Additive Manufacturing of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/poly(ε-caprolactone) Blend Scaffolds for Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Scaffolds Fabrication
2.3. Morphological Characterization
2.4. Thermal Analysis
2.5. Mechanical Testing
2.6. In Vitro Biological Evaluation
2.6.1. Cell Culture
2.6.2. Cell Viability and Proliferation
2.6.3. Morphologic Characterizations by Confocal Laser Scanning Microscopy (CLSM)
2.7. Statistical Analysis
3. Results and Discussion
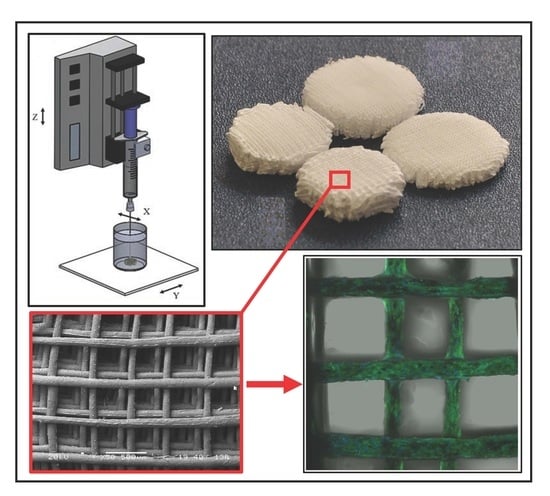
3.1. Additive Manufacturing of Scaffolds
3.2. Morphological Characterization
3.3. Thermal Characterization
3.4. Mechanical Characterization
3.5. Biological Characterization
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Puppi, D.; Chiellini, F.; Dash, M.; Chiellini, E. Biodegradable Polymers for Biomedical Applications. In Biodegradable Polymers: Processing, Degradation and Applications; Felton, G.P., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; pp. 545–604. [Google Scholar]
- Woodruff, M.A.; Lange, C.; Reichert, J.; Berner, A.; Chen, F.; Fratzl, P.; Schantz, J.-T.; Hutmacher, D.W. Bone tissue engineering: From bench to bedside. Mater. Today 2012, 15, 430–435. [Google Scholar] [CrossRef]
- Puppi, D.; Chiellini, F.; Piras, A.M.; Chiellini, E. Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010, 35, 403–440. [Google Scholar] [CrossRef]
- Morelli, A.; Puppi, D.; Chiellini, F. Polymers from Renewable Resources. J. Renew. Mater. 2013, 1, 83–112. [Google Scholar] [CrossRef]
- Doyle, C.; Tanner, E.T.; Bonfield, W. In vitro and in vivo evaluation of polyhydroxybutyrate and of polyhydroxybutyrate reinforced with hydroxyapatite. Biomaterials 1991, 12, 841–847. [Google Scholar] [CrossRef]
- Chen, G.Q.; Wu, Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef] [PubMed]
- Jack, K.S.; Velayudhan, S.; Luckman, P.; Trau, M.; Grøndahl, L.; Cooper-White, J. The fabrication and characterization of biodegradable HA/PHBV nanoparticle-polymer composite scaffolds. Acta Biomater. 2009, 5, 2657–2667. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Kong, L.; Zhang, L.; Gong, Y.; Chen, G.; Zhao, N.; Zhang, X. Improvement of mechanical properties of poly(dl-lactide) films by blending of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Eur. Polym. J. 2006, 42, 764–775. [Google Scholar] [CrossRef]
- Wang, Y.W.; Wu, Q.; Chen, G.Q. Attachment, proliferation and differentiation of osteoblasts on random biopolyester poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds. Biomaterials 2004, 25, 669–675. [Google Scholar] [CrossRef]
- Wang, Y.W.; Yang, F.; Wu, Q.; Cheng, Y.C.; Yu, P.H.; Chen, J.; Chen, G.Q. Effect of composition of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) on growth of fibroblast and osteoblast. Biomaterials 2005, 26, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhu, S.; Chen, Y.; Chang, Z.; Chen, G.; Gong, Y.; Zhao, N.; Zhang, X. Studies on bone marrow stromal cells affinity of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate). Biomaterials 2004, 25, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Ling, Z.; Zhenhu An, Z.; Guoqiang, C.; Yandao, G.; Nanming, Z.; Xiufang, Z. Preparation and evaluation of porous poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) hydroxyapatite composite scaffolds. J. Biomater. Appl. 2008, 22, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, J.M.; Garrido, L.; Quijada-Garrido, I.; Kaschta, J.; Schubert, D.W.; Boccaccini, A.R. Novel poly(hydroxyalkanoates)-based composites containing Bioglass (R) and calcium sulfate for bone tissue engineering. Biomed. Mater. 2012, 7, 054105. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Yang, Y.; Ren, L.; Wang, Y.; Li, Y.; Huang, H. Dielectric behaviors of PHBHHx–BaTiO3 multifunctional composite films. Compos. Sci. Technol. 2012, 72, 370–375. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, X.-L.; Yang, S.-C.; Lin, X.; He, Y.; Yan, C.; Wu, L.; Chen, G.-Q.; Wang, Z.-Y.; Wu, Q. MicroRNAs in the regulation of interfacial behaviors of MSCs cultured on microgrooved surface pattern. Biomaterials 2011, 32, 9207–9217. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, R.; Wang, P.-P.; Jian, J.; Jiang, X.-L.; Yan, C.; Lin, X.; Wu, L.; Chen, G.-Q.; Wu, Q. The differential effects of aligned electrospun PHBHHx fibers on adipogenic and osteogenic potential of MSCs through the regulation of PPARγ signaling. Biomaterials 2012, 33, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-P.; You, M.; Wang, D.; Peng, G.; Wang, Z.; Chen, G.-Q. Fabrication of carbon nanotube (CNT)/poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) nanocomposite films for human mesenchymal stem cell (hMSC) differentiation. Polym. Chem. 2013, 4, 4490–4498. [Google Scholar] [CrossRef]
- ASTM. International F2792—12a Standard Terminology for Additive Manufacturing Technologies; ASTM: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Mota, C.; Puppi, D.; Chiellini, F.; Chiellini, E. Additive manufacturing techniques for the production of tissue engineering constructs. J. Tissue Eng. Regen. Med. 2015, 9, 174–190. [Google Scholar] [CrossRef] [PubMed]
- Leroy, E.; Petit, I.; Audic, J.L.; Colomines, G.; Deterre, R. Rheological characterization of a thermally unstable bioplastic in injection molding conditions. Polym. Degrad. Stab. 2012, 97, 1915–1921. [Google Scholar] [CrossRef]
- Kosorn, W.; Sakulsumbat, M.; Uppanan, P.; Kaewkong, P.; Chantaweroad, S.; Jitsaard, J.; Sitthiseripratip, K.; Janvikul, W. PCL/PHBV blended three dimensional scaffolds fabricated by fused deposition modeling and responses of chondrocytes to the scaffolds. J. Biomed. Mater. Res. B 2016. [Google Scholar] [CrossRef]
- Mota, C.; Wang, S.Y.; Puppi, D.; Gazzarri, M.; Migone, C.; Chiellini, F.; Chen, G.Q.; Chiellini, E. Additive manufacturing of poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyhexanoate] scaffolds for engineered bone development. J. Tissue Eng. Regen. Med. 2017, 11, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Pirosa, A.; Morelli, A.; Chiellini, F. Design, fabrication and characterization of tailored poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyexanoate] scaffolds by Computer-aided Wet-spinning. Rapid Prototyp. J. 2018, 24. unpublished. [Google Scholar]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer-Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, S.; Kong, M.; Geng, W.; Li, R.K.Y.; Song, C.; Kong, D. Phase morphology, physical properties, and biodegradation behavior of novel PLA/PHBHHx blends. J. Biomed. Mater. Res. B 2011, 100B, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Chiono, V.; Ciardelli, G.; Vozzi, G.; Sotgiu, M.G.; Vinci, B.; Domenici, C.; Giusti, P. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/poly(ε-caprolactone) blends for tissue engineering applications in the form of hollow fibers. J. Biomed. Mater. Res. A 2008, 85A, 938–953. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Chong, M.S.K.; Teo, E.Y.; Chen, G.-Q.; Chan, J.K.Y.; Teoh, S.-H. Biocompatibility studies and characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/polycaprolactone blends. J. Biomed. Mater. Res. B 2013, 101B, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Mota, C.; Gazzarri, M.; Dinucci, D.; Gloria, A.; Myrzabekova, M.; Ambrosio, L.; Chiellini, F. Additive manufacturing of wet-spun polymeric scaffolds for bone tissue engineering. Biomed. Microdevices 2012, 14, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.; Puppi, D.; Dinucci, D.; Gazzarri, M.; Chiellini, F. Additive manufacturing of star poly(ε-caprolactone) wet-spun scaffolds for bone tissue engineering applications. J. Bioact. Compat. Polym. 2013, 28, 320–340. [Google Scholar] [CrossRef]
- Dini, F.; Barsotti, G.; Puppi, D.; Coli, A.; Briganti, A.; Giannessi, E.; Miragliotta, V.; Mota, C.; Pirosa, A.; Stornelli, M.R.; et al. Tailored star poly (ε-caprolactone) wet-spun scaffolds for in vivo regeneration of long bone critical size defects. J. Bioact. Compat. Polym. 2016, 31, 15–30. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- ASTM. D1621—10 “Standard Test Method for Compressive Properties of Rigid Cellular Plastics”; ASTM: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Puppi, D.; Piras, A.M.; Chiellini, F.; Chiellini, E.; Martins, A.; Leonor, I.B.; Neves, N.; Reis, R. Optimized electro- and wet-spinning techniques for the production of polymeric fibrous scaffolds loaded with bisphosphonate and hydroxyapatite. J. Tissue Eng. Regen. Med. 2011, 5, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Tuzlakoglu, K.; Alves, C.M.; Mano, J.F.; Reis, R.L. Production and characterization of chitosan fibers and 3-D fiber mesh scaffolds for tissue engineering applications. Macromol. Biosci. 2004, 4, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.E.; Holtorf, H.L.; Reis, R.L.; Mikos, A.G. Influence of the porosity of starch-based fiber mesh scaffolds on the proliferation and osteogenic differentiation of bone marrow stromal cells cultured in a flow perfusion bioreactor. Tissue Eng. 2006, 12, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Malheiro, V.N.; Caridade, S.G.; Alves, N.M.; Mano, J.F. New poly(ε-caprolactone)/chitosan blend fibers for tissue engineering applications. Acta Biomater. 2010, 6, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Neves, S.C.; Moreira Teixeira, L.S.; Moroni, L.; Reis, R.L.; Van Blitterswijk, C.A.; Alves, N.M.; Karperien, M.; Mano, J.F. Chitosan/Poly(ε-caprolactone) blend scaffolds for cartilage repair. Biomaterials 2011, 32, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Dinucci, D.; Bartoli, C.; Mota, C.; Migone, C.; Dini, F.; Barsotti, G.; Carlucci, F.; Chiellini, F. Development of 3D wet-spun polymeric scaffolds loaded with antimicrobial agents for bone engineering. J. Bioact. Compat. Polym. 2011, 26, 478–492. [Google Scholar] [CrossRef]
- Puppi, D.; Chiellini, F. Wet-spinning of Biomedical Polymers: From Single Fibers Production to Additive Manufacturing of 3D Scaffolds. Polym. Int. 2017. [Google Scholar] [CrossRef]
- Puppi, D.; Migone, C.; Grassi, L.; Pirosa, A.; Maisetta, G.; Batoni, G.; Chiellini, F. Integrated three-dimensional fiber/hydrogel biphasic scaffolds for periodontal bone tissue engineering. Polym. Int. 2016, 65, 631–640. [Google Scholar] [CrossRef]
- Puppi, D.; Piras, A.M.; Pirosa, A.; Sandreschi, S.; Chiellini, F. Levofloxacin-loaded star poly(ε-caprolactone) scaffolds by additive manufacturing. J. Mater. Sci. Mater. Med. 2016, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Migone, C.; Morelli, A.; Bartoli, C.; Gazzarri, M.; Pasini, D.; Chiellini, F. Microstructured chitosan/poly(γ-glutamic acid) polyelectrolyte complex hydrogels by computer-aided wet-spinning for biomedical three-dimensional scaffolds. J. Bioact. Compat. Polym. 2016, 31, 531–549. [Google Scholar] [CrossRef]
- Chiellini, F.; Puppi, D.; Piras, A.M.; Morelli, A.; Bartoli, C.; Migone, C. Modelling of pancreatic ductal adenocarcinoma in vitro with three-dimensional microstructured hydrogels. RSC Adv. 2016, 6, 54226–54235. [Google Scholar] [CrossRef]
- Neves, S.C.; Mota, C.; Longoni, A.; Barrias, C.C.; Granja, P.L.; Moroni, L. Additive manufactured polymeric 3D scaffolds with tailored surface topography influence mesenchymal stromal cells activity. Biofabrication 2016, 8, 025012. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Wang, M.; Zhou, W.Y.; Cheung, W.L.; Li, Z.Y.; Lu, W.W. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater. 2010, 6, 4495–4505. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Wang, M. Customized Ca–P/PHBV nanocomposite scaffolds for bone tissue engineering: Design, fabrication, surface modification and sustained release of growth factor. J. R. Soc. Interface 2010, 7, S615–S629. [Google Scholar] [CrossRef] [PubMed]
- Bin, D.; Wai Lam, C.; Min, W. Optimized fabrication of Ca–P/PHBV nanocomposite scaffolds via selective laser sintering for bone tissue engineering. Biofabrication 2011, 3, 015001. [Google Scholar]
- Puppi, D.; Zhang, X.; Yang, L.; Chiellini, F.; Sun, X.; Chiellini, E. Nano/microfibrous polymeric constructs loaded with bioactive agents and designed for tissue engineering applications: A review. J. Biomed. Mater. Res. B 2014, 102, 1562–1579. [Google Scholar] [CrossRef] [PubMed]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Yang, H.-X.; Sun, M.; Zhou, P. Investigation of water diffusion in poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) by generalized two-dimensional correlation ATR–FTIR spectroscopy. Polymer 2009, 50, 1533–1540. [Google Scholar] [CrossRef]
- Ding, C.; Cheng, B.; Wu, Q. DSC analysis of isothermally melt-crystallized bacterial poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) films. J. Therm. Anal. Calorim. 2011, 103, 1001–1006. [Google Scholar] [CrossRef]
- Wang, Y.W.; Wu, Q.; Chen, J.; Chen, G.Q. Evaluation of three-dimensional scaffolds made of blends of hydroxyapatite and poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) for bone reconstruction. Biomaterials 2005, 26, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Wutticharoenmongkol, P.; Pavasant, P.; Supaphol, P. Osteoblastic Phenotype Expression of MC3T3-E1 Cultured on Electrospun Polycaprolactone Fiber Mats Filled with Hydroxyapatite Nanoparticles. Biomacromolecules 2007, 8, 2602–2610. [Google Scholar] [CrossRef] [PubMed]
- Kommareddy, K.P.; Lange, C.; Rumpler, M.; Dunlop, J.W.C.; Manjubala, I.; Cui, J.; Kratz, K.; Lendlein, A.; Fratzl, P. Two stages in three-dimensional in vitro growth of tissue generated by osteoblastlike cells. Biointerphases 2010, 5, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. 2001, 55, 203–216. [Google Scholar] [CrossRef]
- Quarles, L.D.; Yohay, D.A.; Lever, L.W.; Caton, R.; Wenstrup, R.J. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: An in vitro model of osteoblast development. J. Bone Min. Res. 1992, 7, 683–692. [Google Scholar] [CrossRef] [PubMed]







| Sample | F (mL·h−1) | Vdep (mm·min−1) | Fiber Diameter (μm) | Pore Size (μm) |
|---|---|---|---|---|
| PHBHHx | 0.5 | 300 | 88 ± 12 | 485 ± 40 |
| PHBHHx/PCL 3:1 | 1 | 560 | 116 ± 12 | 493 ± 29 |
| PHBHHx/PCL 2:1 | 1 | 560 | 114 ± 15 | 470 ± 46 |
| PHBHHx/PCL 1:1 | 1 | 560 | 120 ± 11 | 484 ± 18 |
| Sample | 1st Decomposition Step | 2nd Decomposition STEP | ||
|---|---|---|---|---|
| Peak (°C) | Weight Loss (%) | Peak (°C) | Weight Loss (%) | |
| PHBHHx | 290.7 ± 1.8 | 97.6 ± 0.9 | - | - |
| PHBHHx/PCL 3:1 | 291.3 ± 2.2 | 74.6 ± 1.2 | 405.9 ± 0.5 | 25.1 ± 0.4 |
| PHBHHx/PCL 2:1 | 289.2 ± 1.4 | 65.4 ± 1.4 | 405.9 ± 0.7 | 34.2 ± 0.5 |
| PHBHHx/PCL 1:1 | 285.2 ± 1.6 | 44.9 ± 0.8 | 405.3 ± 0.8 | 54.6 ± 0.8 |
| PCL raw | - | - | 406.6 ± 0.4 | 99.1 ± 0.4 |
| Sample | 1st Heating | 2nd Heating | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tg1 (°C) | Tm1 (°C) | ΔH1 (J/g) | Tg2 (°C) | Tm2 (°C) | ΔH2 (J/g) | Tg1 (°C) | Tm1 (°C) | ΔH1 (J/g) | Tg2 (°C) | |
| PHBHHx | --- | --- | --- | −0.6 ± 0.2 | 93.7 ± 1.2 | 41.4 ± 1.8 | --- | --- | --- | −1.1 ± 0.6 |
| PHBHHx/PCL 3:1 | −70.7 ± 1.6 | 58.5 ± 0.9 | 18.6 ± 1.2 | −0.2 ± 0.6 | 93.5 ± 1.6 | 18.4 ± 0.9 | −66.2 ± 0.8 | 56.0 ± 1.2 | 15.4 ± 0.8 | −1.1 ± 0.8 |
| PHBHHx/PCL 2:1 | −65.2 ± 1.4 | 60.7 ± 1.2 | 30.2 ± 1.8 | −0.1 ± 0.3 | 94.3 ± 2.1 | 14.9 ± 1.1 | −64.9 ± 1.3 | 55.8 ± 0.4 | 21.1 ± 1.4 | −0.9 ± 0.4 |
| PHBHHx/PCL 1:1 | −60.2 ± 1.4 | 61.3 ± 0.8 | 53.4 ± 2.4 | −0.6 ± 0.2 | 94.2 ± 1.9 | 7.5 ± 0.4 | −63.4 ± 1.4 | 56.3 ± 1.6 | 36.0 ± 1.4 | −1.4 ± 0.5 |
| PCL raw | −61.4 ± 1.1 | 63.4 ± 0.5 | 96.5 ± 2.1 | --- | --- | --- | −64.7 ± 1.6 | 55.9 ± 1.5 | 83.0 ± 2.6 | --- |
| Scaffolds | Compressive Modulus (MPa) | Yield Strain (%) | Yield Stress (MPa) | Stress at 85% Strain (MPa) |
|---|---|---|---|---|
| PHBHHx | 0.16 ± 0.12 | 56.8 ± 9.5 | 0.32 ± 0.02 | 0.47 ± 0.09 |
| PHBHHx/PCL 3:1 | 0.17 ± 0.89 | 35.0 ± 9.4 | 0.18 ± 0.03 | 0.41 ± 0.09 |
| PHBHHx/PCL 2:1 | 0.39 ± 0.14 | 57.9 ± 5.5 | 0.36 ± 0.05 | 0.48 ± 0.05 |
| PHBHHx/PCL 1:1 | 0.37 ± 0.07 | 66.8 ± 5.5 | 0.36 ± 0.02 | 0.51 ± 0.07 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puppi, D.; Morelli, A.; Chiellini, F. Additive Manufacturing of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/poly(ε-caprolactone) Blend Scaffolds for Tissue Engineering. Bioengineering 2017, 4, 49. https://doi.org/10.3390/bioengineering4020049
Puppi D, Morelli A, Chiellini F. Additive Manufacturing of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/poly(ε-caprolactone) Blend Scaffolds for Tissue Engineering. Bioengineering. 2017; 4(2):49. https://doi.org/10.3390/bioengineering4020049
Chicago/Turabian StylePuppi, Dario, Andrea Morelli, and Federica Chiellini. 2017. "Additive Manufacturing of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/poly(ε-caprolactone) Blend Scaffolds for Tissue Engineering" Bioengineering 4, no. 2: 49. https://doi.org/10.3390/bioengineering4020049
APA StylePuppi, D., Morelli, A., & Chiellini, F. (2017). Additive Manufacturing of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/poly(ε-caprolactone) Blend Scaffolds for Tissue Engineering. Bioengineering, 4(2), 49. https://doi.org/10.3390/bioengineering4020049