4D Flow Assessment of Vorticity in Right Ventricular Diastolic Dysfunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Acquisition
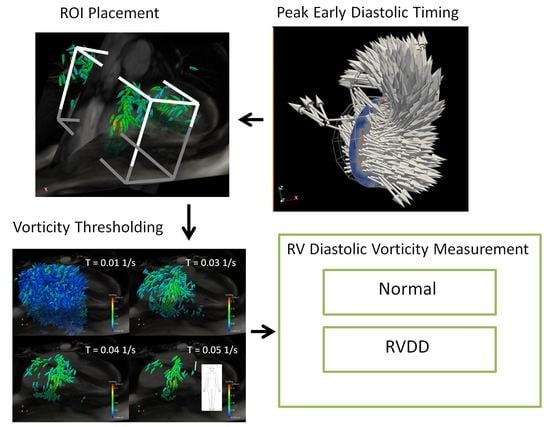
2.2. Preprocessing
2.3. Vorticity Calculation
2.4. Estimation of Cardiac Event Timing
3. Results
3.1. Timing of Cardiac Events
3.2. Interobserver Reliability
3.3. Peak E-Wave Vorticity
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Funding
References
- Ghosh, E.; Kovács, S.J. The Vortex Formation Time to Diastolic Function Relation: Assessment of Pseudonormalized versus Normal Filling. Physiol. Rep. 2013, 1, e00170. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Caracciolo, G.; Kheradvar, A.; Pedrizzetti, G.; Khandheria, B.K.; Narula, J.; Sengupta, P.P. Contrast Echocardiography for Assessing Left Ventricular Vortex Strength in Heart Failure: A Prospective Cohort Study. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Pedrizzetti, G.; Canna, G.L.; Alfieri, O.; Tonti, G. The Vortex—An Early Predictor of Cardiovascular Outcome? Nat. Rev. Cardiol. 2014, 11, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Pasipoularides, A.; Shu, M.; Shah, A.; Womack, M.S.; Glower, D.D. Diastolic Right Ventricular Filling Vortex in Normal and Volume Overload States. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1064–H1072. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, A.G.; Zajac, J.; Eriksson, J.; Dyverfeldt, P.; Bolger, A.F.; Ebbers, T.; Carlhäll, C.-J. 4-D Blood Flow in the Human Right Ventricle. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2344–H2350. [Google Scholar] [CrossRef] [PubMed]
- Markl, M.; Kilner, P.; Ebbers, T. Comprehensive 4D Velocity Mapping of the Heart and Great Vessels by Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. 2011, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- ElBaz, M.S.; Calkoen, E.; Westenberg, J.J.; Lelieveldt, B.P.; Roest, A.; van der Geest, R.J. Three Dimensional Right Ventricular Diastolic Vortex Rings: Characterization and Comparison with Left Ventricular Diastolic Vortex Rings from 4D Flow MRI. J. Cardiovasc. Magn. Reson. 2014, 16 (Suppl. 1), 42. [Google Scholar] [CrossRef]
- Elbaz, M.S.; Calkoen, E.E.; Westenberg, J.J.; Lelieveldt, B.P.; Roest, A.A.; van der Geest, R.J. Vortex Flow during Early and Late Left Ventricular Filling in Normal Subjects: Quantitative Characterization Using Retrospectively-Gated 4D Flow Cardiovascular Magnetic Resonance and Three-Dimensional Vortex Core Analysis. J. Cardiovasc. Magn. Reson. 2014, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- Kilner, P.J.; Yang, G.Z.; Wilkes, A.J.; Mohiaddin, R.H.; Firmin, D.N.; Yacoub, M.H. Asymmetric Redirection of Flow through the Heart. Nature 2000, 404, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, P.M.; Toger, J.; Heiberg, E.; Carlsson, M.; Arheden, H. Quantification of Left and Right Atrial Kinetic Energy Using Four-Dimensional Intracardiac Magnetic Resonance Imaging Flow Measurements. J. Appl. Physiol. 2013, 114, 1472–1481. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Alzate, A.; Kellihan, H.B.; Consigny, D.W.; Niespodzany, E.J.; François, C.J.; Wieben, O.; Chesler, N.C.; Frydrychowicz, A. 4D MR Velocity Mapping Using PC VIPR to Investigate the Hemodynamics of Acute Pulmonary Hypertension in a Dog Model. Proc. Intl. Soc. Mag. Reson. Med. 2011, 19, 1326. [Google Scholar]
- Geiger, J.; Markl, M.; Jung, B.; Grohmann, J.; Stiller, B.; Langer, M.; Arnold, R. 4D-MR Flow Analysis in Patients after Repair for Tetralogy of Fallot. Eur. Radiol. 2011, 21, 1651–1657. [Google Scholar] [CrossRef] [PubMed]
- Francois, C.J.; Srinivasan, S.; Schiebler, M.L.; Reeder, S.B.; Niespodzany, E.; Landgraf, B.R.; Wieben, O.; Frydrychowicz, A. 4D Cardiovascular Magnetic Resonance Velocity Mapping of Alterations of Right Heart Flow Patterns and Main Pulmonary Artery Hemodynamics in Tetralogy of Fallot. J. Cardiovasc. Magn. Reson. 2012, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Markl, M.; Geiger, J.; Kilner, P.J.; Föll, D.; Stiller, B.; Beyersdorf, F.; Arnold, R.; Frydrychowicz, A. Time-Resolved Three-Dimensional Magnetic Resonance Velocity Mapping of Cardiovascular Flow Paths in Volunteers and Patients with Fontan Circulation. Eur. J. Cardiothorac. Surg. 2011, 39, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Hussain, F. On the Identification of a Vortex. J. Fluid Mech. 1995, 285, 69–94. [Google Scholar] [CrossRef]
- Gan, C.T.-J.; Lankhaar, J.-W.; Westerhof, N.; Marcus, J.T.; Becker, A.; Twisk, J.W.R.; Boonstra, A.; Postmus, P.E.; Vonk-Noordegraaf, A. Noninvasively Assessed Pulmonary Artery Stiffness Predicts Mortality in Pulmonary Arterial Hypertension. Chest 2007, 132, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Shiina, Y.; Funabashi, N.; Lee, K.; Daimon, M.; Sekine, T.; Kawakubo, M.; Takahashi, M.; Yajima, R.; Tanabe, N.; Kuriyama, T.; et al. Right Atrium Contractility and Right Ventricular Diastolic Function Assessed by Pulsed Tissue Doppler Imaging Can Predict Brain Natriuretic Peptide in Adults with Acquired Pulmonary Hypertension. Int. J. Cardiol. 2009, 135, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Shiina, Y.; Funabashi, N.; Lee, K.; Daimon, M.; Sekine, T.; Kawakubo, M.; Sekine, Y.; Takahashi, M.; Yajima, R.; Wakatsuki, Y.; et al. Doppler Imaging Predicts Cardiac Events in Chronic Pulmonary Thromboembolism. Int. J. Cardiol. 2009, 133, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Fenster, B.E.; Browning, J.; Schroeder, J.D.; Schafer, M.; Podgorski, C.A.; Smyser, J.; Silveira, L.J.; Buckner, J.K.; Hertzberg, J.R. Vorticity Is a Marker of Right Ventricular Diastolic Dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1087–H1093. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Machiraju, R.; Thompson, D. Detection and Visualization of Vortices. The Visualization Handbook; Academic Press: Cambridge, MA, USA, 2005; pp. 295–309. [Google Scholar]
- Squillacote, A. The Paraview Guide; Kitware, Inc.: Clifton Park, NY, USA, 2008. [Google Scholar]
- McLaughlin, V.V.; Archer, S.L.; Badesch, D.B.; Barst, R.J.; Farber, H.W.; Lindner, J.R.; Mathier, M.A.; McGoon, M.D.; Park, M.H.; Rosenson, R.S.; et al. ACCF/AHA 2009 Expert Consensus Document on Pulmonary HypertensionA Report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association Developed in Collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J. Am. Coll. Cardiol. 2009, 53, 1573–1619. [Google Scholar] [PubMed]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography Endorsed by the European Association of Echocardiography, a Registered Branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar]
- Markl, M.; Chan, F.P.; Alley, M.T.; Wedding, K.L.; Draney, M.T.; Elkins, C.J.; Parker, D.W.; Wicker, R.; Taylor, C.A.; Herfkens, R.J.; et al. Time-Resolved Three-Dimensional Phase-Contrast MRI. J. Magn. Reson. Imaging JMRI 2003, 17, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Bock, J.; Kreher, B.; Hennig, J.; Markl, M. Optimized Pre-Processing of Time-Resolved 2D and 3D Phase Contrast MRI Data. In Proceedings of the 15th Annual Meeting of ISMRM, Berlin, Germany, 19–25 May 2007; p. 3138. [Google Scholar]
- Axel, L.; Morton, D. Correction of Phase Wrapping in Magnetic Resonance Imaging. Med. Phys. 1989, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Petitjean, C.; Zuluaga, M.A.; Bai, W.; Dacher, J.-N.; Grosgeorge, D.; Caudron, J.; Ruan, S.; Ayed, I.B.; Cardoso, M.J.; Chen, H.-C.; et al. Right Ventricle Segmentation from Cardiac MRI: A Collation Study. Med. Image Anal. 2015, 19, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Heiberg, E.; Sjögren, J.; Ugander, M.; Carlsson, M.; Engblom, H.; Arheden, H. Design and Validation of Segment—Freely Available Software for Cardiovascular Image Analysis. BMC Med. Imaging 2010, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, H.; Little, W.C. The Cardiac Cycle and the Physiological Basis of Left Ventricular Contraction, Ejection, Relaxation, and Filling. Heart Fail. Clin. 2008, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]










| Control (n = 14) | RVDD (n = 20) | ||
|---|---|---|---|
| BMI | [kg/m2] | 25 ± 2 | 28 ± 1 |
| CO | [L/min] | 4.3 ± 0.3 | 4.4 ± 0.3 |
| CI | [L/min/m2] | 2.4 ± 0.2 | 2.5 ± 0.2 |
| E/A | [-] | 21.8 ± 0.2 | 1.1 ± 0.1 |
| E/e′ | [-] | 3.4 ± 0.3 | 6.4 ± 0.6 |
| RVEDV | [mL] | 138 ± 9 | 181 ± 17 |
| RVESV | [mL] | 69 ± 6 | 123 ± 17 |
| RVEF | [%] | 50 ± 2 | 35 ± 3 |
| p < 0.05 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p | 0.011 | 0.012 | 0.015 | 0.024 | 0.025 | 0.029 | 0.034 | 0.045 | 0.047 | 0.048 |
| ROI | RA | RA | RA | RA | RA | RA | RH | RA | RA | RH |
| Scaling/Thgreshold Method | SS | SS | SS | SS | TS | TS | TS | SS | TS | SS |
| Thgreshold [L/s] | 0.04 | 0.05 | 0.06 | 0.03 | 0.04 | 0.03 | 0.05 | 0.04 | 0.06 | 0.03 |
| Normal Mean Vorticity [L/s or m2/60] | 709 | 425 | 255 | 1121 | 1826 | 2685 | 1184 | 1353 | 750 | 2051 |
| Normal Standard Deviation | 325 | 219 | 145 | 466 | 886 | 1026 | 712 | 518 | 531 | 707 |
| RVDD Mean Vorticity [L/s or m2/60] | 408 | 222 | 127 | 749 | 1109 | 1870 | 653 | 887 | 387 | 1450 |
| RVDD Standard Deviation | 303 | 208 | 136 | 417 | 834 | 998 | 632 | 785 | 449 | 995 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Browning, J.R.; Hertzberg, J.R.; Schroeder, J.D.; Fenster, B.E. 4D Flow Assessment of Vorticity in Right Ventricular Diastolic Dysfunction. Bioengineering 2017, 4, 30. https://doi.org/10.3390/bioengineering4020030
Browning JR, Hertzberg JR, Schroeder JD, Fenster BE. 4D Flow Assessment of Vorticity in Right Ventricular Diastolic Dysfunction. Bioengineering. 2017; 4(2):30. https://doi.org/10.3390/bioengineering4020030
Chicago/Turabian StyleBrowning, James R., Jean R. Hertzberg, Joyce D. Schroeder, and Brett E. Fenster. 2017. "4D Flow Assessment of Vorticity in Right Ventricular Diastolic Dysfunction" Bioengineering 4, no. 2: 30. https://doi.org/10.3390/bioengineering4020030
APA StyleBrowning, J. R., Hertzberg, J. R., Schroeder, J. D., & Fenster, B. E. (2017). 4D Flow Assessment of Vorticity in Right Ventricular Diastolic Dysfunction. Bioengineering, 4(2), 30. https://doi.org/10.3390/bioengineering4020030