Revolutionizing Utility of Big Data Analytics in Personalized Cardiovascular Healthcare
Abstract
1. Introduction
2. Big Data in Healthcare
2.1. Enhancing CVD Treatment and Research Through Big Data Analytics
2.2. Challenges
3. Applications of Big Data Analytics in Cardiovascular Diseases
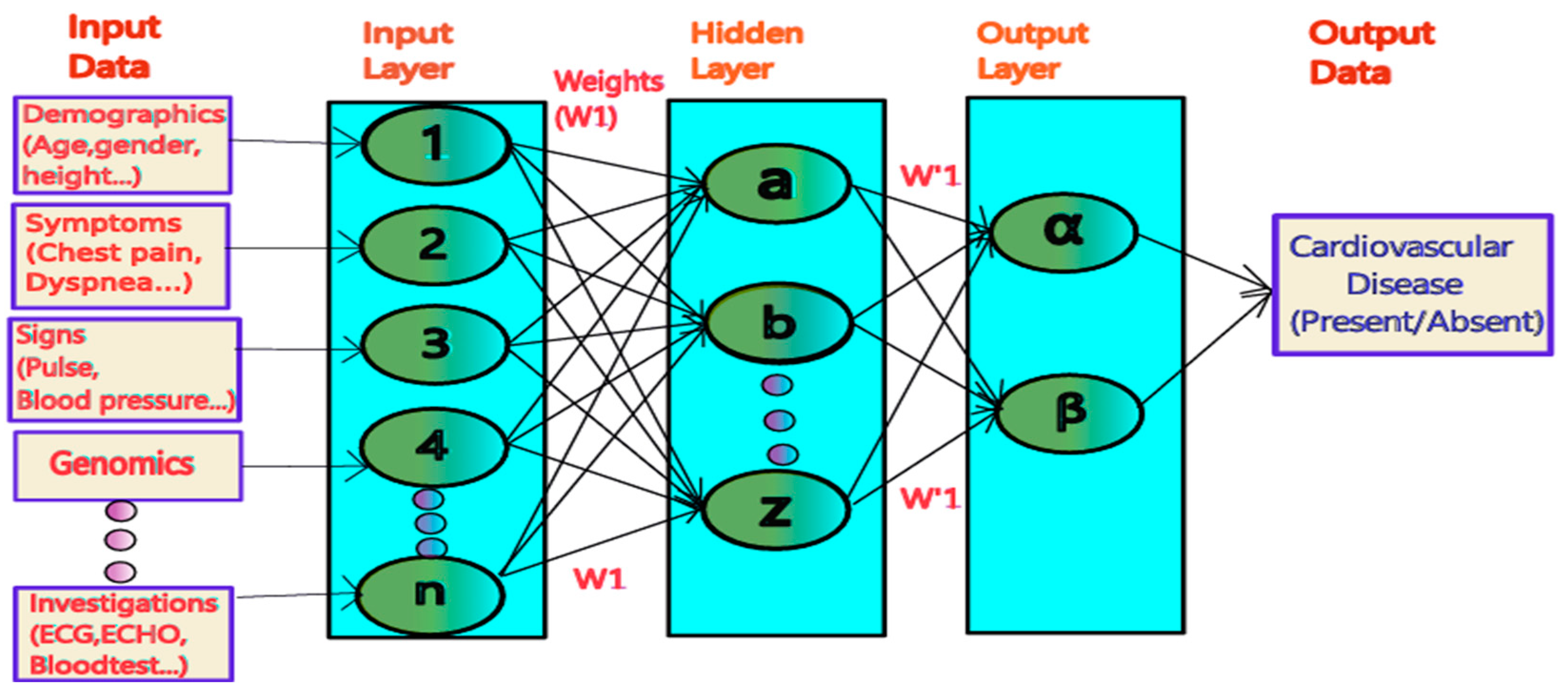
4. Predictive Modelling and Risk Assessment
5. Personalized Medicine
5.1. Role of Genomics and Precision Medicine
5.2. Tailoring Treatments Based on Individual Data
5.3. Using Big Data for Public Health Initiatives
5.4. Identifying Trends and Patterns in CVD
5.5. Improving Disease Detection and Diagnosis
- a.
- Acute Cardiovascular Disease Detection: Pattern recognition via BDA makes it possible to detect acute cardiovascular diseases in early stages with high accuracy. Zhang et al., [53] proposed a multimodal-based strategy by fusing ECG, phonocardiograms, echocardiography, Holter monitors, and biological markers for CAD detection, reaching high diagnostic accuracy based on the complementary information among different data modalities [52].
- b.
- Severity Assessment: The appraisal of cardiovascular disease severity can be greatly improved via the integration of several imaging techniques [52] by combining echocardiography and cardiac MRI to boost the prediction of sudden cardiac death in dilated cardiomyopathy patients. This multimodal method reaches a more complete evaluation of cardiac function and structure and increases accuracy in severity appraisal [54].
- c.
- Early Identification of At-Risk Populations: BDA also enables the early identification of populations at risk for CVD by utilizing lifestyle, genetic, and environmental data. Studies have demonstrated the integration of wearable device data, genomics, and social determinants of health to identify high-risk individuals for atrial fibrillation. This proactive approach facilitates timely preventive interventions, significantly reducing the burden of disease and healthcare costs
6. Drug and Medical Device Safety Surveillance
7. Quality of Care and Performance Measurement
8. Challenges and Future Directions
- 1.
- Informed Consent: Individuals may provide consent for the collection and use of their data without fully understanding the potential future applications, particularly as data can be repurposed, aggregated, and shared across diverse platforms [68] Google’s Project Nightingale collected healthcare data from millions without patient consent, leading to public backlash and calls for stricter transparency and consent protocols. IBM’s AI ethics initiatives emphasize transparency and explainability, requiring that AI decision-making processes be understandable to stakeholders (7 Essential Data Ethics Examples for Businesses in 2025).
- 2.
- Privacy and Confidentiality: Protecting the privacy and confidentiality of sensitive health data is a primary concern in big data applications [69]. While anonymization and de-identification techniques are routinely used, these methods are not foolproof, as advances in data linkage and re-identification techniques have demonstrated that individuals can still be identified by combining disparate data sources [70].
- 3.
- Data Ownership and Control: The issue of data ownership is a central ethical challenge in the big data landscape [71]. This raises questions regarding the rights of data subjects and whether they should be entitled to a share of the benefits that result from the use of their data, particularly in cases where institutions or corporations derive financial or intellectual gains.
- 4.
- Equity and the Big Data Divide: The capacity to harness big data for healthcare innovation is disproportionately concentrated among institutions with advanced technological infrastructure, deep financial resources, and sophisticated analytical expertise. This concentration creates a “big data divide”, wherein institutions and populations with fewer resources may be left behind, exacerbating existing health disparities [72].
- 5.
- Epistemological Challenges: The vast scale of big data in healthcare creates an over-reliance on correlation-driven insights, often without a clear understanding of the underlying causal mechanisms. This presents significant epistemological challenges, as decisions based on superficial correlations may lead to erroneous conclusions and suboptimal interventions [73].
9. Future Directions: Balancing Core Considerations
10. Conclusions
Funding
Conflicts of Interest
References
- Kim, D.S.; Park, J.W. Medical big data: Promise and challenges. Kidney Res. Clin. Pract. 2017, 36, 3–11. Available online: https://www.krcp-ksn.org/journal/view.php?id=10.23876/j.krcp.2017.36.1.3 (accessed on 14 September 2024).
- Silverio, A.; Cavallo, P.; De Rosa, R.; Galasso, G. Big health data and cardiovascular diseases: A challenge for research, an opportunity for clinical care. Front. Med. 2019, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- American Heart Association. Heart disease and stroke statistics—2019 update: A report from the American Heart Association. Circulation 2019, 139, e56–e528.
- Roden, D.M.; Van Driest, S.L.; Wells, Q.S.; Mosley, J.D.; Denny, J.C.; Peterson, J.F. Opportunities and challenges in cardiovascular pharmacogenomics. Circ. Res. 2018, 122, 1176–1190. [Google Scholar] [CrossRef]
- Hammad, R.; Barhoush, M.; Abed-Alguni, B.H. A semantic-based approach for managing healthcare big data: A survey. J. Healthc. Eng. 2020, 2020, 8865808. [Google Scholar] [CrossRef]
- Di Mauro, M.; Greco, M.; Grimaldi, M. A formal definition of big data based on its essential features. Libr. Rev. 2016, 65, 122. Available online: https://www.academia.edu/23962108/A_formal_definition_of_Big_Data_based_on_its_essential_features (accessed on 14 September 2024). [CrossRef]
- Batko, K.; Ślęzak, A. The use of big data analytics in healthcare. J. Big Data 2022, 9, 3. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8733917/ (accessed on 14 September 2024). [CrossRef]
- Toumieux, P.; Chevalier, L.; Sahuguède, S.; Julien-Vergonjanne, A. Optical wireless connected objects for healthcare. Healthc. Technol. Lett. 2015, 2, 118–122. [Google Scholar] [CrossRef]
- Awrahman, B.J.; Aziz Fatah, C.; Hamaamin, M.Y. A review of the role and challenges of big data in healthcare informatics and analytics. Comput. Intell. Neurosci. 2022, 2022, 5317760. [Google Scholar] [CrossRef]
- Poplin, R.; Varadarajan, A.V.; Blumer, K.; Liu, Y.; McConnell, M.V.; Corrado, G.S.; Peng, L.; Webster, D.R. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat. Biomed. Eng. 2018, 2, 158–164. [Google Scholar] [CrossRef]
- Houston Methodist. How Big Data Analytics Can Improve Cardiovascular Medicine; Methodology|Houston Methodist. 2024. Available online: https://read.houstonmethodist.org/how-big-data-analytics-can-improve-cardiovascular-medicine (accessed on 21 August 2024).
- Quazi, S.; Malik, J.A. A systematic review of personalized health applications through human-computer interactions (HCI) on cardiovascular health optimization. J. Cardiovasc. Dev. Dis. 2022, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Gunasekaran, S.; Mathivanan, S.K.; Benjula Anbu Malar, M.B.; Jayagopal, P.; Dalu, G.T. An active learning machine technique-based prediction of cardiovascular heart disease from UCI repository database. Sci. Rep. 2023, 13, 13588. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P.K.; Mohapatra, S.K.; Wu, S.L. SLA-based healthcare big data analysis and computing in cloud networks. J. Parallel Distrib. Comput. 2018, 119, 121–135. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Mohapatra, S.K.; Wu, S.L. Analyzing healthcare big data with prediction for future health condition. IEEE Access 2016, 4, 9786–9799. [Google Scholar] [CrossRef]
- Manimurugan, S.; Almutairi, S.; Aborokbah, M.M.; Narmatha, C.; Ganesan, S.; Chilamkurti, N.; Alzaheb, R.A.; Almoamari, H. Two-stage classification model for the prediction of heart disease using IoMT and artificial intelligence. Sensors 2022, 22, 476. [Google Scholar] [CrossRef]
- Choi, S.Y.; Chung, K. Knowledge process of health big data using MapReduce-based associative mining. Pers. Ubiquitous Comput. 2020, 24, 571–581. [Google Scholar] [CrossRef]
- Safa, M.; Pandian, A.; Gururaj, H.; Ravi, V.; Krichen, M. Real-time healthcare big data analytics model for improved QoS in cardiac disease prediction with IoT devices. Health Technol. 2023, 13, 473–483. [Google Scholar] [CrossRef]
- Mohapatra, S.K. Healthcare big data analysis with artificial neural network for cardiac disease prediction. Electronics 2024, 13, 163. [Google Scholar] [CrossRef]
- Tomlinson, B.; Hu, M.; Waye MM, Y.; Chan, P.; Liu, Z.M. Current status of personalized medicine based on pharmacogenetics in cardiovascular medicine. Expert Rev. Precis. Med. Drug Dev. 2016, 1, 5–8. [Google Scholar] [CrossRef]
- Bazargani, Y.T.; Ugurlu, M.; de Boer, A.; Leufkens, H.G.M.; Mantel-Teeuwisse, A.K. Selection of essential medicines for the prevention and treatment of cardiovascular diseases in low- and middle-income countries. BMC Cardiovasc. Disord. 2018, 18, 126. [Google Scholar] [CrossRef]
- Ozenberger, K.; Alexander, G.C.; Shin, J.I.; Whitsel, E.A.; Qato, D.M. Use of prescription medications with cardiovascular adverse effects among older adults in the United States. Pharmacoepidemiol. Drug Saf. 2022, 31, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, A.L.; Chia, J.; Brett, J.; Pearson, S.A.; Falster, M.O. A nationwide study of multimedicine use in people treated with cardiovascular medicines in Australia. Pharmacotherapy 2022, 42, 828–836. [Google Scholar] [CrossRef]
- Jurgens, C.Y.; Lee, C.S.; Aycock, D.M.; Masterson Creber, R.; Denfeld, Q.E.; DeVon, H.A. State of the science: The relevance of symptoms in cardiovascular disease and research: A scientific statement from the American Heart Association. Circulation 2022, 146, e173–e184. [Google Scholar] [CrossRef] [PubMed]
- Shahid, N.; Rappon, T.; Berta, W. Applications of artificial neural networks in health care organizational decision-making: A scoping review. PLoS ONE 2019, 14, e0212356. [Google Scholar] [CrossRef] [PubMed]
- Dykstra, S.; Satriano, A.; Cornhill, A.K.; Lei, L.Y.; Labib, D.; Mikami, Y. Machine learning prediction of atrial fibrillation in cardiovascular patients using cardiac magnetic resonance and electronic health information. Front. Cardiovasc. Med. 2022, 9, 998558. [Google Scholar] [CrossRef]
- Barbieri, S.; Mehta, S.; Wu, B.; Bharat, C.; Poppe, K.; Jorm, L. Predicting cardiovascular risk from national administrative databases using a combined survival analysis and deep learning approach. Int. J. Epidemiol. 2022, 51, 931–944. [Google Scholar] [CrossRef]
- Ming-Lung Tsai, M.D.; Kuan-Fu Chen, M.D. Harnessing Electronic Health Records and Artificial Intelligence for Enhanced Cardiovascular Risk Prediction: A Comprehensive Review. J. Am. Heart Assoc. 2025, 14, e036946. [Google Scholar] [CrossRef]
- Nghiem, N.; Atkinson, J.; Nguyen, B.P.; Tran-Duy, A.; Wilson, N. Predicting high health-cost users among people with cardiovascular disease using machine learning and nationwide linked social administrative datasets. Health Econ. Rev. 2023, 13, 9. [Google Scholar] [CrossRef]
- Khawar Hussain, H.; Tariq, A.; Yousaf Gill, A. Role of arrodetificial intelligence in cardiovascular health care. J. World Sci. 2023, 2, 583–591. [Google Scholar] [CrossRef]
- Tison, G.H.; Zhang, J.; Delling, F.N.; Deo, R.C. Automated and interpretable patient ECG profiles for disease detection, tracking, and discovery. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005289. [Google Scholar] [CrossRef]
- Iriart, J.A.B. Precision medicine/personalized medicine: A critical analysis of movements in the transformation of biomedicine in the early 21st century. Cad. Saúde Pública 2019, 35, e00153118. [Google Scholar] [CrossRef] [PubMed]
- Al Bataineh, A.; Manacek, S. MLP-PSO Hybrid Algorithm for Heart Disease Prediction. J. Pers. Med. 2022, 12, 1208. [Google Scholar] [CrossRef] [PubMed]
- Pathan, M.S.; Nag, A.; Pathan, M.M.; Dev, S. Analyzing the impact of feature selection on the accuracy of heart disease prediction. Healthc. Anal. 2022, 2, 100060. [Google Scholar] [CrossRef]
- Ozcan, M.; Peker, S. A classification and regression tree algorithm for heart disease modeling and prediction. Healthc. Anal. 2023, 3, 100130. [Google Scholar] [CrossRef]
- Verma, L.; Srivastava, S.; Negi, P. A hybrid data mining model to predict coronary artery disease cases using non-invasive clinical data. J. Med. Syst. 2016, 40, 178. [Google Scholar] [CrossRef]
- Dhingra, L.S.; Shen, M.; Mangla, A.; Khera, R. Cardiovascular Care Innovation through Data-Driven Discoveries in the Electronic Health Record. Am. J. Cardiol. 2023, 203, 136–148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jain, K.K. Personalized management of cardiovascular disorders. Med. Princ. Pract. 2017, 26, 399–414. [Google Scholar] [CrossRef]
- Currie, G.; Delles, C. Precision medicine and personalized medicine in cardiovascular disease. In Sex-Specific Analysis of Cardiovascular Function; Kerkhof, P.L.M., Miller, V.M., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 589–605. [Google Scholar] [CrossRef]
- Lee, M.S.; Flammer, A.J.; Lerman, L.O.; Lerman, A. Personalized medicine in cardiovascular diseases. Korean Circ. J. 2012, 42, 583–591. [Google Scholar] [CrossRef]
- Leopold, J.A.; Loscalzo, J. The emerging role of precision medicine in cardiovascular disease. Circ. Res. 2018, 122, 1302–1315. [Google Scholar] [CrossRef]
- Leopold, J.A.; Maron, B.A.; Loscalzo, J. The application of big data to cardiovascular disease: Paths to precision medicine. J. Clin. Investig. 2020, 130, 29–38. [Google Scholar] [CrossRef]
- European Society of Cardiology. Heart Patients Set to Receive Treatment Tailored to Their Genetic and Health Information; The ESC Press Office: Sophia Antipolis, France, 2024; Available online: https://www.escardio.org/The-ESC/Press-Office/Press-releases/heart-patients-set-to-receive-treatment-tailored-to-their-genetic-and-health-inf (accessed on 12 January 2025).
- Kumar, A.; Jena, P.K.; Behera, S.; Lockey, R.F.; Mohapatra, S.; Mohapatra, S. Multifunctional magnetic nanoparticles for targeted delivery. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Oluwafemidiakhoa. Personalized Medicine 2.0: AI’s Role in Tailoring Treatments; Mr. Plan B Publication: London, UK, 2024; Available online: https://medium.com/mr-plan-publication/personalized-medicine-2-0-ais-role-in-tailoring-treatments-2f2592cdd49c (accessed on 22 August 2024).
- Zhang, Y.; Zhu, L.; Li, X.; Ge, C.; Pei, W.; Zhang, M.; Zhong, M.; Zhu, X.; Lv, K. M2 macrophage exosome-derived lncRNA AK083884 protects mice from CVB3-induced viral myocarditis through regulating PKM2/HIF-1α axis mediated metabolic reprogramming of macrophages. Redox Biol. 2024, 69, 103016. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, J.; Zhong, M.; Wu, Z.; Wan, S.; Li, X.; Zhang, Y.; Lv, K. Nanozyme-enhanced tyramine signal amplification probe for preamplification-free myocarditis-related miRNAs detection. Chem. Eng. J. 2025, 503, 158093. [Google Scholar] [CrossRef]
- Pirillo, A.; Catapano, A.L.; Norata, G.D. Monoclonal antibodies in the management of familial hypercholesterolemia: Focus on PCSK9 and ANGPTL3 inhibitors. Curr. Atheroscler. Rep. 2021, 23, 79. [Google Scholar] [CrossRef]
- Gao, J.; Li, P.; Chen, Z.; Zhang, J. A survey on deep learning for multimodal data fusion. Neural Comput. 2020, 32, 829–864. [Google Scholar] [CrossRef]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef]
- Amal, S.; Safarnejad, L.; Omiye, J.A.; Ghanzouri, I.; Cabot, J.H.; Ross, E.G. Use of multi-modal data and machine learning to improve cardiovascular disease care. Front. Cardiovasc. Med. 2022, 9, 840262. [Google Scholar] [CrossRef]
- Bandera, F.; Baghdasaryan, L.; Mandoli, G.E.; Cameli, M. Multimodality imaging predictors of sudden cardiac death. Heart Fail. Rev. 2020, 25, 427–446. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, K.; Miao, S.; Zhang, X.; Yin, Y.; Wan, C.; Yu, Y.; Hu, J.; Wang, Z.; Shan, T.; et al. Automated detection of cardiovascular disease by electrocardiogram signal analysis: A deep learning system. Cardiovasc. Diagn. Ther. 2020, 10, 227–235. [Google Scholar] [CrossRef]
- Zambrano Chaves, J.M.; Wentland, A.L.; Desai, A.D.; Banerjee, I.; Boutin, R.D.; Maron, D.J.; Rodriguez, F.; Sandhu, A.T.; Jeffrey, R.B.; Rubin, D.; et al. Opportunistic assessment of ischemic heart disease risk using abdominopelvic computed tomography and medical record data: A multimodal explainable artificial intelligence approach. Sci. Rep. 2023, 13, 21034. [Google Scholar] [CrossRef]
- Ali, F.; El-Sappagh, S.; Islam, S.R.; Kwak, D.; Ali, A.; Imran, M.; Kwak, K.-S. A smart healthcare monitoring system for heart disease prediction based on ensemble deep learning and feature fusion. Inf. Fusion 2020, 63, 208–222. [Google Scholar] [CrossRef]
- Lalani, C.; Kunwar, E.M.; Kinard, M.; Dhruva, S.S.; Redberg, R.F. Reporting of death in US Food and Drug Administration medical device adverse event reports in categories other than death. JAMA Intern. Med. 2021, 181, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Rathi, V.K.; Ross, J.S.; Redberg, R.F. Unique device identifiers—Missing in action. JAMA Intern. Med. 2023, 183, 1049–1050. [Google Scholar] [CrossRef] [PubMed]
- Resnic, F.S.; Majithia, A.; Marinac-Dabic, D.; Robbins, S.; Ssemaganda, H.; Hewitt, K. Registry-based prospective, active surveillance of medical-device safety. N. Engl. J. Med. 2017, 376, 526–535. [Google Scholar] [CrossRef]
- Ball, R.; Robb, M.; Anderson, S.A.; Dal Pan, G. The FDA’s Sentinel Initiative–A comprehensive approach to medical product surveillance. Clin. Pharmacol. Ther. 2016, 99, 265–268. [Google Scholar] [CrossRef]
- Wang, X.; Ayakulangara Panickan, V.; Cai, T.; Xiong, X.; Cho, K.; Cai, T. Endovascular aneurysm repair devices as a use case for postmarketing surveillance of medical devices. JAMA Intern. Med. 2023, 183, 1090–1097. [Google Scholar] [CrossRef]
- Ross, J.S.; Blount, K.L.; Ritchie, J.D.; Hodshon, B.; Krumholz, H.M. Post-market clinical research conducted by medical device manufacturers: A cross-sectional survey. Med. Devices 2015, 8, 241–249. [Google Scholar] [CrossRef]
- Santana, J.; Waheed, S. Analysis of medical device reports involving ventilator-related incidents in a clinical setting. J. Clin. Eng. 2022, 47, 107–115. [Google Scholar] [CrossRef]
- Mortazavi, B.J.; Downing, N.S.; Bucholz, E.M.; Dharmarajan, K.; Manhapra, A.; Li, S.-X.; Negahban, S.N.; Krumholz, H.M. Analysis of machine learning techniques for heart failure readmissions. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 629–640. [Google Scholar] [CrossRef]
- Moreira, M.W.L.; Rodrigues, J.J.P.C.; Kumar, N.; Al-Muhtadi, J.; Korotaev, V. Evolutionary radial basis function network for gestational diabetes data analytics. J. Comput. Sci. 2018, 27, 410–417. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1877750317304726 (accessed on 19 August 2024). [CrossRef]
- Abouelmehdi, K.; Beni-Hessane, A.; Khaloufi, H. Big healthcare data: Preserving security and privacy. J. Big Data 2018, 5, 1. [Google Scholar] [CrossRef]
- Bainbridge, M. Big data challenges for clinical and precision medicine. In Big Data, Big Challenges: A Healthcare Perspective: Background, Issues, Solutions, and Research Directions; Househ, M., Kushniruk, A.W., Borycki, E.M., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 17–31. [Google Scholar]
- Choudhury, S.; Fishman, J.R.; McGowan, M.L.; Juengst, E.T. Big data, open science and the brain: Lessons learned from genomics. Front. Hum. Neurosci. 2014, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Schadt, E.E. The changing privacy landscape in the era of big data. Mol. Syst. Biol. 2012, 8, 612. [Google Scholar] [CrossRef]
- Joly, Y.; Dove, E.S.; Knoppers, B.M.; Bobrow, M.; Chalmers, D. Data sharing in the post-genomic world: The experience of the International Cancer Genome Consortium (ICGC) Data Access Compliance Office (DACO). PLoS Comput. Biol. 2012, 8, e1002549. [Google Scholar] [CrossRef]
- Steinsbekk, K.S.; Ursin, L.O.; Skolbekken, J.A.; Solberg, B. We’re not in it for the money: Lay people’s moral intuitions on commercial use of “their” biobank. Med. Health Care Philos. 2013, 16, 151–162. [Google Scholar] [CrossRef]
- Krittanawong, C.; Johnson, K.W.; Hershman, S.G.; Tang, W.H.W. Big data, artificial intelligence, and cardiovascular precision medicine. Expert Rev. Precis. Med. Drug Dev. 2018, 3, 305–317. [Google Scholar] [CrossRef]
- Callebaut, W. Scientific perspectivism: A philosopher of science’s response to the challenge of big data biology. Stud. Hist. Philos. Biol. Biomed. Sci. 2012, 43, 69–80. [Google Scholar] [CrossRef]
- Peddicord, D.; Waldo, A.B.; Boutin, M.; Grande, T.; Gutierrez, L., Jr. A proposal to protect privacy of health information while accelerating comparative effectiveness research. Health Aff. 2010, 29, 2082–2090. [Google Scholar] [CrossRef]
- Rumbold, J.M.; Pierscionek, B.K. Ethical challenges in Big Data health research: A review. Healthcare 2023, 5, 34. [Google Scholar]
- Murdoch, T.B.; Detsky, A.S. The inevitable application of big data to health care. JAMA 2013, 309, 1351–1352. [Google Scholar] [CrossRef]
- Roski, J.; Bo-Linn, G.W.; Andrews, T.A. Creating value in health care through big data: Opportunities and policy implications. Health Aff. 2014, 33, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Obermeyer, Z.; Powers, B.; Vogeli, C.; Mullainathan, S. Dissecting racial bias in an algorithm used to manage the health of populations. Science 2019, 366, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Mittelstadt, B.D.; Floridi, L. The ethics of Big Data: Current and foreseeable issues in biomedical contexts. Sci. Eng. Ethics. 2016, 22, 303–341. [Google Scholar] [CrossRef] [PubMed]
- Groves, P.; Kayyali, B.; Knott, D.; Van Kuiken, S. The ‘Big Data’ Revolution in Healthcare: Accelerating Value and Innovation. McKinsey & Company Report. 2016. Available online: https://www.mckinsey.com (accessed on 14 September 2024).
- Hodge, J.G., Jr.; Gostin, L.O.; Jacobson, P.D. Legal issues concerning electronic health information. JAMA 1999, 282, 1466. [Google Scholar] [CrossRef]
- Emam, K.E.; Jonker, E.; Arbuckle, L.; Malin, B. A systematic review of re-identification attacks on health data. PLoS ONE 2011, 6, e28071. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0028071 (accessed on 2 December 2011). [CrossRef]
- Hoffman, S.; Podgurski, A. Balancing privacy, autonomy, and scientific needs in electronic health records research. SMU Law Rev. 2012, 65, 85–144. [Google Scholar]
- Angst, C.M. Protect my privacy or support the common good? Ethical questions about electronic health information exchanges. J. Bus. Ethics 2009, 90, 169–178. [Google Scholar] [CrossRef]
| Descriptive Analytics | Aims to Examine Past Datasets for Patterns and Trends |
|---|---|
| Predictive analytics | Aims to predict likely outcomes and make evidence-based forecasts using historical data |
| Prescriptive analytics | Utilizes data from diverse sources, such as statistical analyses, machine learning algorithms, and data mining techniques, to predict potential future outcomes and determine the most optimal course of action |
| Diagnostic analytics | Analysing historical and real-time data to identify the underlying causes |
| Related Works | Big Data | Map Reduce | Cloud | Cardiac Healthcare Data | ECG Data |
|---|---|---|---|---|---|
| Sahoo P.K. et al., 2018 [14] | ✓ | × | ✓ | ✓ | × |
| Sahoo P.K. et al., 2016 [15] | ✓ | ✓ | ✓ | ✓ | × |
| Manimurugun et al., 2022 [16] | ✓ | × | ✓ | ✓ | × |
| Choi et al., 2020 [17] | ✓ | ✓ | × | × | × |
| Safa et al., 2023 [18] | ✓ | × | × | ✓ | × |
| Mohapatra et al., 2024 [19] | ✓ | ✓ | ✓ | ✓ | ✓ |
| Authors | Dataset | Algorithm Type | Analysis | Number of Features | Accuracy (%) |
|---|---|---|---|---|---|
| Srinivasan et al., 2023 [13] | UCI respository | Learning vector quantization (LVQ) | Classification | 10 | 98 |
| AI Bataineh & Manacek 2022 [33] | Heart disease | Multilayer Perceptron (MLP) + PSO | Classification | 13 | 84 |
| AI Bataineh & Manacek 2022 [33] | Heart disease | Recurrent neural network (RNN) + long short-term memory (LSTM) | Classification | 14 | 95 |
| Pathan M.S et al., 2022 [34] (77) | Cardiovascular Disease (CVD) and Framingham | MLP, support vector classifier | Classification | 12 (CVD) | 74 (CVD) |
| 11 (Fram) | 71 (Fram) | ||||
| Ozcan M et al., 2023 [35] | Cleveland, Hungarian, Switzerland, Long Beach VA Stalog Dataset | Classification and regression tree (CART) | Classification and Regression | 11 | 87 |
| Verma L et. al., 2016 [36] | Department of Cardiology, IGMC | Multinominal logistic regression (MLR) | Classification | 26 | 98 |
| Challenge with BDA Usage | Descriptions | Scientific Evidence/Implications | Citation |
|---|---|---|---|
| Data Privacy and Security | Data safety, patient identifiers, and data breaches might raise concerns about compliance with regulatory agencies. | Studies show that unauthorized access to health data can lead to loss of trust, legal consequences, and delays in adopting analytics. Privacy-preserving models (e.g., federated learning) are being explored. | [74] |
| Integration of Data Sources | BDA uses heterogeneous data sources that combine information on demographics, clinical, anthropometric, lifestyle and risk factors, genomics, metabolomics, and imaging tools. This complexity adds variations in format. | Research highlights difficulties in achieving interoperability across electronic health records (EHRs), devices, and databases. Standards like FHIR are being developed to address this. | [75] |
| Infrastructure Costs | BDA can be a cost-sensitive technique due to the requirements of high computational power, storage, and skilled professionals. | Studies estimate significant upfront and ongoing costs for hospitals and research institutions. Cloud-based solutions can help mitigate infrastructure burdens but may raise additional concerns about data governance. | [76] |
| Algorithm Bias and Accuracy | Data gaps, inaccurate datasets, and poorly coded heterogeneous data can generate misleading and biased algorithms. Often, CVD’s determinants are contextual and a lack of Indigenous data might build inaccurate models for various populations. | Evidence shows that the underrepresentation of certain populations in datasets can lead to biased outcomes. Initiatives to increase diversity in data collection and ethical AI practices are essential. | [77] |
| Ethical and Legal Challenges | Ambiguities around ownership, consent, and the ethical use of patient data complicate the deployment of analytics in healthcare. | Researchers highlight the importance of clear legal frameworks and ethical guidelines. For example, consent models for the secondary use of data in research remain a contested issue. | [78] |
| Resource requirements | BDA is an emerging area requiring expertise from diverse fields (for BDA in the area of CVD: data scientists, clinicians). | Reports emphasize the shortage of professionals trained in both healthcare and data analytics. Education and training programs integrating both domains are critical for capacity building. | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, P.; Sharma, P.; Sharma, K.; Varma, V.; Patel, V.; Sarvaiya, J.; Tavethia, J.; Mehta, S.; Bhadania, A.; Patel, I.; et al. Revolutionizing Utility of Big Data Analytics in Personalized Cardiovascular Healthcare. Bioengineering 2025, 12, 463. https://doi.org/10.3390/bioengineering12050463
Sharma P, Sharma P, Sharma K, Varma V, Patel V, Sarvaiya J, Tavethia J, Mehta S, Bhadania A, Patel I, et al. Revolutionizing Utility of Big Data Analytics in Personalized Cardiovascular Healthcare. Bioengineering. 2025; 12(5):463. https://doi.org/10.3390/bioengineering12050463
Chicago/Turabian StyleSharma, Praneel, Pratyusha Sharma, Kamal Sharma, Vansh Varma, Vansh Patel, Jeel Sarvaiya, Jonsi Tavethia, Shubh Mehta, Anshul Bhadania, Ishan Patel, and et al. 2025. "Revolutionizing Utility of Big Data Analytics in Personalized Cardiovascular Healthcare" Bioengineering 12, no. 5: 463. https://doi.org/10.3390/bioengineering12050463
APA StyleSharma, P., Sharma, P., Sharma, K., Varma, V., Patel, V., Sarvaiya, J., Tavethia, J., Mehta, S., Bhadania, A., Patel, I., & Shah, K. (2025). Revolutionizing Utility of Big Data Analytics in Personalized Cardiovascular Healthcare. Bioengineering, 12(5), 463. https://doi.org/10.3390/bioengineering12050463






