Mitigation of Humic Acid Inhibition in Anaerobic Digestion of Cellulose by Addition of Various Salts
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Set-Up
| Experimental Group | Abbreviation | Avicel g/L COD | HA g/L | Added Salt (mM) |
|---|---|---|---|---|
| Blank (negative control) | - | 0 | 0 | 0 |
| Avicel (positive control) | C | 1 | 0 | 0 |
| Avicel + HA (inhibition group) | I | 1 | 5 | 0 |
| Avicel + HA + KCL (Salt addition group) | K | 1 | 5 | 5 |
| Avicel + HA + NaCl (Salt addition group) | Na | 1 | 5 | 5 |
| Avicel + HA + CaCl2 (Salt addition group) | Ca | 1 | 5 | 5 |
| Avicel + HA + MgCl2 (Salt addition group) | Mg | 1 | 5 | 5 |
| Avicel + HA + FeCl3 (Salt addition group) | Fe | 1 | 5 | 5 |
2.2. Monitoring Methane Production
2.3. Analytical Methods
3. Results and Discussion
3.1. Hydrolysis, Acidogenesis and Methanogenesis Efficiencies

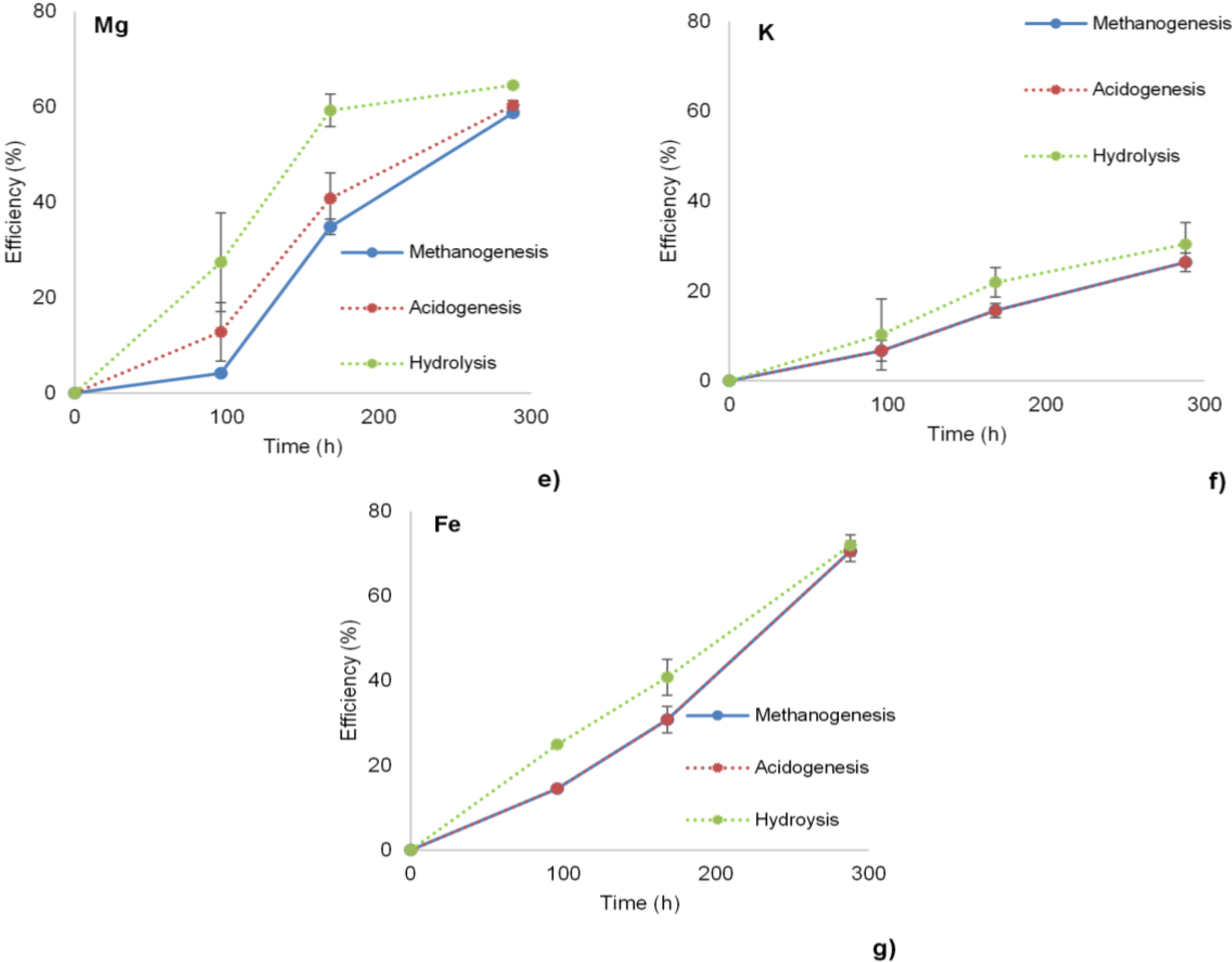
3.2. Methane Yield and Methane Production Rates


3.3. Effects of Salt Addition
| Cations (mg/L) | |||||
|---|---|---|---|---|---|
| Samples | K+ | Na+ | Ca2+ | Mg2+ | Fe2+ + Fe3+ |
| C-i | 116.03 | 217.4 | 24.81 | 12.947 | <0.001 |
| C-f | 85.6 ± 0.44 | 160.71 ± 0.26 | 14.51 ± 0.38 | 10.42 ± 0.01 | <0.001 |
| I-i | 166.11 | 525.97 | 85.33 | 13.54 | 32.05 |
| I-f | 137.47 ± 4.03 | 523.22 ± 17.8 | 91.58 ± 1.35 | 13.47 ± 0.55 | 32.85 ± 1.71 |
| K-i | 306.515 | 514.01 | 60.165 | 12.168 | 23.437 |
| K-f | 298.67 ± 0.29 | 494.31 ± 0.28 | 61.83 ± 3.2 | 12.80 ± 0.78 | 31.31 ± 2.9 |
| Na-i | 141.59 | 717.24 | 80.57 | 13.1 | 25.46 |
| Na-f | 142.1 ± 0.26 | 679.24 ± 11 | 82.81 ± 0.12 | 14.44 ± 0.4 | 37.35 ± 0.4 |
| Ca-i | 128.63 | 501.2 | 285.08 | 6.48 | 32.05 |
| Ca-f | 123.71 ± 1.71 | 470.98 ± 9.16 | 32.63 ± 0.93 | 4.89 ± 0.11 | 0.27 ± 0.01 |
| Mg-i | 127.87 | 517.42 | 99.331 | 134.958 | 32.047 |
| Mg-fi | 107.3 ± 2.24 | 424.56 ± 8.56 | 9.51 ± 0.24 | 117.31 ± 0.3 | <0.001 |
| Fe-i | 124.95 | 688.05 | 68.40 | 10.84 | 347.38 |
| Fe-f | 145.39 ± 0.52 | 643.30 ± 3.69 | 89.59 ± 0.04 | 15.35 ± 0.21 | 55.44 ± 1.50 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Johnstone, N.; Haščič, I.; Popp, D. Renewable energy policies and technological innovation: Evidence based on patent counts. Environ. Resour. Econ. 2010, 45, 133–155. [Google Scholar] [CrossRef]
- Appels, L.; Lauwers, J.; Degrève, J.; Helsen, L.; Lievens, B.; Willems, K.; van Impe, J.; Dewil, R. Anaerobic digestion in global bio-energy production: Potential and research challenges. Renew. Sustain. Energy Rev. 2011, 15, 4295–4301. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ellegaard, L. Codigestion of manure and organic wastes in centralized biogas plants. Appl. Biochem. Biotechnol. 2003, 109, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Raven, R.P.J.M.; Gregersen, K.H. Biogas plants in Denmark: Successes and setbacks. Renew. Sustain. Energy Rev. 2007, 11, 116–132. [Google Scholar] [CrossRef]
- Ma, J.; Frear, C.; Wang, Z.-W.; Yu, L.; Zhao, Q.; Li, X.; Chen, S. A simple methodology for rate-limiting step determination for anaerobic digestion of complex substrates and effect of microbial community ratio. Bioresour. Technol. 2013, 134, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Vavilin, V.A.; Fernandez, B.; Palatsi, J.; Flotats, X. Hydrolysis kinetics in anaerobic degradation of particulate organic material: An overview. Waste Manag. 2008, 28, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, S.; Cartagena, M.C.; Arce, A. Influence of lignin on the methanization of lignocellulosic wastes. Biomass 1990, 21, 43–54. [Google Scholar] [CrossRef]
- Gunaseelan, V.N. Anaerobic digestion of biomass for methane production: A review. Biomass Bioenergy 1997, 13, 83–114. [Google Scholar] [CrossRef]
- Pabón-Pereira, C.P.; de Vries, J.W.; Slingerland, M.A.; Zeeman, G.; van Lier, J.B. Impact of crop-manure ratios on energy production and fertilizing characteristics of liquid and solid digestate during codigestion. Environ. Technol. 2014, 35, 2427–2434. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.V. Hydrolysis Inhibition of Complex Biowaste. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2010. [Google Scholar]
- Saiz-Jimenez, C.; Hermosin, B.; Ortega-Calvo, J.J. Pyrolysis/methylation: A method for structural elucidation of the chemical nature of aquatic humic substances. Water Res. 1993, 27, 1693–1696. [Google Scholar] [CrossRef]
- Tipping, E. Cation Binding by Humic Substances; Cambridge University Press: Cambridge, UK, 2002; Volume 12. [Google Scholar]
- Li, H.; Li, Y.; Jin, Y.; Zou, S.; Li, C. Recovery of sludge humic acids with alkaline pretreatment and its impact on subsequent anaerobic digestion. J. Chem. Technol. Biotechnol. 2014, 89, 707–713. [Google Scholar] [CrossRef]
- Rolando, C.; Elba, V.; Carlos, R. Anaerobic mono-digestion of Turkey manure: Efficient revaluation to obtain methane and soil conditioner. J. Water Resour. Prot. 2011, 3, 584–589. [Google Scholar] [CrossRef]
- Fernandes, T.; van Lier, J.; Zeeman, G. Humic acid-like and fulvic acid-like inhibition on the hydrolysis of cellulose and tributyrin. Bioenergy Res. 2014. [Google Scholar] [CrossRef]
- Brons, H.J.; Field, J.A.; Lexmond, W.A.C.; Lettinga, G. Influence of humic acids on the hydrolysis of potato protein during anaerobic digestion. Agric. Wastes 1985, 13, 105–114. [Google Scholar] [CrossRef]
- Matilainen, A.; Vepsäläinen, M.; Sillanpää, M. Natural organic matter removal by coagulation during drinking water treatment: A review. Adv. Colloid Interface Sci. 2010, 159, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Renou, S.; Givaudan, J.G.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Li, A.; Zhou, Y. Selective removal of DOM on anion-exchange resin from water. In Functions of Natural Organic Matter in Changing Environment; Xu, J., Wu, J., He, Y., Eds.; Springer: South Holland, The Netherlands, 2013; pp. 921–924. [Google Scholar]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, L.; Guwy, A.; Jenicek, P.; Kalyuzhnui, S.; van Lier, J. Anaerobic Biodegradation, Activity and Inhibition (ABAI). In Proceedings of the Task Group Meeting, Prague, Czech, 9–10 October 2006; Institute of Environment & Resources, Technical University of Denmark: Kongens Lyngby, Denmark, 2007.
- Badshah, M.; Lam, D.M.; Liu, J.; Mattiasson, B. Use of an automatic methane potential test system for evaluating the biomethane potential of sugarcane bagasse after different treatments. Bioresour. Technol. 2012, 114, 262–269. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association. Water Environment Federation (APHA). Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- O’Sullivan, C.; Burrell, P.C.; Clarke, W.P.; Blackall, L.L. The effect of biomass density on cellulose solubilisation rates. Bioresour. Technol. 2008, 99, 4723–4731. [Google Scholar] [CrossRef] [PubMed]
- Kugelman, I.J.; McCarty, P.L. Cation toxicity and stimulation in anaerobic waste treatment. J. Water Pollut. Control Fed. 1965, 37, 97–116. [Google Scholar]
- Raposo, F.; de la Rubia, M.A.; Fernández-Cegrí, V.; Borja, R. Anaerobic digestion of solid organic substrates in batch mode: An overview relating to methane yields and experimental procedures. Renew. Sustain. Energy Rev. 2011, 16, 861–877. [Google Scholar] [CrossRef]
- Chen, Y.; Jay, J.C.; Kurt, S.C. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Liu, Y.; Boone, D.R. Effects of salinity on methanogenic decomposition. Bioresour. Technol. 1991, 35, 271–273. [Google Scholar] [CrossRef]
- Tipping, E.; Lofts, S.; Sonke, J. Humic ion-binding model VII: A revised parameterisation of cation-binding by humic substances. Environ. Chem. 2011, 8, 225–235. [Google Scholar] [CrossRef]
- Weber, K.A.; Achenbach, L.A.; Coates, J.D. Microorganisms pumping iron: Anaerobic microbial iron oxidation and reduction. Nat. Rev. Microbiol. 2006, 4, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Kara, F.; Gurakan, G.; Sanin, F. Monovalent cations and their influence on activated sludge floc chemistry, structure, and physical characteristics. Biotechnol. Bioeng. 2008, 100, 231–239. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azman, S.; Khadem, A.F.; Zeeman, G.; Van Lier, J.B.; Plugge, C.M. Mitigation of Humic Acid Inhibition in Anaerobic Digestion of Cellulose by Addition of Various Salts. Bioengineering 2015, 2, 54-65. https://doi.org/10.3390/bioengineering2020054
Azman S, Khadem AF, Zeeman G, Van Lier JB, Plugge CM. Mitigation of Humic Acid Inhibition in Anaerobic Digestion of Cellulose by Addition of Various Salts. Bioengineering. 2015; 2(2):54-65. https://doi.org/10.3390/bioengineering2020054
Chicago/Turabian StyleAzman, Samet, Ahmad F. Khadem, Grietje Zeeman, Jules B. Van Lier, and Caroline M. Plugge. 2015. "Mitigation of Humic Acid Inhibition in Anaerobic Digestion of Cellulose by Addition of Various Salts" Bioengineering 2, no. 2: 54-65. https://doi.org/10.3390/bioengineering2020054





