang, Z.; Li, S.; Bai, Y.; Xu, H.; Surgery, M. Osteoconduction of different sizes of anorganic bone particles in a model of guided bone regeneration. Br. J. Oral Maxillofac. Surg. 2011, 49, 37–41. [Google Scholar] [CrossRef]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implant. Res. 2010, 21, 567–576. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [CrossRef] [PubMed]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2001, 22, 87–96. [Google Scholar] [CrossRef]
- Misch, C.M. Autogenous bone: Is it still the gold standard? Implant Dent. 2010, 19, 361. [Google Scholar] [CrossRef]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52 (Suppl. 2), S18–S22. [Google Scholar] [CrossRef]
- Ferraz, M.P. Bone Grafts in Dental Medicine: An Overview of Autografts, Allografts and Synthetic Materials. Materials 2023, 16, 4117. [Google Scholar] [CrossRef] [PubMed]
- Gil, L.F.; Nayak, V.V.; Benalcázar Jalkh, E.B.; Tovar, N.; Chiu, K.J.; Salas, J.C.; Marin, C.; Bowers, M.; Freitas, G.; Mbe Fokam, D.C.J.J.o.B.M.R.P.B.A.B. Laddec® versus Bio-Oss®: The effect on the healing of critical-sized defect–Calvaria rabbit model. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2022, 110, 2744–2750. [Google Scholar] [CrossRef]
- Shibuya, N.; Jupiter, D.C. Bone graft substitute: Allograft and xenograft. Clin. Podiatr. Med. Surg. 2015, 32, 21–34. [Google Scholar] [CrossRef]
- Rapone, B.; Inchingolo, A.D.; Trasarti, S.; Ferrara, E.; Qorri, E.; Mancini, A.; Montemurro, N.; Scarano, A.; Inchingolo, A.M.; Dipalma, G.; et al. Long-Term Outcomes of Implants Placed in Maxillary Sinus Floor Augmentation with Porous Fluorohydroxyapatite (Algipore® FRIOS®) in Comparison with Anorganic Bovine Bone (Bio-Oss®) and Platelet Rich Plasma (PRP): A Retrospective Study. J. Clin. Med. 2022, 11, 2491. [Google Scholar] [CrossRef]
- Richardson, C.R.; Mellonig, J.T.; Brunsvold, M.A.; McDonnell, H.T.; Cochran, D.L. Clinical evaluation of Bio-Oss®: A bovine-derived xenograft for the treatment of periodontal osseous defects in humans. J. Clin. Periodontol. 1999, 26, 421–428. [Google Scholar] [CrossRef]
- Cordaro, L.; Bosshardt, D.D.; Palattella, P.; Rao, W.; Serino, G.; Chiapasco, M. Maxillary sinus grafting with Bio-Oss® or Straumann® Bone Ceramic: Histomorphometric results from a randomized controlled multicenter clinical trial. Clin. Oral Implant. Res. 2008, 19, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Xu, J.; Han, X.-H.; Cui, F.-Z.; Zhang, D.-S.; Huang, S.-Y. Clinical efficacy of mineralized collagen (MC) versus anorganic bovine bone (Bio-Oss) for immediate implant placement in esthetic area: A single-center retrospective study. BMC Oral Health 2021, 21, 390. [Google Scholar] [CrossRef]
- Piattelli, M.; Favero, G.A.; Scarano, A.; Orsini, G.; Piattelli, A. Bone reactions to anorganic bovine bone (Bio-Oss) used in sinus augmentation procedures: A histologic long-term report of 20 cases in humans. Int. J. Oral Maxillofac. Implant. 1999, 14, 835–840. [Google Scholar]
- Roldan, L.; Isaza, C.; Ospina, J.; Montoya, C.; Domínguez, J.; Orrego, S.; Correa, S. A Comparative Study of HA/DBM Compounds Derived from Bovine and Porcine for Bone Regeneration. J. Funct. Biomater. 2023, 14, 439. [Google Scholar] [CrossRef] [PubMed]
- Perić Kačarević, Z.; Kavehei, F.; Houshmand, A.; Franke, J.; Smeets, R.; Rimashevskiy, D.; Wenisch, S.; Schnettler, R.; Jung, O.; Barbeck, M. Purification processes of xenogeneic bone substitutes and their impact on tissue reactions and regeneration. Int. J. Artif. Organs 2018, 41, 789–800. [Google Scholar] [CrossRef]
- Pereira, R.d.S.; de Carvalho, M.V.N.B.; Hochuli-Vieira, E.; Statkievicz, C.; Pereira Santos, D.L.; Augusto Neto, R.T.; Pinto, C.d.F.S.; Bennardo, F.; Mourão, C.F.J.M. Histomorphometric and Micro-CT Evaluation of Cerabone and Bio-Oss in Maxillary Sinus Lifting: A Randomized Clinical Trial. Medicina 2024, 60, 1834. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xiang, S.; Liu, J.; Ye, L.; Cao, Z.; Pan, J.J.O.S. Combigraft versus Bio-Oss/Bio-Gide in alveolar ridge preservation: A prospective randomized controlled trial. Oral Surg. 2022, 15, 163–169. [Google Scholar] [CrossRef]
- Titsinides, S.; Agrogiannis, G.; Karatzas, T. Bone grafting materials in dentoalveolar reconstruction: A comprehensive review. Jpn. Dent. Sci. Rev. 2019, 55, 26–32. [Google Scholar] [CrossRef]
- Seo, Y.H.; Hwang, S.H.; Kim, Y.N.; Kim, H.J.; Bae, E.B.; Huh, J.B. Bone Reconstruction Using Two-Layer Porcine-Derived Bone Scaffold Composed of Cortical and Cancellous Bones in a Rabbit Calvarial Defect Model. Int. J. Mol. Sci. 2022, 23, 2647. [Google Scholar] [CrossRef]
- Singh, R.; Mahesh, L.; Shukla, S.J.I.J.O.I.C.R. Infections resulting from bone grafting biomaterials. Int. J. Oral Implant. Clin. Res. 2013, 4, 68–71. [Google Scholar] [CrossRef]
- Collagen Matrix, I. Dental Repair Solutions. 15. Available online: https://regenity.com/solution/dental/ (accessed on 21 May 2024).
- Bergamo, E.T.P.; Balderrama, Í.d.F.; Ferreira, M.R.; Spielman, R.; Slavin, B.V.; Torroni, A.; Tovar, N.; Nayak, V.V.; Slavin, B.R.; Coelho, P.G.; et al. Osteogenic differentiation and reconstruction of mandible defects using a novel resorbable membrane: An in vitro and in vivo experimental study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 1966–1978. [Google Scholar] [CrossRef] [PubMed]
- Benic, G.I.; Hammerle, C.H. Horizontal bone augmentation by means of guided bone regeneration. Periodontology 2000 2014, 66, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Mataliotakis, G.I.; Calori, G.M.; Giannoudis, P.V. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Hammerle, C.H.; Jung, R.E. Bone augmentation by means of barrier membranes. Periodontology 2000 2003, 33, 36–53. [Google Scholar] [CrossRef]
- Khan, R.S.; Aslam, M.; Ucer, C.; Wright, S. Success of Xenografts in Alveolar Ridge Preservation Based on Histomorphometric Outcomes. Dent. J. 2023, 11, 215. [Google Scholar] [CrossRef]
- Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Animal models for periodontal regeneration and peri-implant responses. Periodontology 2000 2015, 68, 66–82. [Google Scholar] [CrossRef]
- Sennerby, L.; Persson, L.G.; Berglundh, T.; Wennerberg, A.; Lindhe, J.J.C.I.D.; Research, R. Implant stability during initiation and resolution of experimental periimplantitis: An experimental study in the dog. Clin. Implant. Dent. Relat. Res. 2005, 7, 136–140. [Google Scholar] [CrossRef]
- Gotfredsen, K.; Berglundh, T.; Lindhe, J. Bone reactions at implants subjected to experimental peri-implantitis and static load: A study in the dog. J. Clin. Periodontol. 2002, 29, 144–151. [Google Scholar] [CrossRef]
- Pellegrini, G.; Seol, Y.J.; Gruber, R.; Giannobile, W.V. Pre-clinical models for oral and periodontal reconstructive therapies. J. Dent. Res. 2009, 88, 1065–1076. [Google Scholar] [CrossRef]
- Miyauchi, Y.; Izutani, T.; Teranishi, Y.; Iida, T.; Nakajima, Y.; Xavier, S.P.; Baba, S. Healing Patterns of Non-Collagenated Bovine and Collagenated Porcine Xenografts Used for Sinus Floor Elevation: A Histological Study in Rabbits. J. Funct. Biomater. 2022, 13, 276. [Google Scholar] [CrossRef]
- Hwang, S.H.; Moon, K.; Du, W.; Cho, W.T.; Huh, J.B.; Bae, E.B. Effect of Porcine- and Bovine-Derived Xenografts with Hydroxypropyl Methylcellulose for Bone Formation in Rabbit Calvaria Defects. Materials 2023, 16, 1850. [Google Scholar] [CrossRef]
- Lai, V.J.; Michalek, J.E.; Liu, Q.; Mealey, B.L. Ridge preservation following tooth extraction using bovine xenograft compared with porcine xenograft: A randomized controlled clinical trial. J. Periodontol. 2020, 91, 361–368. [Google Scholar] [CrossRef]
- Schwarz, F.; Herten, M.; Ferrari, D.; Wieland, M.; Schmitz, L.; Engelhardt, E.; Becker, J. Guided bone regeneration at dehiscence-type defects using biphasic hydroxyapatite+beta tricalcium phosphate (Bone Ceramic®) or a collagen-coated natural bone mineral (BioOss Collagen®): An immunohistochemical study in dogs. Int. J. Oral Maxillofac. Surg. 2007, 36, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-I.; Yang, C.; Kim, Y.-T.; Kim, M.-S.; Lee, J.-S.; Choi, S.-H.; Jung, U.-W. Space maintenance using crosslinked collagenated porcine bone grafted without a barrier membrane in one-wall intrabony defects. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1454–1461. [Google Scholar] [CrossRef]
- Park, S.J.; Rahman, M.M.; Lee, J.; Kang, S.W.; Kim, S. Investigation of Bone Regeneration Efficacy of New Bovine Bone Minerals in a Canine Mandibular Critical Defect Model. Adv. Healthc. Mater. 2023, 12, e2202942. [Google Scholar] [CrossRef] [PubMed]
- Bee, S.L.; Hamid, Z.A.A.J.J.o.B.M.R.P.B.A.B. Asymmetric resorbable-based dental barrier membrane for periodontal guided tissue regeneration and guided bone regeneration: A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 2157–2182. [Google Scholar] [CrossRef] [PubMed]
- Juodzbalys, G.; Raustia, A.M.; Kubilius, R. A 5-year follow-up study on one-stage implants inserted concomitantly with localized alveolar ridge augmentation. J. Oral Rehabil. 2007, 34, 781–789. [Google Scholar] [CrossRef]
- Beretta, M.; Cicciù, M.; Poli, P.P.; Rancitelli, D.; Bassi, G.; Grossi, G.B.; Maiorana, C. A Retrospective Evaluation of 192 Implants Placed in Augmented Bone: Long-Term Follow-Up Study. J. Oral Implantol. 2015, 41, 669–674. [Google Scholar] [CrossRef]
- Schmitt, C.M.; Moest, T.; Lutz, R.; Neukam, F.W.; Schlegel, K.A. Anorganic bovine bone (ABB) vs. autologous bone (AB) plus ABB in maxillary sinus grafting. A prospective non-randomized clinical and histomorphometrical trial. Clin. Oral Implant. Res. 2015, 26, 1043–1050. [Google Scholar] [CrossRef]
- Lutz, R.; Berger-Fink, S.; Stockmann, P.; Neukam, F.W.; Schlegel, K.A. Sinus floor augmentation with autogenous bone vs. a bovine-derived xenograft—A 5-year retrospective study. Clin. Oral Implant. Res. 2015, 26, 644–648. [Google Scholar] [CrossRef]
- Choi, J.W.; Hwang, S.S.; Yun, P.Y.; Kim, Y.K. Horizontal ridge augmentation with porcine bone-derived grafting material: A long-term retrospective clinical study with more than 5 years of follow-up. J. Korean Assoc. Oral Maxillofac. Surg. 2023, 49, 324–331. [Google Scholar] [CrossRef]
- Araújo, M.G.; Lindhe, J. Ridge preservation with the use of Bio-Oss® collagen: A 6-month study in the dog. Clin. Oral Implant. Res. 2009, 20, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Mario Roccuzzo, D.J.I.J.P.R.D. Long-term stability of soft tissues following alveolar ridge preservation: 10-year results of a prospective study around nonsubmerged implants. Int. J. Periodontics Restor. Dent. 2014, 34, 795–804. [Google Scholar] [CrossRef]
- Ignatius, A.; Blessing, H.; Liedert, A.; Schmidt, C.; Neidlinger-Wilke, C.; Kaspar, D.; Friemert, B.; Claes, L. Tissue engineering of bone: Effects of mechanical strain on osteoblastic cells in type I collagen matrices. Biomaterials 2005, 26, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Gresita, A.; Raja, I.; Petcu, E.; Hadjiargyrou, M. Collagen-Coated Hyperelastic Bone Promotes Osteoblast Adhesion and Proliferation. Materials 2023, 16, 6996. [Google Scholar] [CrossRef]
- Paknejad, M.; Rokn, A.R.; Yaghobee, S.; Moradinejad, P.; Heidari, M.; Mehrfard, A.J.J.o.D. Effects of two types of anorganic bovine bone on bone regeneration: A histological and histomorphometric study of rabbit calvaria. J. Dent. 2014, 11, 687. [Google Scholar]
- Schliephake, H.; Dard, M.; Planck, H.; Hierlemann, H.; Jakob, A. Guided bone regeneration around endosseous implants using a resorbable membrane vs a PTFE membrane. Clin. Oral Implant. Res. 2000, 11, 230–241. [Google Scholar] [CrossRef]
- Hämmerle, C.H.; Brägger, U.; Bürgin, W.; Lang, N.P. The effect of subcrestal placement of the polished surface of ITI implants on marginal soft and hard tissues. Clin. Oral Implant. Res. 1996, 7, 111–119. [Google Scholar] [CrossRef]
- Avera, S.P.; Stampley, W.A.; McAllister, B.S. Histologic and clinical observations of resorbable and nonresorbable barrier membranes used in maxillary sinus graft containment. Int. J. Oral Maxillofac. Implant. 1997, 12, 88–94. [Google Scholar]
- Lee, J.H.; Yi, G.S.; Lee, J.W.; Kim, D.J. Physicochemical characterization of porcine bone-derived grafting material and comparison with bovine xenografts for dental applications. J. Periodontal Implant. Sci. 2017, 47, 388–401. [Google Scholar] [CrossRef]
- Pimentel, I.; Henriques, B.; Silva, F.; Carvalho, O.; Teughels, W.; Ozcan, M.; Souza, J.C.M. Morphological aspects and distribution of granules composed of deproteinized bovine bone or human dentin into a putty mixture: An in vitro study. Head Face Med. 2023, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Tapety, F.I.; Amizuka, N.; Uoshima, K.; Nomura, S.; Maeda, T. A histological evaluation of the involvement of Bio-Oss® in osteoblastic differentiation and matrix synthesis. Clin. Oral Implant. Res. 2004, 15, 315–324. [Google Scholar] [CrossRef]
- Mukasheva, F.; Adilova, L.; Dyussenbinov, A.; Yernaimanova, B.; Abilev, M.; Akilbekova, D. Optimizing scaffold pore size for tissue engineering: Insights across various tissue types. Front. Bioeng. Biotechnol. 2024, 12, 1444986. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.; Fernando, A.; Martins, G.; Freitas, J.; Judas, F.; Figueiredo, H. Effect of the calcination temperature on the composition and microstructure of hydroxyapatite derived from human and animal bone. Ceram. Int. 2010, 36, 2383–2393. [Google Scholar] [CrossRef]
- Mano, T.; Akita, K.; Fukuda, N.; Kamada, K.; Kurio, N.; Ishikawa, K.; Miyamoto, Y. Histological comparison of three apatitic bone substitutes with different carbonate contents in alveolar bone defects in a beagle mandible with simultaneous implant installation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Wenz, B.; Oesch, B.; Horst, M. Analysis of the risk of transmitting bovine spongiform encephalopathy through bone grafts derived from bovine bone. Biomaterials 2001, 22, 1599–1606. [Google Scholar] [CrossRef]
- Moreno-Gonzalez, I.; Soto, C. Misfolded protein aggregates: Mechanisms, structures and potential for disease transmission. Semin. Cell Dev. Biol. 2011, 22, 482–487. [Google Scholar] [CrossRef]
- Scicchitano, L.J.I. Bovine spongiform encephalopathy and Creutzfeldt-Jakob disease: Background and implications for nursing practice. Insight 2004, 29, 17, 19–21. [Google Scholar]
- Sogal, A.; Tofe, A.J.J.o.p. Risk assessment of bovine spongiform encephalopathy transmission through bone graft material derived from bovine bone used for dental applications. J. Periodontol. 1999, 70, 1053–1063. [Google Scholar] [CrossRef]
- Li, S.-T.; Chen, H.-C.; Yuen, D. Method of Preparing Porous Carbonate Apatite from Natural Bone. 2015. Available online: https://patents.google.com/patent/US20150250921A1/en (accessed on 11 December 2023).
- De Stavola, L.; Tunkel, J.; Dentistry, R. Results of vertical bone augmentation with autogenous bone block grafts and the tunnel technique: A clinical prospective study of 10 consecutively treated patients. Int. J. Periodontics Restor. Dent. 2013, 33, 651. [Google Scholar] [CrossRef]
- Sanz-Sánchez, I.; Sanz-Martín, I.; Ortiz-Vigón, A.; Molina, A.; Sanz, M. Complications in bone-grafting procedures: Classification and management. Periodontology 2000 2022, 88, 86–102. [Google Scholar] [CrossRef] [PubMed]
- Hasson, O. Augmentation of deficient lateral alveolar ridge using the subperiosteal tunneling dissection approach. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 103, e14–e19. [Google Scholar] [CrossRef] [PubMed]
- Nevins, M.L.; Camelo, M.; Nevins, M.; Schupbach, P.; Friedland, B.; Camelo, J.M.B.; Kim, D.M.J.I.J.o.P.; Dentistry, R. Minimally invasive alveolar ridge augmentation procedure (tunneling technique) using rhPDGF-BB in combination with three matrices: A case series. Int. J. Periodontics Restor. Dent. 2009, 29, 370. [Google Scholar]
- Lo Giudice, R.; Puleio, F.; Rizzo, D.; Alibrandi, A.; Lo Giudice, G.; Centofanti, A.; Fiorillo, L.; Di Mauro, D.; Nicita, F. Comparative Investigation of Cutting Devices on Bone Blocks: An SEM Morphological Analysis. Appl. Sci. 2019, 9, 351. [Google Scholar] [CrossRef]
- Zhu, L.; Du, X.; Fu, G.; Wang, L.; Huang, H.; Wu, X.; Xu, B.J.B.O.H. Efficacy of different forms of concentrated growth factors combined with deproteinized bovine bone minerals in guided bone regeneration: A randomized clinical trial. BMC Oral Health 2025, 25, 320. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cervino, G.; Galindo-Moreno, P.; Herford, A.S.; Spagnuolo, G.; Cicciù, M.J.B.R.I. Growth factors in oral tissue engineering: New perspectives and current therapeutic options. BioMed Res. Int. 2021, 2021, 8840598. [Google Scholar] [CrossRef]


| Time (In Vivo) | Group | Bone (%) | Space (%) | Graft (%) |
|---|---|---|---|---|
| 6 weeks | DBB | 31.39 ± 8.41 | 61.91 ± 9.47 | 6.72 ± 2.17 |
| DPB | 39.76 ± 10.30 | 51.03 ± 11.60 | 9.20 ± 2.66 | |
| 12 weeks | DBB | 37.82 ± 8.41 | 53.71 ± 9.47 | 8.46 ± 2.17 |
| DPB | 46.11 ± 10.30 | 44.26 ± 11.60 | 9.61 ± 2.66 | |
| 24 weeks | DBB | 38.10 ± 8.41 | 59.29 ± 9.47 | 2.60 ± 2.17 |
| DPB | 48.54 ± 10.30 | 46.88 ± 11.60 | 4.57 ± 2.66 | |
| 48 weeks | DBB | 71.04 ± 8.41 | 24.24 ± 9.47 | 4.70 ± 2.17 |
| DPB | 68.38 ± 10.30 | 23.81 ± 11.60 | 7.79 ± 2.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slavin, B.V.; Nayak, V.V.; Parra, M.; Spielman, R.D.; Torquati, M.S.; Iglesias, N.J.; Coelho, P.G.; Witek, L. Comparative Evaluation of Bovine- and Porcine-Deproteinized Grafts for Guided Bone Regeneration: An In Vivo Study. Bioengineering 2025, 12, 459. https://doi.org/10.3390/bioengineering12050459
Slavin BV, Nayak VV, Parra M, Spielman RD, Torquati MS, Iglesias NJ, Coelho PG, Witek L. Comparative Evaluation of Bovine- and Porcine-Deproteinized Grafts for Guided Bone Regeneration: An In Vivo Study. Bioengineering. 2025; 12(5):459. https://doi.org/10.3390/bioengineering12050459
Chicago/Turabian StyleSlavin, Blaire V., Vasudev Vivekanand Nayak, Marcelo Parra, Robert D. Spielman, Matteo S. Torquati, Nicholas J. Iglesias, Paulo G. Coelho, and Lukasz Witek. 2025. "Comparative Evaluation of Bovine- and Porcine-Deproteinized Grafts for Guided Bone Regeneration: An In Vivo Study" Bioengineering 12, no. 5: 459. https://doi.org/10.3390/bioengineering12050459
APA StyleSlavin, B. V., Nayak, V. V., Parra, M., Spielman, R. D., Torquati, M. S., Iglesias, N. J., Coelho, P. G., & Witek, L. (2025). Comparative Evaluation of Bovine- and Porcine-Deproteinized Grafts for Guided Bone Regeneration: An In Vivo Study. Bioengineering, 12(5), 459. https://doi.org/10.3390/bioengineering12050459







