Characterization of Chlorhexidine-Loaded Calcium-Hydroxide Microparticles as a Potential Dental Pulp-Capping Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
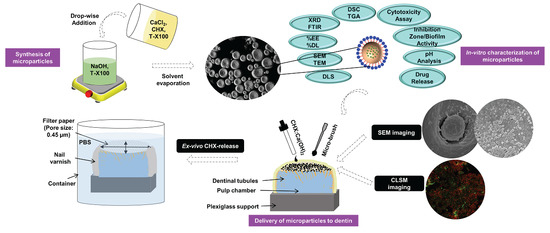
2.2. Synthesis of Microparticles
2.3. Morphological Features
2.4. Encapsulation-Efficiency and Drug-Loading
2.5. Spectral and Thermal Analyses
2.6. Evaluation of pH
2.7. Cytotoxicity Assay
2.8. Agar-Diffusion Bacterial Inhibition Zone Test
2.9. Preparation of Dentin-Specimens for Microparticles Application
2.10. Microparticles Application to Surface-Prepared Dentin-Substrates
2.11. Biofilm Formation and Live/Dead Cell Assay
2.12. CHX Release-Profiles
2.13. Statistical Analysis
3. Results
3.1. Morphological Features
3.2. Encapsulation-Efficiency and Drug-Loading
3.3. Spectral Analyses
3.4. Thermal Properties
3.5. Evaluation of pH
3.6. Cytotoxicity Assay
3.7. Bacterial Inhibition Zone
3.8. Microparticles Application to Dentin-Substrates
3.9. Biofilm Attachment/Confocal Microscopy Imaging
3.10. In Vitro CHX-Release Profiles
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Ethical approval & Informed consent
References
- Ghoddusi, J.; Forghani, M.; Parisai, I. New approaches in vital pulp therapy in permanent teeth. Iran. Endod. J. 2013, 9, 15–22. [Google Scholar] [PubMed]
- Zhang, W.; Yelick, P.C. Vital pulp therapy—Current progress of dental pulp regeneration and revascularization. Int. J. Dent. 2010, 2010, 856087. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, L.; Pameijer, C.H.; Emerich, K.; Adamowicz-Klepalska, B. Histological evaluation of mineral trioxide aggregate and calcium hydroxide in direct pulp capping of human immature permanent teeth. Am. J. Dent. 2008, 21, 262–266. [Google Scholar] [PubMed]
- Harandi, A.; Forghani, M.; Ghoddusi, J. Vital pulp therapy with three different pulpotomy agents in immature molars: A case report. Iran. Endod. J. 2013, 8, 145–148. [Google Scholar] [PubMed]
- Hermann, B.W. Kalziumhydroxid als Mittel zum Behandeln und Fullen von Zahnwurzelkanälen. Med. Diss. Univ. Würzburg 1920. (In German) [Google Scholar]
- Mohammadi, Z.; Shalavi, S.; Yazdizadeh, M. Antimicrobial activity of calcium hydroxide in endodontics: A review. Chonnam. Med. J. 2012, 48, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Eidelman, E.; Finn, S.; Koulourides, T. Remineralization of carious dentin treated with calcium hydroxide. J. Dent. Child. 1964, 32, 218–225. [Google Scholar]
- Stanley, H.; Lundy, T. Dycal therapy for pulp exposures. Oral Surg. Oral Med. Oral Pathol. 1972, 34, 818–827. [Google Scholar] [CrossRef]
- Tronstad, L.; Mjör, I.A. Pulp reactions to calcium hydroxide-containing materials. Oral Surg. Oral Med. Oral Pathol. 1972, 33, 961–965. [Google Scholar] [CrossRef]
- Graham, L.; Cooper, P.R.; Cassidy, N.; Nor, J.E.; Sloan, A.J.; Smith, A.J. The effect of calcium hydroxide on solubilisation of bio-active dentine matrix components. Biomaterials 2006, 27, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Accorinte, M.; Loguercio, A.D.; Reis, A.; Carneiro, E.; Grande, R.; Murata, S.; Holland, R. Response of human dental pulp capped with MTA and calcium hydroxide powder. Oper. Dent. 2008, 33, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.; Sübay, R.; Ostro, E.; Suzuki, S.; Suzuki, S. Tunnel defects in dentin bridges: Their formation following direct pulp capping. Oper. Dent. 1995, 21, 4–11. [Google Scholar]
- Pameijer, C.; Norval, G. Pulp Capping with an Experimental Hemostatic Agent and Calcium Hydroxide. J. Dent. Res. 2002, 81, A237. [Google Scholar]
- Boushell, L.W.; Swift, J.; Edward, J. Dentin Bonding: Matrix Metalloproteinases and Chlorhexidine. J. Esthet. Restor. Dent. 2011, 23, 347–352. [Google Scholar] [CrossRef] [PubMed]
- De Magalhaes Silveira, C.F.; Cunha, R.S.; Fontana, C.E.; de Martin, A.S.; de Almeida Gomes, B.P.F.; Motta, R.H.L.; da Silveira Bueno, C.E. Assessment of the antibacterial activity of calcium hydroxide combined with chlorhexidine paste and other intracanal medications against bacterial pathogens. Eur. J. Dent. 2011, 5, 1–7. [Google Scholar]
- Rathke, A.; Meisohle, D.; Bokelmann, J.; Haller, B. Antibacterial activity of calcium hydroxide and chlorhexidine containing points against Fusobacterium nucleatum and Parvimonas micra. Eur. J. Dent. 2012, 6, 434–439. [Google Scholar] [PubMed]
- Mohammadi, Z.; Abbott, P.V. Antimicrobial substantivity of root canal irrigants and medicaments: A review. Aust. Endod. J. 2009, 35, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Haenni, S.; Schmidlin, P.; Mueller, B.; Sener, B.; Zehnder, M. Chemical and antimicrobial properties of calcium hydroxide mixed with irrigating solutions. Int. Endod. J. 2003, 36, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Almyroudi, A.; Mackenzie, D.; McHugh, S.; Saunders, W. The effectiveness of various disinfectants used as endodontic intracanal medications: An in vitro study. J. Endod. 2002, 28, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Basrani, B.; Tjäderhane, L.; Santos, J.M.; Pascon, E.; Grad, H.; Lawrence, H.P.; Friedman, S. Efficacy of chlorhexidine-and calcium hydroxide–containing medicaments against Enterococcus faecalis in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 96, 618–624. [Google Scholar] [CrossRef]
- Taglieri, G.; Mondelli, C.; Daniele, V.; Pusceddu, E.; Trapananti, A. Synthesis and X-ray diffraction analyses of calcium hydroxide nanoparticles in aqueous suspension. Adv. Mater. Phys. Chem. 2013, 3, 108–112. [Google Scholar] [CrossRef]
- Carretti, E.; Chelazzi, D.; Rocchigiani, G.; Baglioni, P.; Poggi, G.; Dei, L. Interactions between nanostructured calcium hydroxide and acrylate copolymers: Implications in cultural heritage conservation. Langmuir 2013, 29, 9881–9890. [Google Scholar] [CrossRef] [PubMed]
- Vitkov, L.; Hannig, M.; Krautgartner, W.; Herrmann, M.; Fuchs, K.; Klappacher, M.; Hermann, A. Ex vivo gingival-biofilm consortia. Lett. Appl. Microbiol. 2005, 41, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Navarro, C.; Suzuki, A.; Ruiz-Agudo, E. Alcohol dispersions of calcium hydroxide nanoparticles for stone conservation. Langmuir 2013, 29, 11457–11470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jia, Z.; Srinivasan, M. Application of direct covalent molecular assembly in the fabrication of polyimide ultrathin films. Langmuir 2005, 21, 3389–3395. [Google Scholar] [CrossRef] [PubMed]
- Villalaín, J.; Gómez-Fernandez, J.C.; Prieto, M.J. Structural information on probe solubilization in micelles by FT—IR spectroscopy. J. Colloid Interface Sci. 1988, 124, 233–237. [Google Scholar] [CrossRef]
- Daniele, V.; Taglieri, G. Synthesis of Ca(OH)2 nanoparticles with the addition of Triton X-100. Protective treatments on natural stones: Preliminary results. J. Cult. Herit. 2012, 13, 40–46. [Google Scholar] [CrossRef]
- Kim, T.; Olek, J. Effects of sample preparation and interpretation of thermogravimetric curves on calcium hydroxide in hydrated pastes and mortars. Transp. Res. Rec. 2012, 2290, 10–18. [Google Scholar] [CrossRef]
- Gooch, J.W.; Johnston, A.W.; Johnston, A.F. Broad Spectrum Antimicrobial Purification Materials and Methods for Purifying Fluids. US Patents US7427409 B2, 11 December 2008. [Google Scholar]
- Stanley, H.R. Pulp capping: Conserving the dental pulp—Can it be done? Is it worth it? Oral Surg. Oral Med. Oral Pathol. 1989, 68, 628–639. [Google Scholar] [CrossRef]
- Stanley, H. Criteria for standardizing and increasing credibility of direct pulp capping studies. Am. J. Dent. 1998, 11, S17–S34. [Google Scholar] [PubMed]
- Qureshi, A.; Soujanya, E.; Nandakumar, P. Recent advances in pulp capping materials: An overview. J. Clin. Diagn. Res. 2014, 8, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Freire, L.G.; Carvalho, C.N.; Ferrari, P.H.P.; Siqueira, E.L.; Gavini, G. Influence of dentin on pH of 2% chlorhexidine gel and calcium hydroxide alone or in combination. Dent. Traumatol. 2010, 26, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Tarquinio, S.; Demarco, F.; Piva, E.; Rivero, E. The influence of haemostatic agents on healing of healthy human dental pulp tissue capped with calcium hydroxide. Int. Endod. J. 2006, 39, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Bal, C.; Alacam, A.; Tuzuner, T.; Tirali, R.E.; Baris, E. Effects of antiseptics on pulpal healing under calcium hydroxide pulp capping: A pilot study. Eur. J. Dent. 2011, 5, 265–272. [Google Scholar] [PubMed]
- Hörsted-Bindslev, P.; Vilkinis, V.; Sidlauskas, A. Direct capping of human pulps with a dentin bonding system or with calcium hydroxide cement. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 96, 591–600. [Google Scholar] [CrossRef]
- Strom, T.; Arora, A.; Osborn, B.; Karim, N.; Komabayashi, T.; Liu, X. Endodontic release system for apexification with calcium hydroxide microspheres. J. Dent. Res. 2012, 91, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Taglieri, G.; Mondelli, C.; Daniele, V.; Pusceddu, E.; Scoccia, G. Synthesis, Textural and structural properties of calcium hydroxide nanoparticles in hydro-alcoholic suspension. Adv. Mater. Phys. Chem. 2014, 4, 50–59. [Google Scholar] [CrossRef]
- Samanta, A.; Chanda, D.K.; Das, P.S.; Ghosh, J.; Mukhopadhyay, A.K.; Dey, A. Synthesis of Nano Calcium Hydroxide in Aqueous Medium. J. Am. Ceram. Soc. 2015, 99, 787–795. [Google Scholar] [CrossRef]
- Doi, K.; Aki, H. Chlorhexidine Gluconate-coNtaining, Stabilized Aqueous Pharmaceutical Preparations. US Patents US5908865 A, 1 June 1999. [Google Scholar]
- Osorio, R.; Osorio, E.; Medina-Castillo, A.L.; Toledano, M. Polymer Nanocarriers for Dentin Adhesion. J. Dent. Res. 2014, 93, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Balagopal, S.; Arjunkumar, R. Chlorhexidine: The gold standard antiplaque agent. J. Pharm. Sci. Res. 2013, 5, 270–274. [Google Scholar]
- Violich, D.; Chandler, N. The smear layer in endodontics—A review. Int. Endod. J. 2010, 43, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Kubinek, R.; Zapletalova, Z.; Vujtek, M.; Novotný, R.; Chmelickova, H. Examination of Dentin Surface Using AFM and SEM; Formatex Reserch Center: Badajoz, Spain, 2007; pp. 593–598. [Google Scholar]
- Schuurs, A.; Gruythuysen, R.; Wesselink, P. Pulp capping with adhesive resin-based composite vs. calcium hydroxide: A review. Dent. Traumatol. 2000, 16, 240–250. [Google Scholar] [CrossRef]
- Signoretti, F.G.C.; de Almeida Gomes, B.P.F.; Montagner, F.; Tosello, F.B.; Jacinto, R.C. Influence of 2% chlorhexidine gel on calcium hydroxide ionic dissociation and its ability of reducing endotoxin. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Trevino, E.G.; Patwardhan, A.N.; Henry, M.A.; Perry, G.; Dybdal-Hargreaves, N.; Hargreaves, K.M.; Diogenes, A. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J. Endod. 2011, 37, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.-M.; Jeon, S.H.; Park, J.-Y.; Chung, J.-H.; Choung, Y.-H.; Choung, P.-H. Dental stem cell therapy with calcium hydroxide in dental pulp capping. Tissue Eng. Part A 2010, 16, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Karaöz, E.; Demircan, P.C.; Sağlam, Ö.; Aksoy, A.; Kaymaz, F.; Duruksu, G. Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochem. Cell Biol. 2011, 136, 455–473. [Google Scholar] [CrossRef] [PubMed]
- Greenhill, J.D.; Pashley, D.H. The effects of desensitizing agents on the hydraulic conductance of human dentin in vitro. J. Dent. Res. 1981, 60, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Duncan, H.; Pitt Ford, T.; Luder, H. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: A randomized controlled trial. Int. Endod. J. 2008, 41, 128–150. [Google Scholar] [PubMed]
- Furey, A.; Hjelmhaug, J.; Lobner, D. Toxicity of Flow Line, Durafill VS, and Dycal to dental pulp cells: Effects of growth factors. J. Endod. 2010, 36, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.; Hassan, R.; Riad, M. Evaluation of the antibacterial efficacy of different bioactive lining and pulp capping agents. Tanta Dent. J. 2015, 12, 132–139. [Google Scholar] [CrossRef]
- Poggio, C.; Beltrami, R.; Colombo, M.; Ceci, M.; Dagna, A.; Chiesa, M. In vitro antibacterial activity of different pulp capping materials. J. Clin. Exp. Dent. 2015, 7, e584–e588. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Lopes, H.P.; de Uzeda, M. Recontamination of coronally unsealed root canals medicated with camphorated paramonochlorophenol or calcium hydroxide pastes after saliva challenge. J. Endod. 1998, 24, 11–14. [Google Scholar] [CrossRef]
- Athanassiadis, B.; Abbott, P.; Walsh, L.J. The use of calcium hydroxide, antibiotics and biocides as antimicrobial medicaments in endodontics. Aust. Dent. J. 2007, 52 (Suppl. 1), S64–S82. [Google Scholar] [CrossRef] [PubMed]
- Kanisavaran, Z.M. Chlorhexidine gluconate in endodontics: An update review. Int. Dent. J. 2008, 58, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Shalavi, S. Is Chlorhexidine an Ideal Vehicle for Calcium Hydroxide? A Microbiologic Review. Iran. Endod. J. 2012, 7, 115–122. [Google Scholar] [PubMed]
- Stevens, R.H.; Grossman, L.I. Evaluation of the antimicrobial potential of calcium hydroxide as an intracanal medicament. J. Endod. 1983, 9, 372–374. [Google Scholar] [CrossRef]
- Koruyucu, M.; Topcuoglu, N.; Tuna, E.B.; Ozel, S.; Gencay, K.; Kulekci, G.; Seymen, F. An assessment of antibacterial activity of three pulp capping materials on Enterococcus faecalis by a direct contact test: An in vitro study. Eur. J. Dent. 2015, 9, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Diffusion in biofilms. J. Bacteriol. 2003, 185, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Eliades, G.; Palaghias, G.; Vougiouklakis, G. Effect of acidic conditioners on dentin morphology, molecular composition and collagen conformation in situ. Dent. Mater. 1997, 13, 24–33. [Google Scholar] [CrossRef]
- Cerda-Cristerna, B.I.; Breceda-Leija, A.; Méndez-González, V.; Chavarría-Bolaños, D.; Flores-Reyes, H.; Garrocho-Rangel, A.; Komabayashi, T.; Wadajkar, A.S.; Pozos-Guillén, A.J. Sustained release of calcium hydroxide from poly (dl-lactide-co-glycolide) acid microspheres for apexification. Odontology 2015, 104, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Yagui, C.O.; Pessoa, A., Jr.; Tavares, L.C. Micellar solubilization of drugs. J. Pharm. Pharm. Sci. 2005, 8, 147–163. [Google Scholar] [PubMed]
- Kim, J.; Uchiyama, T.; Carrilho, M.; Agee, K.A.; Mazzoni, A.; Breschi, L.; Carvalho, R.M.; Tjaderhane, L.; Looney, S.; Wimmer, C.; et al. Chlorhexidine binding to mineralized versus demineralized dentin powder. Dent. Mater. 2010, 26, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, A.; Hadakar, G.S.; Raju, O. Comparative evaluation of pH and antibacterial effect of various calcium hydroxide combinations on E. faecalis and its effect on root strength: An in vitro study. Contemp. Clin. Dent. 2012, 3, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Njeh, A.; Uzunoğlu, E.; Ardila-Osorio, H.; Simon, S.; Berdal, A.; Kellermann, O.; Goldberg, M. Reactionary and reparative dentin formation after pulp capping: Hydrogel vs. Dycal. Evid. Based Endod. 2016, 1, 1–9. [Google Scholar] [CrossRef]
- Estrela, C.; Holland, R. Calcium hydroxide: Study based on scientific evidences. J. Appl. Oral Sci. 2003, 11, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Carrilho, M.R.; Carvalho, R.M.; Sousa, E.N.; Nicolau, J.; Breschi, L.; Mazzoni, A.; Tjäderhane, L.; Tay, F.R.; Agee, K.; Pashley, D.H. Substantivity of chlorhexidine to human dentin. Dent. Mater. 2010, 26, 779–785. [Google Scholar] [CrossRef] [PubMed]









| Formulations | z-Average Diameter (μm) | Zeta Potential (ζ) (mV) | Polydispersity Index (PDI) | Encapsulation Efficiency EE (%) | Drug Loading (DL) (%) | Microparticle Recovery (%) |
|---|---|---|---|---|---|---|
| Ca(OH)2/Blank | 5.3 ± 0.2 A | 2.19 ± 0.4 A | 0.789 ± 0.038 A | - | - | 40.26 ± 2.6 A |
| CHX:Ca(OH)2/25 mg | 1.8 ± 0.5 B | 23.52 ± 4.5 B | 0.347 ± 0.010 B | 39.16 ± 1.6 A | 8.80 ± 6.1 A | 38.05 ± 3.5 A |
| CHX:Ca(OH)2/50 mg | 1.4 ± 0.3 B | 35.97 ± 8.6 C | 0.319 ± 0.093 B | 62.34 ± 2.4 B | 20.53 ± 3.4 B | 30.78 ± 1.9 B |
| Bacterial Strains Used | Diameters of Inhibition Zones (cm)* Measured at: | ||||||
|---|---|---|---|---|---|---|---|
| Ca(OH)2/Blank | CHX:Ca(OH)2/25 mg | CHX:Ca(OH)2/50 mg | Commercial Ca(OH)2 Powder | Dycal | |||
| MP | CHX +ve Control | MP | CHX +ve Control | ||||
| S. mutans | 0.72±0.05 A | 1.2±.0.37 B | 2.3±0.52 C | 1.8±0.28 D | 2.5±0.46 C | 0.62±0.18 AD | 0.48±0.15 D |
| E. faecalis | 0.49±0.13 A | 0.95±.0.24 B | 1.4±0.32 C | 1.1±0.31 B | 2.1±0.37 D | 0.46±0.17 A | 0.34±0.09 A |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priyadarshini, B.M.; Selvan, S.T.; Narayanan, K.; Fawzy, A.S. Characterization of Chlorhexidine-Loaded Calcium-Hydroxide Microparticles as a Potential Dental Pulp-Capping Material. Bioengineering 2017, 4, 59. https://doi.org/10.3390/bioengineering4030059
Priyadarshini BM, Selvan ST, Narayanan K, Fawzy AS. Characterization of Chlorhexidine-Loaded Calcium-Hydroxide Microparticles as a Potential Dental Pulp-Capping Material. Bioengineering. 2017; 4(3):59. https://doi.org/10.3390/bioengineering4030059
Chicago/Turabian StylePriyadarshini, Balasankar M., Subramanian T. Selvan, Karthikeyan Narayanan, and Amr S. Fawzy. 2017. "Characterization of Chlorhexidine-Loaded Calcium-Hydroxide Microparticles as a Potential Dental Pulp-Capping Material" Bioengineering 4, no. 3: 59. https://doi.org/10.3390/bioengineering4030059
APA StylePriyadarshini, B. M., Selvan, S. T., Narayanan, K., & Fawzy, A. S. (2017). Characterization of Chlorhexidine-Loaded Calcium-Hydroxide Microparticles as a Potential Dental Pulp-Capping Material. Bioengineering, 4(3), 59. https://doi.org/10.3390/bioengineering4030059