Characterizing the Impact of Fabrication Methods on Mechanically Tunable Gelatin Hydrogels for Cardiac Fibrosis Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Polydimethylsiloxane (PDMS) Stamp Preparation
2.2. Glass Preparation for Gelatin Thin Film Chips
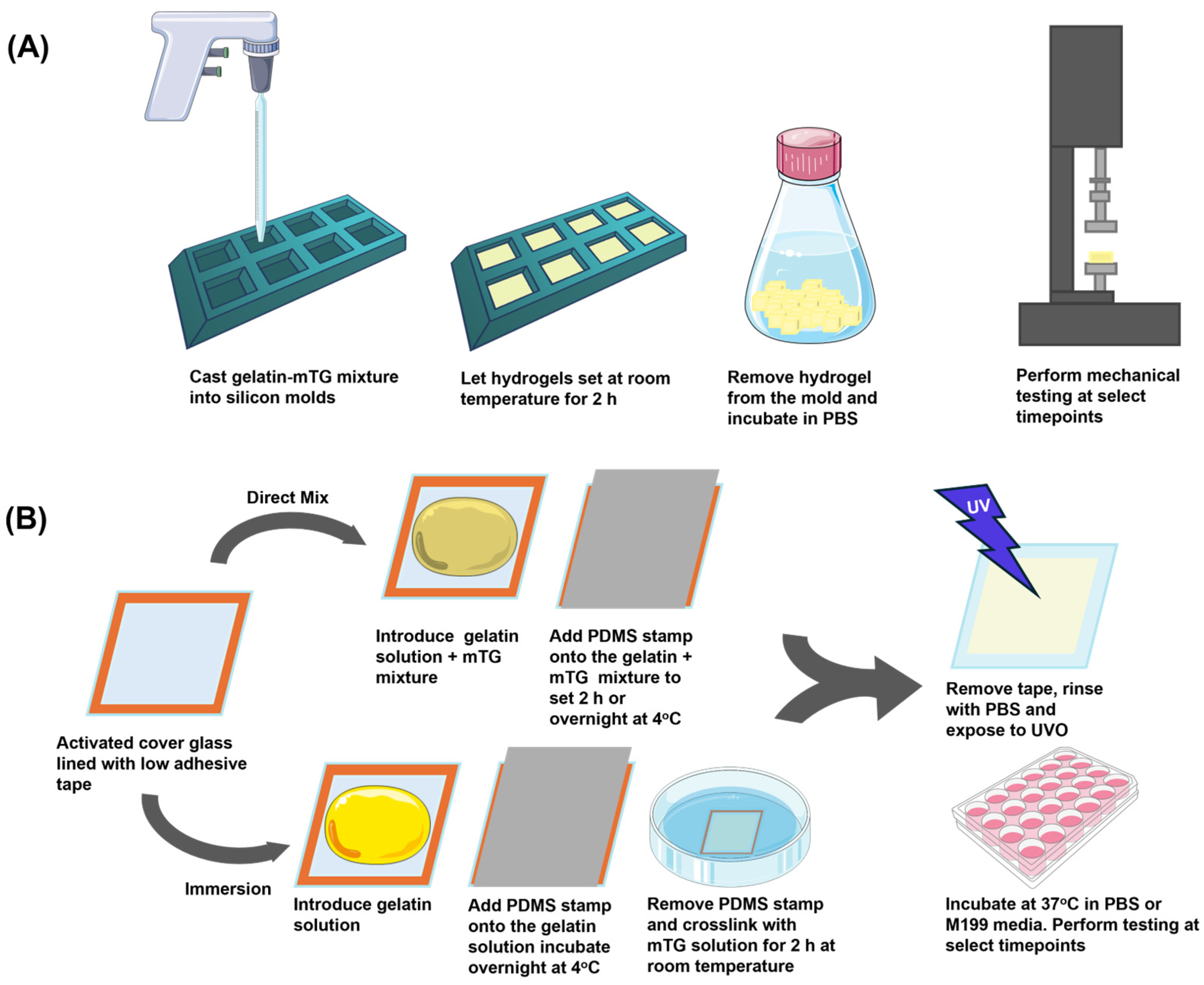
2.3. Gelatin Hydrogel Preparation
2.3.1. Direct Mix Gelatin Crosslinking
2.3.2. Immersion Gelatin Crosslinking
2.4. Piuma Nanonindentation
2.5. Instron Mechanical Testing
2.6. Cardiac Fibroblast Culture
2.7. Immunocytochemistry and Analysis
2.8. Statistical Analysis
3. Results
3.1. Elastic Modulus Studies
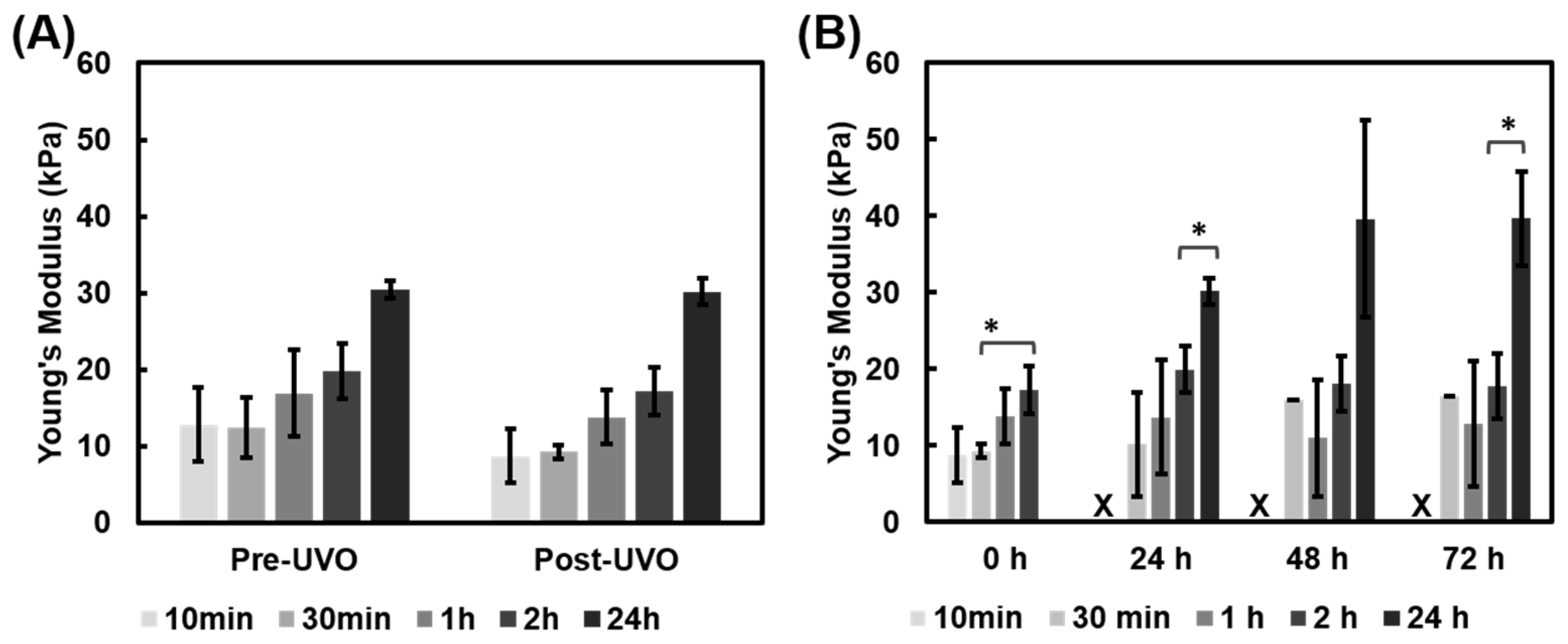
3.1.1. UVO Impact on Gelatin Film Modulus
3.1.2. Mechanical Properties of Gelatin Film Chips Using Piuma
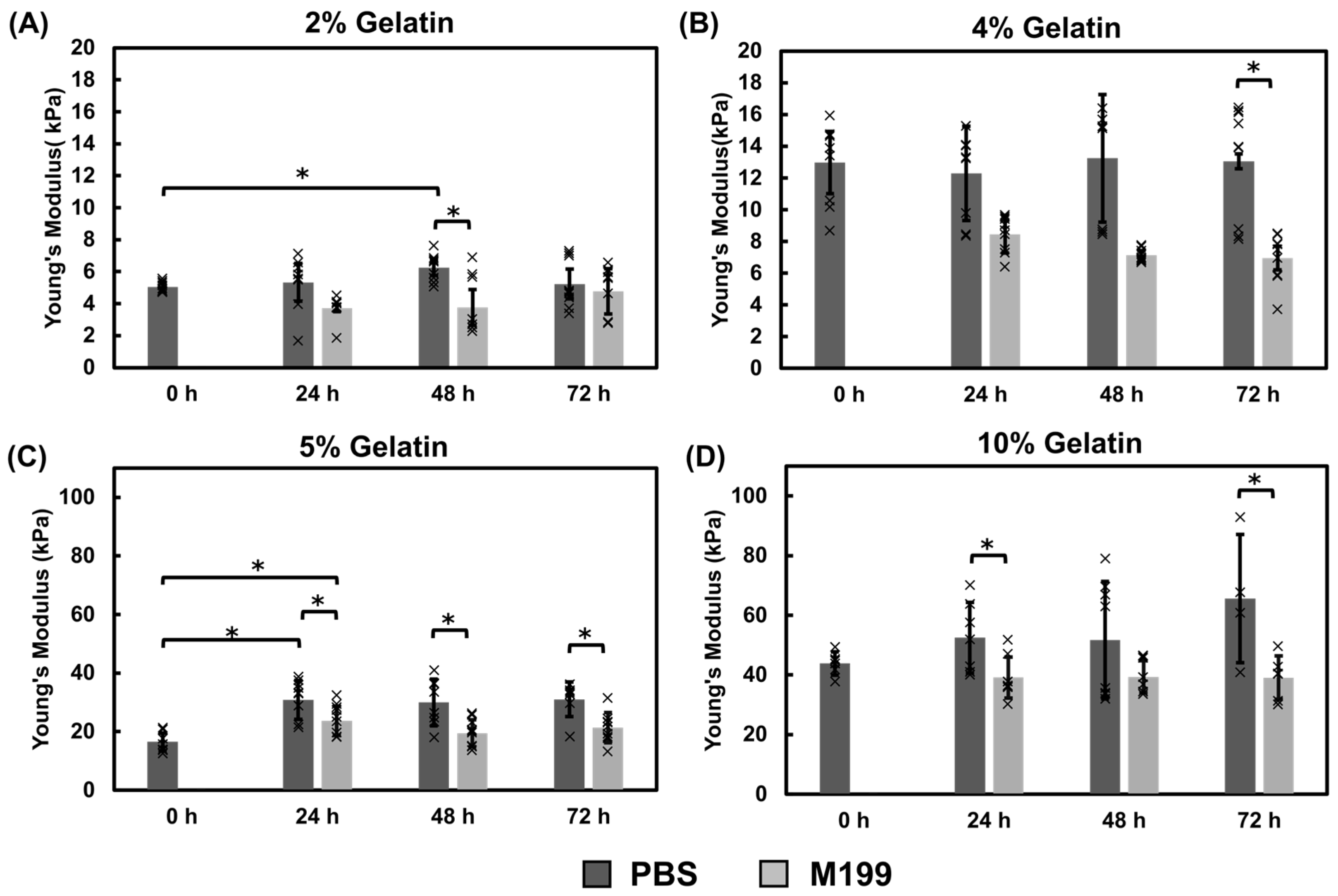
Mechanical Properties of Gelatin Films Using the Immersion Crosslinking Method
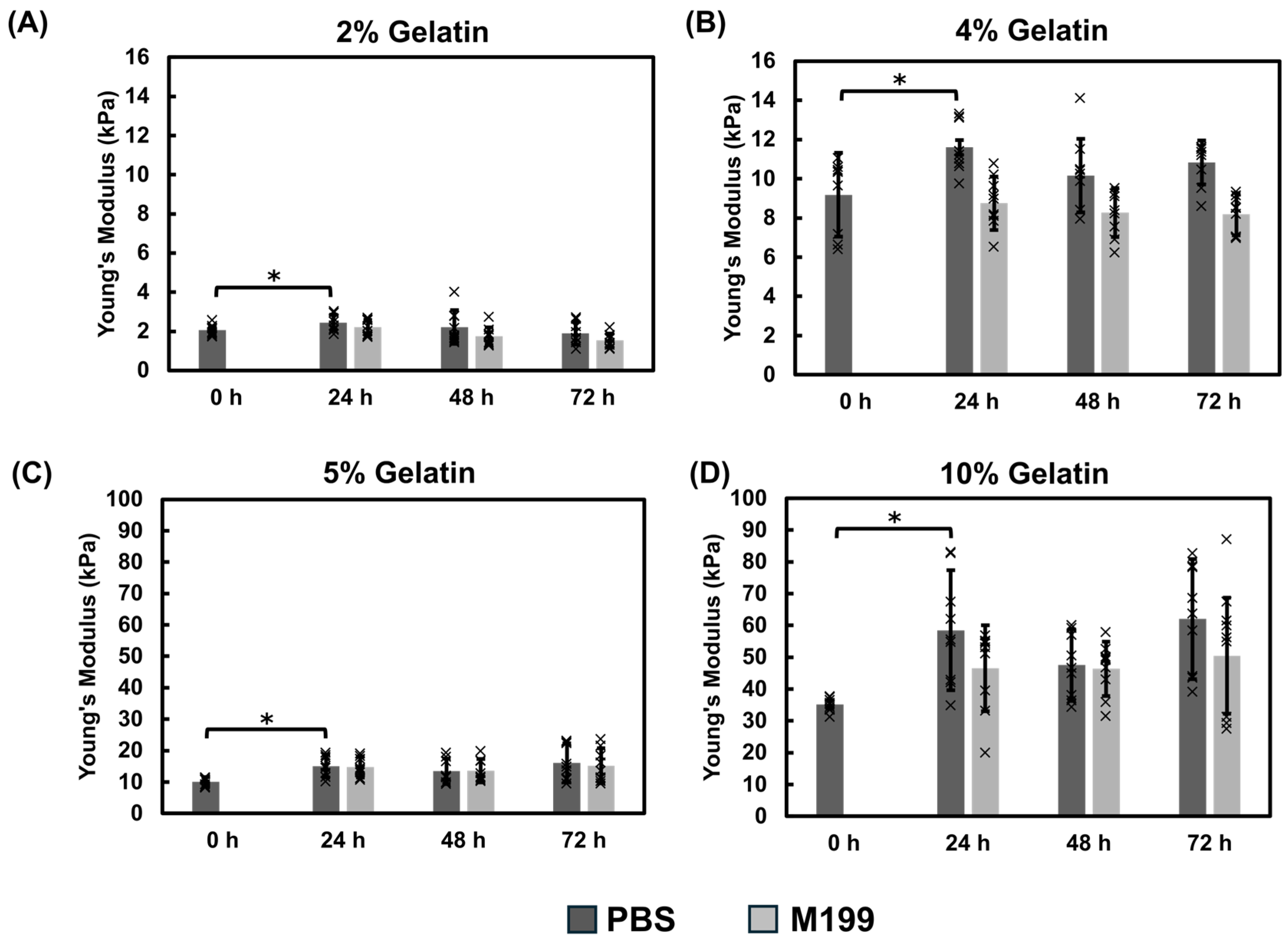
Mechanical Properties of Gelatin Films Using the Direct Mix Crosslinking Method
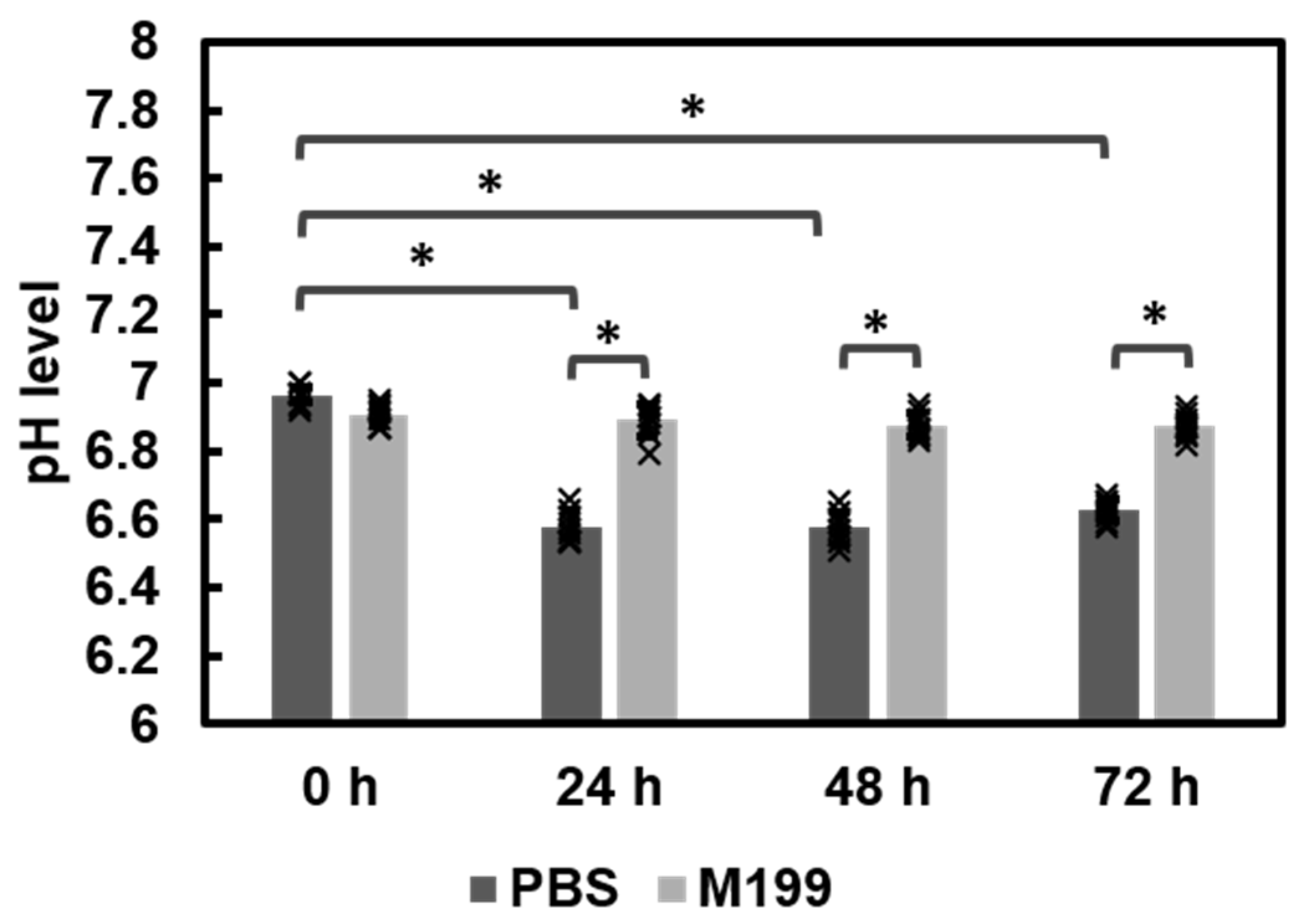
3.1.3. Comparison of Fabrication Methods and pH Effects
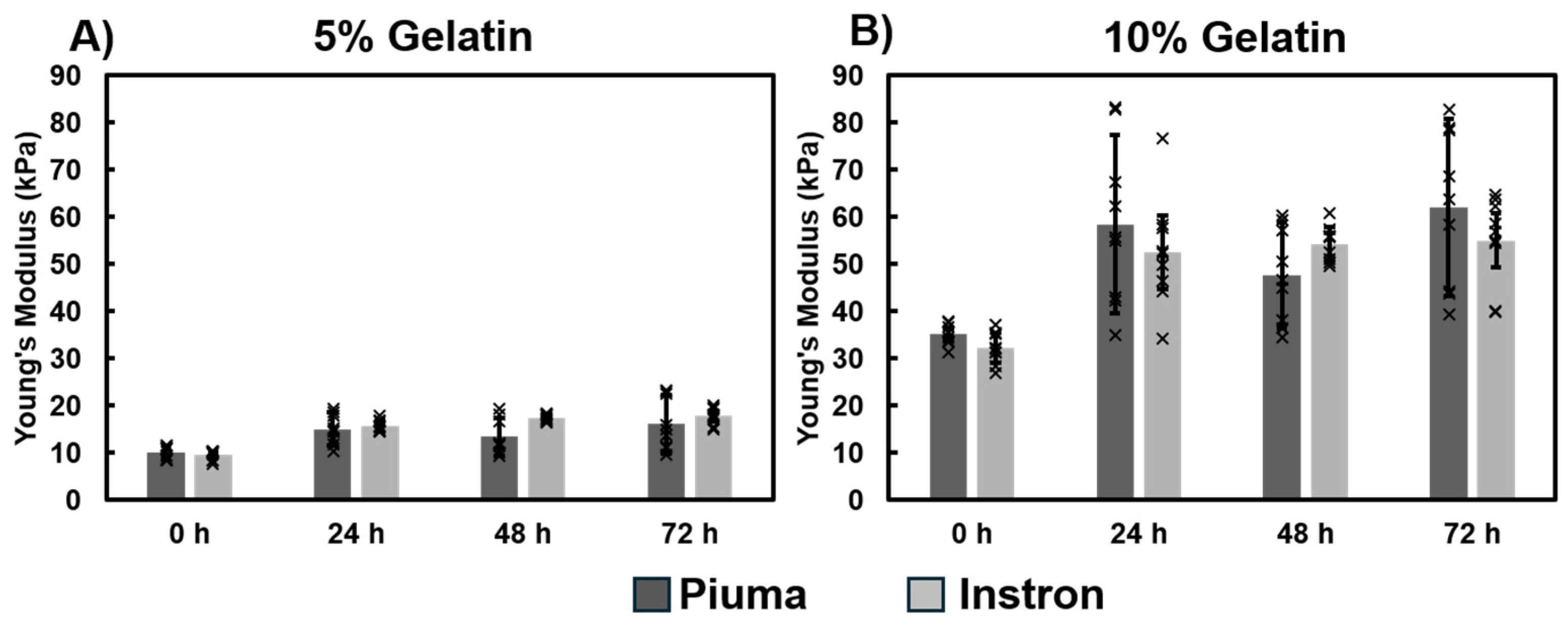
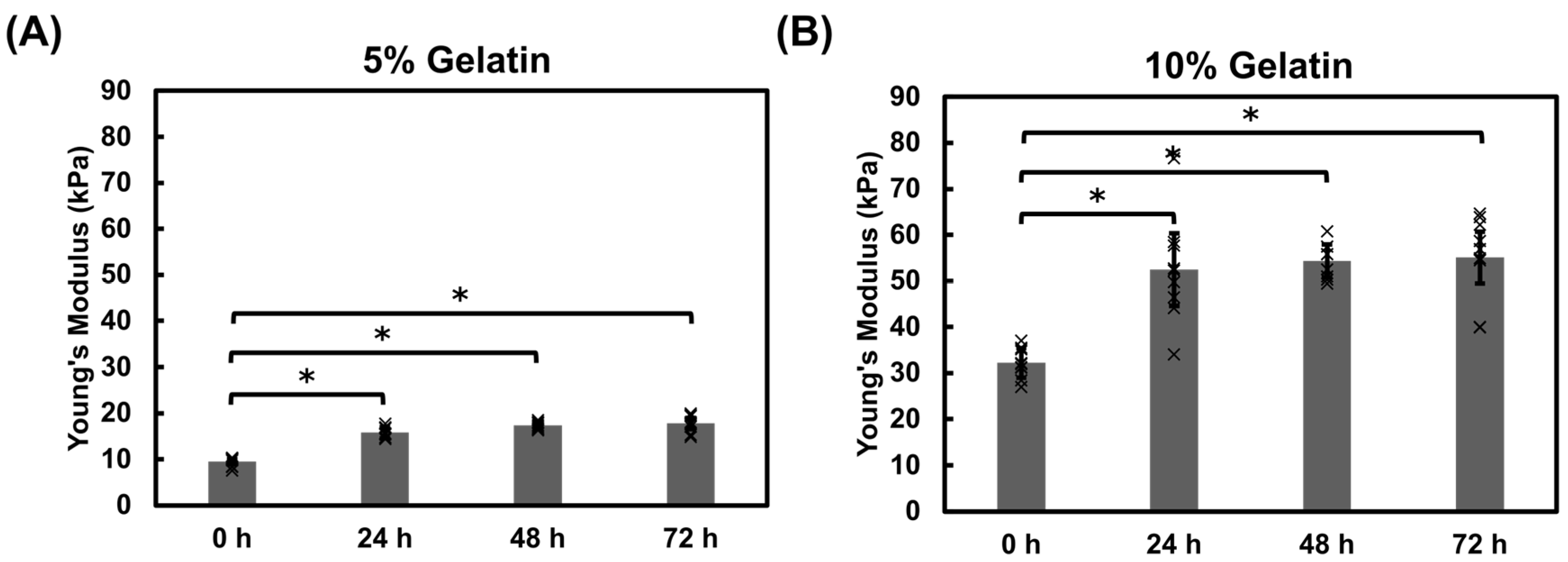
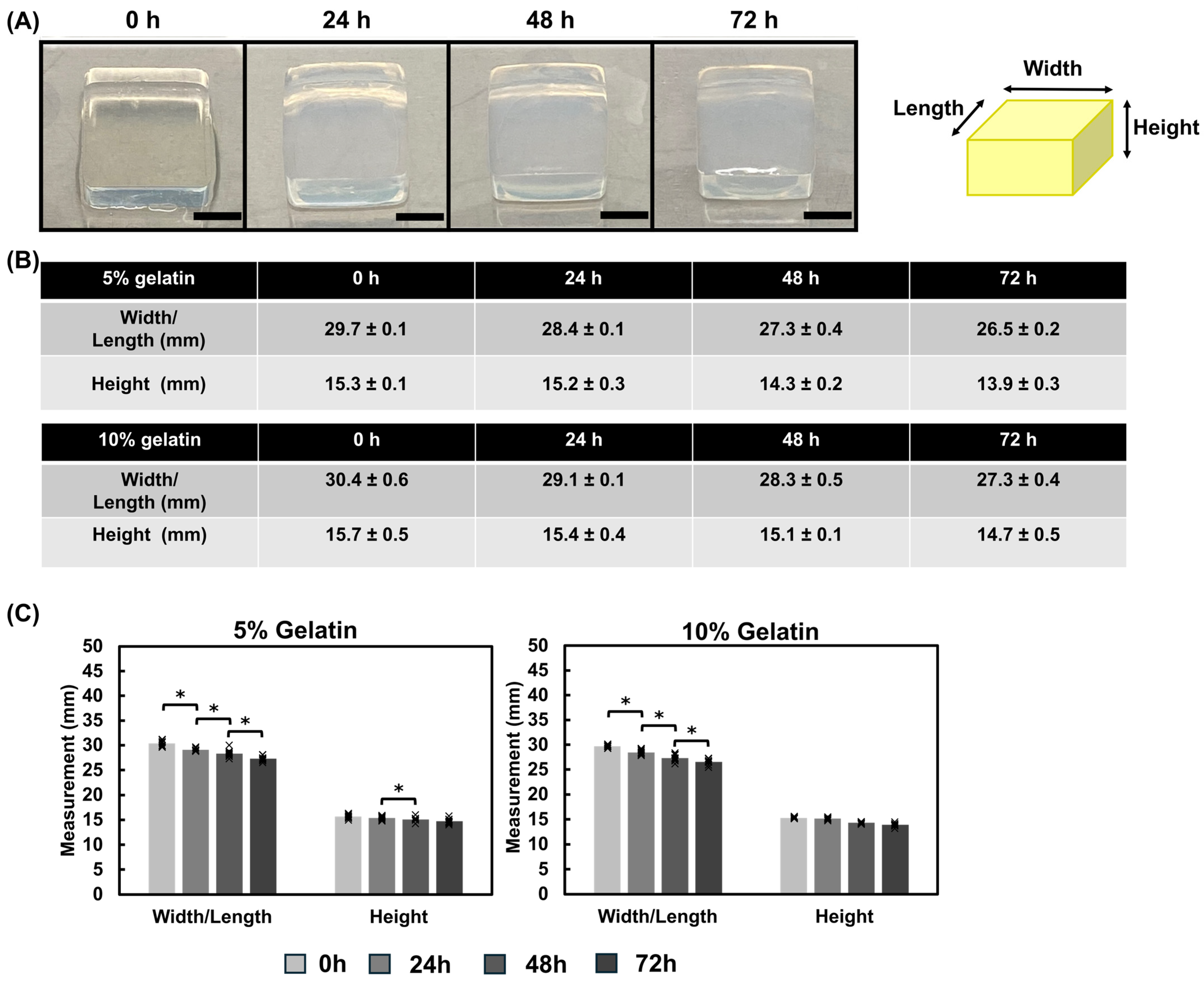
3.1.4. Mechanical Properties of Gelatin Hydrogel Cubes Using Instron
3.2. Assessing the Mechanical Impact of Modulus Cardiac Fibroblasts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECM | Extracellular Matrix |
| PDMS | Polydimethylsiloxane |
| mTG | Microbial transglutaminase |
| UVO | Ultraviolet Ozone |
| SMA | Smooth Muscle Actin |
| SD | Standard deviation |
| FDA | Food and Drug Administration |
| min | Minute(s) |
| h | Hours (s) |
| M199 | Medium 199 |
| PBS | Phosphate Buffered Saline |
| GelMA | Gelatin Methacryloyl |
| w/w | Weight/weight |
Appendix A
Appendix A.1. Assessing mTG Incubation for Crosslinking

| Modulus based on Crosslinking Method | |||
|---|---|---|---|
| [Gelatin] w/v | [mTG] w/v | Direct Mix | Immersion |
| 2% | 1% | 2.2 ± 0.2 kPa (Piuma, PBS) | 5.5 ± 0.5 kPa (Piuma, PBS) |
| 1.8 ± 0.3 kPa (Piuma, M199) | 4.1 ± 0.6 kPa (Piuma, M199) | ||
| 4% | 0.8% | 10.4 ± 1.0 kPa (Piuma, PBS) | 13.0 ± 0.4 kPa (Piuma, PBS) |
| 8.4 ± 0.3 kPa (Piuma, M199) | 7.5 ± 0.8 kPa (Piuma, M199) | ||
| 5% | 0.8% | 13.7 ± 2.6 kPa (Piuma, PBS) | 26.9 ± 8.6 kPa (Piuma, PBS) |
| 14.5 ± 0.8 kPa (Piuma, M199) | 21.6 ± 5.1 kPa (Piuma, M199) | ||
| 15.1 ± 3.9 kPa (Instron, PBS) | |||
| 10% | 1% | 50.8 ± 12.1 kPa (Piuma, PBS) | 52.2 ± 16.0 kPa (Piuma, PBS) |
| 47.8 ± 2.3 kPa (Piuma, M199) | 39.2 ± 6.2 kPa (Piuma, M199) | ||
| 48.5 ± 10.9 kPa (Instron, PBS) | |||
Appendix A.2. Enzymatic Degradation of Hydrogels

References
- Bowers, S.L.K.; Banerjee, I.; Baudino, T.A. The extracellular matrix: At the center of it all. J. Mol. Cell Cardiol. 2010, 48, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, A.W.; Alford, P.W.; Jin, H.; Ripplinger, C.M.; Werdich, A.A.; Sheehy, S.P.; Grosberg, A.; Parker, K.K. Controlling the contractile strength of engineered cardiac muscle by hierarchal tissue architecture. Biomaterials 2012, 33, 5732–5741. [Google Scholar] [CrossRef] [PubMed]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-chips: Into the next decade. Nat. Rev. Drug Discov. 2021, 20, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Nahak, B.K.; Mishra, A.; Preetam, S.; Tiwari, A. Advances in Organ-on-a-Chip Materials and Devices. ACS Appl. Bio Mater. 2022, 5, 3576–3607. [Google Scholar] [CrossRef]
- Monteduro, A.G.; Rizzato, S.; Caragnano, G.; Trapani, A.; Giannelli, G.; Maruccio, G. Organs-on-chips technologies–A guide from disease models to opportunities for drug development. Biosens. Bioelectron. 2023, 231, 115271. [Google Scholar] [CrossRef]
- Alarake, N.Z.; Frohberg, P.; Groth, T.; Pietzsch, M. Mechanical Properties and Biocompatibility of in Situ Enzymatically Cross-Linked Gelatin Hydrogels. Int. J. Artif. Organs 2017, 40, 159–168. [Google Scholar] [CrossRef]
- Zaupa, A. Physical Crosslinking of Gelatin: A Supramolecular Approach to Biomaterials. Ph.D. Thesis, Universität Potsdam, Potsdam, Germany, 2010. [Google Scholar]
- Paguirigan, A.L.; Beebe, D.J. Protocol for the fabrication of enzymatically crosslinked gelatin microchannels for microfluidic cell culture. Nat. Protoc. 2007, 2, 1782–1788. [Google Scholar] [CrossRef]
- Noshadi, I.; Hong, S.; Sullivan, K.E.; Sani, E.S.; Portillo-Lara, R.; Tamayol, A.; Shin, S.R.; Gao, A.E.; Stoppel, W.L.; Black, L.D., III; et al. In vitro and in vivo analysis of visible light crosslinkable gelatin methacryloyl (GelMA) hydrogels. Biomater. Sci. 2017, 5, 2093–2105. [Google Scholar] [CrossRef]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. Photocrosslinkable Gelatin Hydrogel for Epidermal Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 108–118. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Rubini, K.; Roveri, N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 2001, 22, 763–768. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Roveri, N.; Rubini, K. Stabilization of gelatin films by crosslinking with genipin. Biomaterials 2002, 23, 4827–4832. [Google Scholar] [CrossRef] [PubMed]
- Lien, S.-M.; Li, W.-T.; Huang, T.-J. Genipin-crosslinked gelatin scaffolds for articular cartilage tissue engineering with a novel crosslinking method. Mater. Sci. Eng. C 2008, 28, 36–43. [Google Scholar] [CrossRef]
- Ng, W.-C.; Lokanathan, Y.; Fauzi, M.B.; Baki, M.M.; Zainuddin, A.A.; Phang, S.J.; Azman, M. In vitro evaluation of genipin-crosslinked gelatin hydrogels for vocal fold injection. Sci. Rep. 2023, 13, 5128. [Google Scholar] [CrossRef]
- Taghdi, M.H.; Al-Masawa, M.E.; Muttiah, B.; Fauzi, M.B.; Law, J.X.; Zainuddin, A.A.; Lokanathan, Y. Three-Dimensional Bioprinted Gelatin—Genipin Hydrogels Enriched with hUCMSC-Derived Small Extracellular Vesicles for Regenerative Wound Dressings. Polymers 2025, 17, 1163. [Google Scholar] [CrossRef] [PubMed]
- Merk, M.; Chirikian, O.; Adlhart, C. 3D PCL/Gelatin/Genipin Nanofiber Sponge as Scaffold for Regenerative Medicine. Materials 2021, 14, 2006. [Google Scholar] [CrossRef] [PubMed]
- Ermis, M. Photo-crosslinked gelatin methacrylate hydrogels with mesenchymal stem cell and endothelial cell spheroids as soft tissue substitutes. J. Mater. Res. 2021, 36, 176–190. [Google Scholar] [CrossRef]
- De Moor, L.; Smet, J.; Plovyt, M.; Bekaert, B.; Vercruysse, C.; Asadian, M.; De Geyter, N.; Van Vlierberghe, S.; Dubruel, P.; Declercq, H. Engineering microvasculature by 3D bioprinting of prevascularized spheroids in photo-crosslinkable gelatin. Biofabrication 2021, 13, 045021. [Google Scholar] [CrossRef]
- Tigner, T.J.; Rajput, S.; Gaharwar, A.K.; Alge, D.L. Comparison of Photo Cross Linkable Gelatin Derivatives and Initiators for Three-Dimensional Extrusion Bioprinting. Biomacromolecules 2020, 21, 454–463. [Google Scholar] [CrossRef]
- Basara, G.; Yue, X.; Zorlutuna, P. Dual Crosslinked Gelatin Methacryloyl Hydrogels for Photolithography and 3D Printing. Gels 2019, 5, 34. [Google Scholar] [CrossRef]
- Chau, D.Y.; Collighan, R.J.; Verderio, E.A.; Addy, V.L.; Griffin, M. The cellular response to transglutaminase-cross-linked collagen. Biomaterials 2005, 26, 6518–6529. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.N.; Starchenko, A.; Williams, R.M.; Bonassar, L.J.; Reinhart-King, C.A. Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior. Acta Biomater. 2013, 9, 4635–4644. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.G. The role of matrix stiffness in regulating cell behavior. Hepatology 2008, 47, 1394–1400. [Google Scholar] [CrossRef]
- Yeung, T.; Georges, P.C.; Flanagan, L.A.; Marg, B.; Ortiz, M.; Funaki, M.; Zahir, N.; Ming, W.; Weaver, V.; Janmey, P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskelet. 2005, 60, 24–34. [Google Scholar] [CrossRef]
- Querceto, S.; Santoro, R.; Gowran, A.; Grandinetti, B.; Pompilio, G.; Regnier, M.; Tesi, C.; Poggesi, C.; Ferrantini, C.; Pioner, J.M. The harder the climb the better the view: The impact of substrate stiffness on cardiomyocyte fate. J. Mol. Cell Cardiol. 2022, 166, 36–49. [Google Scholar] [CrossRef]
- Byfield, F.J.; Reen, R.K.; Shentu, T.-P.; Levitan, I.; Gooch, K.J. Endothelial actin and cell stiffness is modulated by substrate stiffness in 2D and 3D. J. Biomech. 2009, 42, 1114–1119. [Google Scholar] [CrossRef]
- Tian, G.; Ren, T. Mechanical stress regulates the mechanotransduction and metabolism of cardiac fibroblasts in fibrotic cardiac diseases. Eur. J. Cell Biol. 2023, 102, 151288. [Google Scholar] [CrossRef]
- Di, X.; Gao, X.; Peng, L.; Ai, J.; Jin, X.; Qi, S.; Li, H.; Wang, K.; Luo, D. Cellular mechanotransduction in health and diseases: From molecular mechanism to therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 282. [Google Scholar] [CrossRef]
- Pasqualini, F.S.; Nesmith, A.P.; Horton, R.E.; Sheehy, S.P.; Parker, K.K. Mechanotransduction and Metabolism in Cardiomyocyte Microdomains. BioMed Res. Int. 2016, 2016, 4081638. [Google Scholar] [CrossRef]
- Orr, A.W.; Helmke, B.P.; Blackman, B.R.; Schwartz, M.A. Mechanisms of mechanotransduction. Dev. Cell 2006, 10, 11–20. [Google Scholar] [CrossRef]
- Nakayama, K.H.; Hou, L.; Huang, N.F. Role of extracellular matrix signaling cues in modulating cell fate commitment for cardiovascular tissue engineering. Adv. Healthc. Mater. 2014, 3, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fan, P.; Jin, F.; Huang, G.; Guo, X.; Xu, F. Dynamic and static biomechanical traits of cardiac fibrosis. Front. Bioeng. Biotechnol. 2022, 10, 1042030. [Google Scholar] [CrossRef] [PubMed]
- Antonovaite, N. Exploring the Mechanical Microenvironment of the Brain by Dynamic Indentation. Ph.D. Thesis, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands, 2021. [Google Scholar]
- Anseth, K.S.; Bowman, C.N.; Brannon-Peppas, L. Mechanical properties of hydrogels and their experimental determination. Biomaterials 1996, 17, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Horton, R.E.; Yadid, M.; McCain, M.L.; Sheehy, S.P.; Pasqualini, F.S.; Park, S.-J.; Cho, A.; Campbell, P.; Parker, K.K. Angiotensin II Induced Cardiac Dysfunction on a Chip. PLoS ONE 2016, 11, e0146415. [Google Scholar] [CrossRef]
- Shihan, M.H.; Novo, S.G.; Le Marchand, S.J.; Wang, Y.; Duncan, M.K. A simple method for quantitating confocal fluorescent images. Biochem. Biophys. Rep. 2021, 25, 100916. [Google Scholar] [CrossRef]
- Herum, K.M.; Choppe, J.; Kumar, A.; Engler, A.J.; McCulloch, A.D. Mechanical regulation of cardiac fibroblast profibrotic phenotypes. Mol. Biol. Cell 2017, 28, 1871–1882. [Google Scholar] [CrossRef]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef]
- Landry, N.M.; Rattan, S.G.; Dixon, I.M.C. An Improved Method of Maintaining Primary Murine Cardiac Fibroblasts in Two-Dimensional Cell Culture. Sci. Rep. 2019, 9, 12889. [Google Scholar] [CrossRef]
- Van Putten, S.; Shafieyan, Y.; Hinz, B. Mechanical control of cardiac myofibroblasts. J. Mol. Cell Cardiol. 2016, 93, 133–142. [Google Scholar] [CrossRef]
- Mammoto, T.; Ingber, D.E. Mechanical control of tissue and organ development. Development 2010, 137, 1407–1420. [Google Scholar] [CrossRef]
- Kuo, J.C. Mechanotransduction at focal adhesions: Integrating cytoskeletal mechanics in migrating cells. J. Cell Mol. Med. 2013, 17, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Mechanobiology and diseases of mechanotransduction. Ann. Med. 2003, 35, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Ting, M.S.; Travas-Sejdic, J.; Malmström, J. Modulation of hydrogel stiffness by external stimuli: Soft materials for mechanotransduction studies. J. Mater. Chem. B 2021, 9, 7578–7596. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; An, S.; Bae, J.; Kim, J.; Kang, J.-S.; Park, S. Development of microbial transglutaminase-crosslinked gelatin hydrogel gratings with structural stability under high-stretching conditions for induction of muscle hypertrophy. J. Ind. Eng. Chem. 2023, 124, 348–357. [Google Scholar] [CrossRef]
- Sasaki, S.; Suzuki, T.; Morikawa, K.; Matsusaki, M.; Sato, K. Fabrication of a Gelatin-Based Microdevice for Vascular Cell Culture. Micromachines 2022, 14, 107. [Google Scholar] [CrossRef]
- Cosson, S.; Lutolf, M.P. Hydrogel microfluidics for the patterning of pluripotent stem cells. Sci. Rep. 2014, 4, 4462. [Google Scholar] [CrossRef]
- Ando, H.; Adachi, M.; Umeda, K.; Matsuura, A.; Nonaka, M.; Uchio, R.; Tanaka, H.; Motoki, M. Purification and Characteristics of a Novel Transglutaminase Derived from Microorganisms. Agric. Biol. Chem. 1989, 53, 2613–2617. [Google Scholar] [CrossRef]
- Cui, L.; Du, G.; Zhang, D.; Chen, J. Thermal stability and conformational changes of transglutaminase from a newly isolated Streptomyces hygroscopicus. Bioresour. Technol. 2008, 99, 3794–3800. [Google Scholar] [CrossRef]
- Powers, J.D.; McCulloch, A.D. Biomechanical signals regulating the structure of the heart. Curr. Opin. Physiol. 2022, 25, 100482. [Google Scholar] [CrossRef]
- Ghazal, R.; Wang, M.; Liu, D.; Tschumperlin, D.J.; Pereira, N.L. Cardiac Fibrosis in the Multi-Omics Era: Implications for Heart Failure. Circ. Res. 2025, 136, 773–802. [Google Scholar] [CrossRef]
- Saha, K.; Keung, A.J.; Irwin, E.F.; Li, Y.; Little, L.; Schaffer, D.V.; Healy, K.E. Substrate modulus directs neural stem cell behavior. Biophys. J. 2008, 95, 4426–4438. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xiao, Z.; Ren, X.; Long, H.; Qian, H.; Ma, K.; Guo, Y. Enzymatically crosslinked gelatin hydrogel promotes the proliferation of adipose tissue-derived stromal cells. PeerJ 2016, 4, e2497. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zohar, R.; McCulloch, C.A. Multiple roles of α-smooth muscle actin in mechanotransduction. Exp. Cell Res. 2006, 312, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Shinde, A.V.; Humeres, C.; Frangogiannis, N.G. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim. Biophys. Acta 2017, 1863, 298–309. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Fibroblast-Extracellular Matrix Interactions in Tissue Fibrosis. Curr. Pathobiol. Rep. 2016, 4, 11–18. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, R.; Zhao, S.; Li, B.; Wang, S.; Lu, W.; Zhu, D. Mechanically Tough and Conductive Hydrogels Based on Gelatin and Z–Gln–Gly Generated by Microbial Transglutaminase. Polymers 2024, 16, 999. [Google Scholar] [CrossRef]
- Trengove, A.; Duchi, S.; Onofrillo, C.; O’Connell, C.D.; Di Bella, C.; O’Connor, A.J. Microbial Transglutaminase Improves ex vivo Adhesion of Gelatin Methacryloyl Hydrogels to Human Cartilage. Front. Med. Technol. 2021, 3, 773673. [Google Scholar] [CrossRef]



| [Gelatin] (w/v) | [mTG] (w/v) | Testing Parameters |
|---|---|---|
| 2% | 1% | Crosslinking method: Immersion vs. Direct mix |
| 4% | 0.8% | UVO exposure impact |
| 5% | 0.8% | Incubation solution: PBS vs. M199 |
| 10% | 1% | Mechanical: Piuma vs. Instron |
| Pros | Cons | |
|---|---|---|
| Instron | Measurement time duration Handles a wide range of materials and loads Versatile (compression, tension, flexural, cyclic testing) | Amount of material needed for measurements Can be destructive to samples Less sensitive for soft materials Larger instrument footprint |
| Piuma | Less sample required for measurements Possibility for higher resolution Lower cost alternative to AFM Measure in physiological conditions Automation for sample mapping Minimally Destructive Semi-portable | Optimized for air measurements Calibration time Cost of probes Probe to probe variability Sensitivity to vibrations and sample heterogeneity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folh, J.; Tran, P.L.D.; Horton, R.E. Characterizing the Impact of Fabrication Methods on Mechanically Tunable Gelatin Hydrogels for Cardiac Fibrosis Studies. Bioengineering 2025, 12, 759. https://doi.org/10.3390/bioengineering12070759
Folh J, Tran PLD, Horton RE. Characterizing the Impact of Fabrication Methods on Mechanically Tunable Gelatin Hydrogels for Cardiac Fibrosis Studies. Bioengineering. 2025; 12(7):759. https://doi.org/10.3390/bioengineering12070759
Chicago/Turabian StyleFolh, Jordyn, Phan Linh Dan Tran, and Renita E. Horton. 2025. "Characterizing the Impact of Fabrication Methods on Mechanically Tunable Gelatin Hydrogels for Cardiac Fibrosis Studies" Bioengineering 12, no. 7: 759. https://doi.org/10.3390/bioengineering12070759
APA StyleFolh, J., Tran, P. L. D., & Horton, R. E. (2025). Characterizing the Impact of Fabrication Methods on Mechanically Tunable Gelatin Hydrogels for Cardiac Fibrosis Studies. Bioengineering, 12(7), 759. https://doi.org/10.3390/bioengineering12070759








