Handheld Device Adapted to Smartphone Cameras for the Measurement of Sodium Ion Concentrations at Saliva-Relevant Levels via Fluorescence
Abstract
:1. Introduction
2. Experimental Section
2.1. Sodium Green Indicator Dye
2.2. Test Solutions
2.3. Sample Holder, Chamber and Smartphone Covers


2.4. Laboratory Microplate Fluorescence Instrument
2.5. Image Processing
2.6. Computational
3. Results and Discussion
3.1. Sodium Ion Detection Using Fluorescence
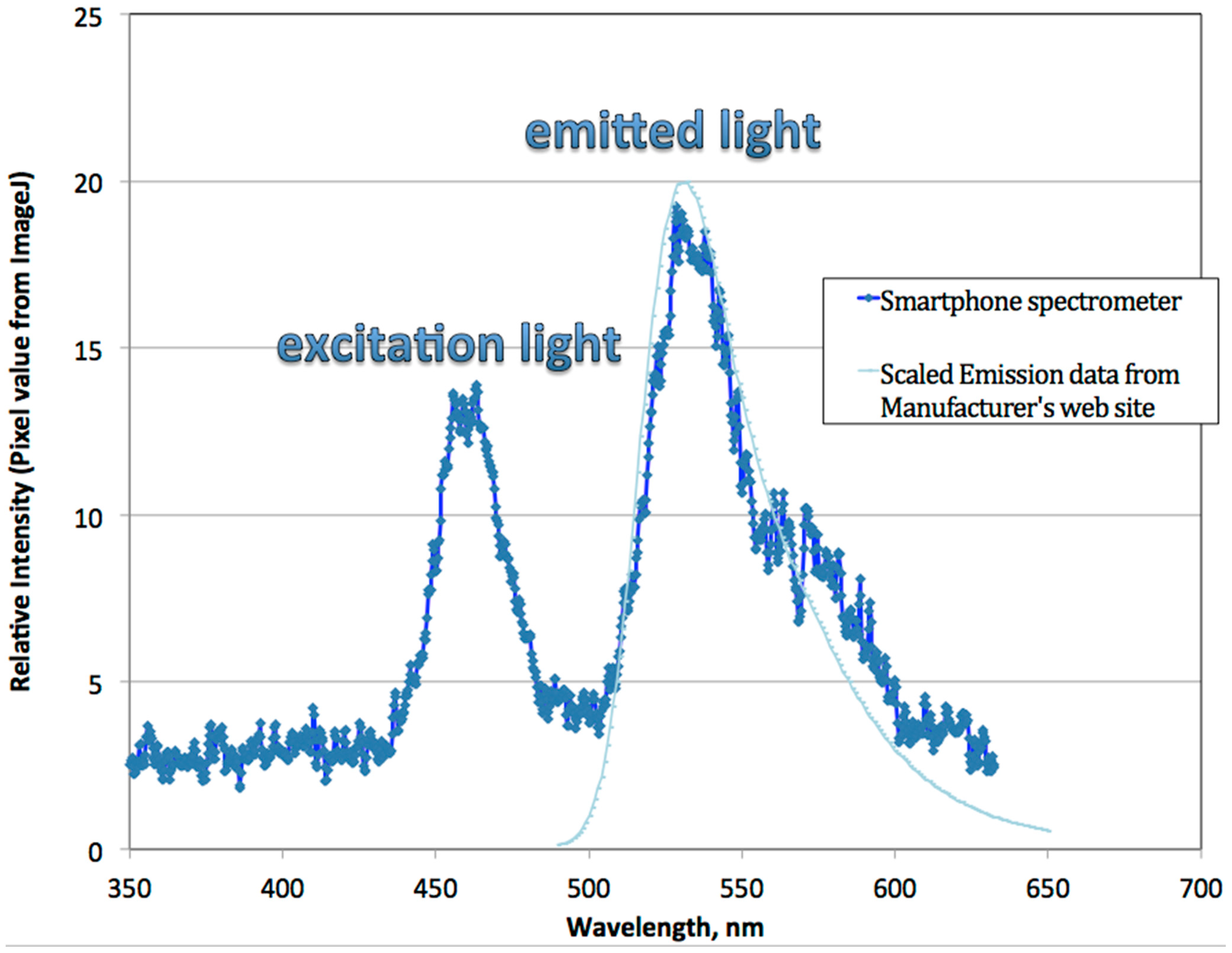


3.2. Discussion
3.2.1. Utility of Diffraction Grating for Avoiding Pixel Saturation

3.2.2. Chromatic Dispersion and Aberrations




4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marques, M.; Loebenberg, R.; Almukainzi, M. Simulated biological fluids with possible application in dissolution testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Life Technologies, Sodium Green™ Indicator in The Molecular Probes Handbook. Available online: http://www.lifetechnologies.com/us/en/home/references/molecular-probes-the-handbook/indicators-for-na-k-cl-and-miscellaneous-ions/fluorescent-na-and-k-indicators.html (accessed on 2 March 2015).
- Kaushik, A.; Vasudev, A.; Arya, S.K.; Pasha, S.K.; Bhansali, S. Recent advances in cortisol sensing technologies for point-of-care application. Biosens. Bioelectron. 2014, 53, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Motamayel, Ahmadi F.; davoodi, P.; Dalband, M.; Hendi, S.S. Saliva as a mirror of the body health. DJH 2010, 1, 1–15. [Google Scholar]
- Pels, E. Oral hygiene status and selected saliva biomarkers in children with acute lymphoblastic leukaemia during anticancer therapy. J. Leuk. 2013. [Google Scholar] [CrossRef]
- Hall, J.E. Guyton and Hall Textbook of Medical Physiology; Elsevier: Amsterdam, Netherlands, 2011. [Google Scholar]
- MathWorks. Converting Color Data between Color Spaces. Available online: http://www.mathworks.com/help/images/converting-color-data-between-color-spaces.html (accessed on 16 December 2014).
- Malon, R.S.P.; Sadir, S.; Balakrisdhnan, M.; Corcoles, E.P. Saliva-based biosensors: Noninvasive monitoring tool for clinical diagnostics. BioMed Res. Int. 2014, 2014, 20. [Google Scholar] [CrossRef] [PubMed]
- Blicharz, T.; Rissin, D.; Bowden, M.; Hayman, R.; DiCesare, C.; Bhatia, J.; Grand-Pierre, N.; Siqueira, W.L.; Helmerhorst, E.J.; Loscalzo, J.; et al. Use of colorimetric test strips for monitoring the effect of hemodialysis on salivary nitrite and uric acid in patients with end-stage renal disease: A proof of principle. Clin. Chem. 2008, 54, 1473–1489. [Google Scholar] [CrossRef] [PubMed]
- Marston, P.L.; Trinh, E.H. Hyperbolic umbilic diffraction catastrophe and rainbow scattering from spheroidal drops. Nature 1984, 312, 529–531. [Google Scholar] [CrossRef]
- García, A.A.; Nuñez, L.; John, C.; Hadish, H.; Orioke, V.; Mujica, V. Application of Newton’s zero order caustic for analysis and measurement: Part-I absorbance. Int. Res. J. Pure Appl. Chem. 2013, 4, 46–59. [Google Scholar] [CrossRef]
- Yang, M.; Wu, Y.; Sheng, X.; Fang Ren, K. Comparison of scattering diagrams of large non-spherical particles calculated by VCRM and MLFMA. J. Quant. Spectrosc. Radiat. Transf. 2015. [Google Scholar] [CrossRef]
- Yu, H. Laser Beam Interaction with Spheroidal Droplets: Computation and Measurement. Available online: http://tuprints.ulb.tu-darmstadt.de/3714/7/Dissertation.pdf (accessed on 6 November 2013).
- Public Lab. Spectrometer. Available online: http://publiclab.org/wiki/spectrometer (accessed on 6 November 2013).
- Szymacinski, H.; Lakowicz, J.R. Sodium green as a potential probe for intracellular sodium imaging based on fluorescence lifetime. Anal. Biochem. 1997, 250, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, D.; Kenneth, L.; Yu, H.; Clark, P.; Lin, Y.; George, S. Label-Free biodetection using a smartphone. Lab Chip 2013, 13, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Kofler, J.; Arnold, N. Axially symmetric focusing as a cuspoid diffraction catatastrophe: Scalar and vector cases and comparison with the theory of Mie. Phys. Rev. B 2006, 73, 17. [Google Scholar] [CrossRef]
- Sosa-Martinez, H.; Gutierrez-Vega, J.C. Optical forces on a Mie spheroidal particles aribitrarily oriented in a counterpropagating trap. J. Opt. Soc. Am. B 2009, 26, 2109–2116. [Google Scholar] [CrossRef]
- Palima, D.; Gluckstad, J. Gearing up for optical microrobotics: Micromanipulation and actuation of synthetic microstructures for optical forces. Laser Photonics Rev. 2013, 7, 478–494. [Google Scholar] [CrossRef]
- Berry, M.V.; Howls, C.J. Axial and focal-plane diffraction catastrophe integrals. J. Phys. A Math. Theor. 2010, 43, 1751–8113. [Google Scholar] [CrossRef]
- Thaning, A.; Friberg, A.T.; Popov, S.Y.; Jaroszeqicz, Z. Design of diffractive axicons producing uniform line images in Gaussian Schell-model illumination. J. Opt. Soc. Am. A Opt. Image. Sci. Vis. 2002, 19, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Graf, T.; Moloney, J.; Venkataramani, S. Asymptotic analysis of weakly nonlinear Bessel-Gauss beams. Phys. D: Nonlinear Phenom. 2013, 243, 32–44. [Google Scholar] [CrossRef]
- Thaning, A.; Jaroszeqicz, Z.; Friberg, A.T. Diffraction axicons in oblique illuminations: Analysis and experiments and comparison with elliptical axicons. Appl. Opt. 2003, 42, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Duocastella, M.; Arnold, C.B. Bessel and annular beams for materials processing. Laser Photonics 2012, 6, 607–621. [Google Scholar] [CrossRef]
- Marston, P.L.; Kaduchak, G. Generalized rainbows and unfolded glories of oblate drops: Organization for multiple internal reflections and extensions of cusps into Alexander’s dark band. Appl. Opt. 1994, 33, 4702–4713. [Google Scholar] [CrossRef] [PubMed]
- Marston, P.L. Catastrophe optics of spheroidal drops and generalized rainbows. J. Quant. Spectrosc. Radiat. Transf. 1999, 63, 341–351. [Google Scholar] [CrossRef]
- Masmali, A.M.; Purslow, C.; Murphy, P.J. The tear ferning test: A simple clinical technique to evaluate the ocular tear film. Clin. Exp. Optom. 2014, 97, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Kofler, J. Focusing of light in axially symmetric systems within the wave optics approximation. Graduate Engineering Degree Thesis, Johannes Kepler University, Linz, Austria, 2004. [Google Scholar]
- Stamnes, J.J. Waves in Focal Regions; Hilger, A., Ed.; Bristol and Boston: Boston, MA, USA, 1986. [Google Scholar]
- Thompson, K.P. Multinodal fifth-order optical aberrations of optical systems without rotational symmetry: The comatic aberrations. J. Opt. Soc. Am. A 2010, 27, 1490–1504. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipowicz, M.; Garcia, A. Handheld Device Adapted to Smartphone Cameras for the Measurement of Sodium Ion Concentrations at Saliva-Relevant Levels via Fluorescence. Bioengineering 2015, 2, 122-138. https://doi.org/10.3390/bioengineering2020122
Lipowicz M, Garcia A. Handheld Device Adapted to Smartphone Cameras for the Measurement of Sodium Ion Concentrations at Saliva-Relevant Levels via Fluorescence. Bioengineering. 2015; 2(2):122-138. https://doi.org/10.3390/bioengineering2020122
Chicago/Turabian StyleLipowicz, Michelle, and Antonio Garcia. 2015. "Handheld Device Adapted to Smartphone Cameras for the Measurement of Sodium Ion Concentrations at Saliva-Relevant Levels via Fluorescence" Bioengineering 2, no. 2: 122-138. https://doi.org/10.3390/bioengineering2020122





