Microdevice Platform for In Vitro Nervous System and Its Disease Model
Abstract
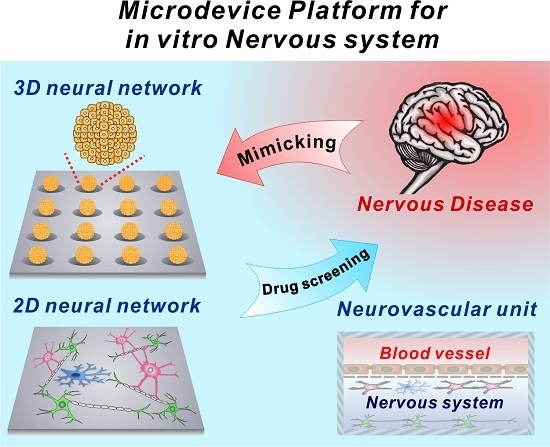
:1. Introduction
2. In Vivo Mimicking Central Nervous System Model on a Microdevice
2.1. Two-Dimensional Neural Network on a Microdevice
2.2. Three-Dimensional Neural Network on a Microdevice
2.3. In Vitro Neurovascular Unit Models in the Microfluidic Device
3. Neuronal Disease Models on the Nervous System-On-A-Chip
3.1. Neurodegenerative Disease Models on the Nervous System-On-A-Chip
3.2. Neuroinflammation Models on the Nervous System-On-A-Chip
3.3. Metastatic Brain Tumor Model on the Nervous System-On-A-Chip
4. Future Directions
4.1. Electrochemical Detection of Neurotransmitter
4.2. Multielectrode Array Based Electrical Signal Detection
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Martin, J.B. Molecular basis of the neurodegenerative disorders. N. Engl. J. Med. 1999, 340, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Laurie, C.; Mosley, R.L.; Gendelman, H.E. Oxidative stress and the pathogenesis of neurodegenerative disorders. Int. Rev. Neurobiol. 2007, 82, 297–325. [Google Scholar] [PubMed]
- Association, A.S. 2016 alzheimer’s disease facts and figures. Alzheimer Dement. 2016, 12, 459–509. [Google Scholar]
- Hanger, D.P.; Anderton, B.H.; Noble, W. Tau phosphorylation: The therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 2009, 15, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, D.M.; Morris, J.C.; Goate, A.M. Alzheimer’s disease: The challenge of the second century. Sci Transl. Med. 2011, 3, 77sr1. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Wolburg, H.; Lippoldt, A. Tight junctions of the blood-brain barrier: Development, composition and regulation. Vasc. Pharmacol. 2002, 38, 323–337. [Google Scholar] [CrossRef]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood-brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.L.; Staddon, J.M. The cell biology of the blood-brain barrier. Annu. Rev. Neurosci. 1999, 22, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.P.; Hardy, J.; Fischbeck, K.H. Toxic proteins in neurodegenerative disease. Science 2002, 296, 1991–1995. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, T.R.; Carvalho, A.C.; Jurkiewicz, A.; Frussa-Filho, R.; Smaili, S.S. Mitochondrial calcium, oxidative stress and apoptosis in a neurodegenerative disease model induced by 3-nitropropionic acid. J. Neurochem. 2004, 88, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Sherer, T.B.; Betarbet, R.; Stout, A.K.; Lund, S.; Baptista, M.; Panov, A.V.; Cookson, M.R.; Greenamyre, J.T. An in vitro model of parkinson’s disease: Linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J. Neurosci. 2002, 22, 7006–7015. [Google Scholar] [PubMed]
- Mestas, J.; Hughes, C.C. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Pamies, D.; Hartung, T.; Hogberg, H.T. Biological and medical applications of a brain-on-a-chip. Exp. Biol. Med. 2014, 239, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Haring, A.P.; Sontheimer, H.; Johnson, B.N. Microphysiological human brain and neural systems-on-a-chip: Potential alternatives to small animal models and emerging platforms for drug discovery and personalized medicine. Stem Cell. Rev. 2017, 13, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Reverse engineering human pathophysiology with organs-on-chips. Cell 2016, 164, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Bhise, N.S.; Ribas, J.; Manoharan, V.; Zhang, Y.S.; Polini, A.; Massa, S.; Dokmeci, M.R.; Khademhosseini, A. Organ-on-a-chip platforms for studying drug delivery systems. J. Control. Release 2014, 190, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Polini, A.; Prodanov, L.; Bhise, N.S.; Manoharan, V.; Dokmeci, M.R.; Khademhosseini, A. Organs-on-a-chip: A new tool for drug discovery. Expert Opin. Drug Discov. 2014, 9, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Caplin, J.D.; Granados, N.G.; James, M.R.; Montazami, R.; Hashemi, N. Microfluidic organ-on-a-chip technology for advancement of drug development and toxicology. Adv. Healthc. Mater. 2015, 4, 1426–1450. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Lee, J.; Shin, W.; Choi, J.W.; Kim, H.J. Priming nanoparticle-guided diagnostics and therapeutics towards human organs-on-chips microphysiological system. Nano Converg. 2016, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Biederer, T.; Sara, Y.; Mozhayeva, M.; Atasoy, D.; Liu, X.R.; Kavalali, E.T.; Sudhof, T.C. Syncam, a synaptic adhesion molecule that drives synapse assembly. Science 2002, 297, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Biederer, T.; Scheiffele, P. Mixed-culture assays for analyzing neuronal synapse formation. Nat. Protoc. 2007, 2, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Charles, P.; Tait, S.; Faivre-Sarrailh, C.; Barbin, G.; Gunn-Moore, F.; Denisenko-Nehrbass, N.; Guennoc, A.M.; Girault, J.A.; Brophy, P.J.; Lubetzki, C. Neurofascin is a glial receptor for the paranodin/caspr-contactin axonal complex at the axoglial junction. Curr. Biol. 2002, 12, 217–220. [Google Scholar] [CrossRef]
- Park, J.; Koito, H.; Li, J.; Han, A. Multi-compartment neuron-glia co-culture platform for localized cns axon-glia interaction study. Lab Chip 2012, 12, 3296–3304. [Google Scholar] [CrossRef] [PubMed]
- Brewer, B.M.; Shi, M.; Edd, J.F.; Webb, D.J.; Li, D. A microfluidic cell co-culture platform with a liquid fluorocarbon separator. Biomed. Microdevices 2014, 16, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Odawara, A.; Gotoh, M.; Suzuki, I. Control of neural network patterning using collagen gel photothermal etching. Lab Chip 2013, 13, 2040–2046. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, Z.; Huang, J.; Lin, X.; Luo, R.; Chen, C.H.; Shi, P. Neuroarray: A universal interface for patterning and interrogating neural circuitry with single cell resolution. Sci. Rep. 2014, 4, 4784. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Majumdar, D.; Gao, Y.; Brewer, B.M.; Goodwin, C.R.; McLean, J.A.; Li, D.; Webb, D.J. Glia co-culture with neurons in microfluidic platforms promotes the formation and stabilization of synaptic contacts. Lab Chip 2013, 13, 3008–3021. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, Y.; Owen, S.C.; Shoichet, M.S. Polymers used to influence cell fate in 3d geometry: New trends. Prog. Polym. Sci. 2012, 37, 645–658. [Google Scholar] [CrossRef]
- Li, R.H.; Altreuter, D.H.; Gentile, F.T. Transport characterization of hydrogel matrices for cell encapsulation. Biotechnol. Bioeng. 1996, 50, 365–373. [Google Scholar] [CrossRef]
- Xu, T.; Molnar, P.; Gregory, C.; Das, M.; Boland, T.; Hickman, J.J. Electrophysiological characterization of embryonic hippocampal neurons cultured in a 3d collagen hydrogel. Biomaterials 2009, 30, 4377–4383. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.H.; Lee, J.M.; Chung, B.G. Hydrogel-encapsulated 3d microwell array for neuronal differentiation. Biomed. Mater. 2016, 11, 015019. [Google Scholar] [CrossRef] [PubMed]
- Kunze, A.; Giugliano, M.; Valero, A.; Renaud, P. Micropatterning neural cell cultures in 3d with a multi-layered scaffold. Biomaterials 2011, 32, 2088–2098. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Im, S.K.; Oh, S.J.; Jeong, S.; Yoon, E.S.; Lee, C.J.; Choi, N.; Hur, E.M. Anisotropically organized three-dimensional culture platform for reconstruction of a hippocampal neural network. Nat. Commun. 2017, 8, 14346. [Google Scholar] [CrossRef] [PubMed]
- Chwalek, K.; Tang-Schomer, M.D.; Omenetto, F.G.; Kaplan, D.L. In vitro bioengineered model of cortical brain tissue. Nat. Protoc. 2015, 10, 1362–1373. [Google Scholar] [CrossRef] [PubMed]
- Tang-Schomer, M.D.; White, J.D.; Tien, L.W.; Schmitt, L.I.; Valentin, T.M.; Graziano, D.J.; Hopkins, A.M.; Omenetto, F.G.; Haydon, P.G.; Kaplan, D.L. Bioengineered functional brain-like cortical tissue. Proc. Natl. Acad. Sci. USA 2014, 111, 13811–13816. [Google Scholar] [CrossRef] [PubMed]
- Booth, R.; Kim, H. Characterization of a microfluidic in vitro model of the blood-brain barrier (mubbb). Lab Chip 2012, 12, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, Y.; Obermeier, B.; Cotleur, A.; Sano, Y.; Kanda, T.; Ransohoff, R.M. An in vitro blood-brain barrier model combining shear stress and endothelial cell/astrocyte co-culture. J. Neurosci. Methods 2014, 232, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.D.; Khafagy, E.-S.; Khanafer, K.; Takayama, S.; ElSayed, M.E. Organization of endothelial cells, pericytes, and astrocytes into a 3d microfluidic in vitro model of the blood–brain barrier. Mol. Pharm. 2016, 13, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Pensabene, V.; Markov, D.A.; Allwardt, V.; Neely, M.D.; Shi, M.; Britt, C.M.; Hoilett, O.S.; Yang, Q.; Brewer, B.M.; et al. Recreating blood-brain barrier physiology and structure on chip: A novel neurovascular microfluidic bioreactor. Biomicrofluidics 2015, 9, 054124. [Google Scholar] [CrossRef] [PubMed]
- Achyuta, A.K.; Conway, A.J.; Crouse, R.B.; Bannister, E.C.; Lee, R.N.; Katnik, C.P.; Behensky, A.A.; Cuevas, J.; Sundaram, S.S. A modular approach to create a neurovascular unit-on-a-chip. Lab Chip 2013, 13, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Blaauwgeers, H.G.; Holtkamp, G.M.; Rutten, H.; Witmer, A.N.; Koolwijk, P.; Partanen, T.A.; Alitalo, K.; Kroon, M.E.; Kijlstra, A.; van Hinsbergh, V.W.; et al. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am. J. Pathol. 1999, 155, 421–428. [Google Scholar] [CrossRef]
- Abbott, N.J.; Ronnback, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Hinderer, S.; Layland, S.L.; Schenke-Layland, K. Ecm and ecm-like materials - biomaterials for applications in regenerative medicine and cancer therapy. Adv. Drug Deliv. Rev. 2016, 97, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Jarjour, A.A.; Zhang, H.; Bauer, N.; Ffrench-Constant, C.; Williams, A. In vitro modeling of central nervous system myelination and remyelination. Glia 2012, 60, 1–12. [Google Scholar] [CrossRef] [PubMed]
- El-Ali, J.; Sorger, P.K.; Jensen, K.F. Cells on chips. Nature 2006, 442, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Kida, Y.S. In vitro reconstruction of neuronal networks derived from human ips cells using microfabricated devices. PLoS ONE 2016, 11, e0148559. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pevny, L.; Lovell-Badge, R.; Smith, A. Generation of purified neural precursors from embryonic stem cells by lineage selection. Curr. Biol. 1998, 8, 971–974. [Google Scholar] [CrossRef]
- Babu, H.; Ramirez-Rodriguez, G.; Fabel, K.; Bischofberger, J.; Kempermann, G. Synaptic network activity induces neuronal differentiation of adult hippocampal precursor cells through bdnf signaling. Front. Neurosci. 2009, 3, 49. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Keller, G. Differentiation of embryonic stem cells to clinically relevant populations: Lessons from embryonic development. Cell 2008, 132, 661–680. [Google Scholar] [CrossRef] [PubMed]
- Singec, I.; Knoth, R.; Meyer, R.P.; Maciaczyk, J.; Volk, B.; Nikkhah, G.; Frotscher, M.; Snyder, E.Y. Defining the actual sensitivity and specificity of the neurosphere assay in stem cell biology. Nat. Methods 2006, 3, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.H.; Martin, J.; Elia, J.; Flippin, J.; Paramban, R.I.; Hefferan, M.P.; Vidal, J.G.; Mu, Y.; Killian, R.L.; Israel, M.A.; et al. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS ONE 2011, 6, e17540. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Park, J.; Lee, S.H. Size-controllable networked neurospheres as a 3d neuronal tissue model for alzheimer’s disease studies. Biomaterials 2013, 34, 2938–2946. [Google Scholar] [CrossRef] [PubMed]
- Kato-Negishi, M.; Morimoto, Y.; Onoe, H.; Takeuchi, S. Millimeter-sized neural building blocks for 3d heterogeneous neural network assembly. Adv. Healthc. Mater. 2013, 2, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Valadez, A.V.; Zuo, P.; Nie, Z. Microfluidic 3d cell culture: Potential application for tissue-based bioassays. Bioanalysis 2012, 4, 1509–1525. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.F.; Zhang, Y.; Ho, Y.P.; Chiu, Y.L.; Jung, Y.; Leong, K.W. Rapid formation of multicellular spheroids in double-emulsion droplets with controllable microenvironment. Sci. Rep. 2013, 3, 3462. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Chung, B.G.; Lee, D.H.; Khademhosseini, A.; Kim, J.H.; Lee, S.H. Controlled-size embryoid body formation in concave microwell arrays. Biomaterials 2010, 31, 4296–4303. [Google Scholar] [CrossRef] [PubMed]
- DingleYu-Ting, L.; BoutinMolly, E.; ChirilaAnda, M.; LiviLiane, L.; LabriolaNicholas, R.; JakubekLorin, M.; MorganJeffrey, R.; DarlingEric, M.; KauerJulie, A. Three-dimensional neural spheroid culture: An in vitro model for cortical studies. Tissue Eng. Part C Methods 2015, 21, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, B.K.; Jeong, G.S.; Hyun, J.K.; Lee, C.J.; Lee, S.H. Three-dimensional brain-on-a-chip with an interstitial level of flow and its application as an in vitro model of alzheimer’s disease. Lab Chip 2015, 15, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Velazquez Sanchez, C.; Chen, M.; Morin, P.J.; Wells, J.M.; Hanlon, E.B.; Xia, W. Three dimensional human neuro-spheroid model of alzheimer’s disease based on differentiated induced pluripotent stem cells. PLoS ONE 2016, 11, e0163072. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Friess, W.; Schlapp, M. Sterilization of gentamicin containing collagen/plga microparticle composites. Eur. J. Pharm. Biopharm. 2006, 63, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Peter, S.J.; Lyman, M.D.; Lai, H.L.; Leite, S.M.; Tamada, J.A.; Uyama, S.; Vacanti, J.P.; Langer, R.; Mikos, A.G. In vitro and in vivo degradation of porous poly(dl-lactic-co-glycolic acid) foams. Biomaterials 2000, 21, 1837–1845. [Google Scholar] [CrossRef]
- Oh, S.H.; Kang, S.G.; Kim, E.S.; Cho, S.H.; Lee, J.H. Fabrication and characterization of hydrophilic poly(lactic-co-glycolic acid)/poly(vinyl alcohol) blend cell scaffolds by melt-molding particulate-leaching method. Biomaterials 2003, 24, 4011–4021. [Google Scholar] [CrossRef]
- Rowlands, A.S.; Lim, S.A.; Martin, D.; Cooper-White, J.J. Polyurethane/poly(lactic-co-glycolic) acid composite scaffolds fabricated by thermally induced phase separation. Biomaterials 2007, 28, 2109–2121. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Feng, X.; Mao, T.; Feng, X.; Ouyang, H.W.; Zhao, G.; Chen, F. Engineering of human tracheal tissue with collagen-enforced poly-lactic-glycolic acid non-woven mesh: A preliminary study in nude mice. Br. J. Oral. Maxillofac. Surg. 2007, 45, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Stevens, L.; Thompson, B.C.; Gilmore, K.J.; Gorkin, R., 3rd; Stewart, E.M.; Panhuis, M.; Romero-Ortega, M.; Wallace, G.G. 3d printing of layered brain-like structures using peptide modified gellan gum substrates. Biomaterials 2015, 67, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Blood vessels and nerves: Common signals, pathways and diseases. Nat. Rev. Genet. 2003, 4, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Carmignoto, G.; Gomez-Gonzalo, M. The contribution of astrocyte signalling to neurovascular coupling. Brain Res. Rev. 2010, 63, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Filosa, J.A.; Blanco, V.M. Neurovascular coupling in the mammalian brain. Exp. Physiol. 2007, 92, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Villringer, A.; Dirnagl, U. Coupling of brain activity and cerebral blood flow: Basis of functional neuroimaging. Cerebrovasc. Brain Metab. Rev. 1994, 7, 240–276. [Google Scholar]
- Shaw, T.G.; Mortel, K.F.; Meyer, J.S.; Rogers, R.L.; Hardenberg, J.; Cutaia, M.M. Cerebral blood flow changes in benign aging and cerebrovascular disease. Neurology 1984, 34, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.C.; Edvinsson, L.; MacKenzie, E.T. The concept of coupling blood flow to brain function: Revision required? Ann. Neurol. 1987, 22, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Audus, K.L.; Borchardt, R.T. Characterization of an in vitro blood-brain barrier model system for studying drug transport and metabolism. Pharm. Res. 1986, 3, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.L.; Hall, D.E.; Porter, S.; Barbu, K.; Cannon, C.; Horner, H.C.; Janatpour, M.; Liaw, C.W.; Manning, K.; Morales, J.; et al. A cell culture model of the blood-brain barrier. J. Cell Biol. 1991, 115, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Cecchelli, R.; Dehouck, B.; Descamps, L.; Fenart, L.; Buee-Scherrer, V.V.; Duhem, C.; Lundquist, S.; Rentfel, M.; Torpier, G.; Dehouck, M.P. In vitro model for evaluating drug transport across the blood-brain barrier. Adv. Drug Deliv. Rev. 1999, 36, 165–178. [Google Scholar] [CrossRef]
- Wilhelm, I.; Krizbai, I.A. In vitro models of the blood-brain barrier for the study of drug delivery to the brain. Mol. Pharm. 2014, 11, 1949–1963. [Google Scholar] [CrossRef] [PubMed]
- Hatherell, K.; Couraud, P.-O.; Romero, I.A.; Weksler, B.; Pilkington, G.J. Development of a three-dimensional, all-human in vitro model of the blood–brain barrier using mono-, co-, and tri-cultivation transwell models. J. Neurosci. Methods 2011, 199, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Deli, M.A.; Nakao, S.; Honda, M.; Hayashi, K.; Nakaoke, R.; Kataoka, Y.; Niwa, M. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cell. Mol. Neurobiol. 2007, 27, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Deli, M.A.; Kawaguchi, H.; Shimizudani, T.; Shimono, T.; Kittel, A.; Tanaka, K.; Niwa, M. A new blood–brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem. Int. 2009, 54, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Takata, F.; Dohgu, S.; Yamauchi, A.; Matsumoto, J.; Machida, T.; Fujishita, K.; Shibata, K.; Shinozaki, Y.; Sato, K.; Kataoka, Y. In vitro blood-brain barrier models using brain capillary endothelial cells isolated from neonatal and adult rats retain age-related barrier properties. PLoS ONE 2013, 8, e55166. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, K.; Tanaka, K.; Nakagawa, S.; Thuy, D.H.D.; Ujifuku, K.; Kamada, K.; Hayashi, K.; Matsuo, T.; Nagata, I.; Niwa, M. Initial contact of glioblastoma cells with existing normal brain endothelial cells strengthen the barrier function via fibroblast growth factor 2 secretion: A new in vitro blood–brain barrier model. Cell. Mol. Neurobiol. 2013, 33, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Yeon, J.H.; Na, D.; Choi, K.; Ryu, S.W.; Choi, C.; Park, J.K. Reliable permeability assay system in a microfluidic device mimicking cerebral vasculatures. Biomed. Microdevices 2012, 14, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Prabhakarpandian, B.; Shen, M.C.; Nichols, J.B.; Mills, I.R.; Sidoryk-Wegrzynowicz, M.; Aschner, M.; Pant, K. Sym-bbb: A microfluidic blood brain barrier model. Lab Chip 2013, 13, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Kim, H.N.; Im, S.K.; Chung, S.; Kang, J.Y.; Choi, N. Collagen-based brain microvasculature model in vitro using three-dimensional printed template. Biomicrofluidics 2015, 9, 024115. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Toepke, M.W.; Beebe, D.J. Pdms absorption of small molecules and consequences in microfluidic applications. Lab Chip 2006, 6, 1484–1486. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Blurton-Jones, M.; Rhee, S.W.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. A microfluidic culture platform for cns axonal injury, regeneration and transport. Nat. Methods 2005, 2, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Hellman, A.N.; Vahidi, B.; Kim, H.J.; Mismar, W.; Steward, O.; Jeon, N.L.; Venugopalan, V. Examination of axonal injury and regeneration in micropatterned neuronal culture using pulsed laser microbeam dissection. Lab Chip 2010, 10, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Dollé, J.-P.; Morrison III, B.; Schloss, R.S.; Yarmush, M.L. Brain-on-a-chip microsystem for investigating traumatic brain injury: Axon diameter and mitochondrial membrane changes play a significant role in axonal response to strain injuries. Technology 2014, 2, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Hashimoto, T.; Wong, E.; Hori, Y.; Wood, L.B.; Zhao, L.; Haigis, K.M.; Hyman, B.T.; Irimia, D. Microfluidic chemotaxis platform for differentiating the roles of soluble and bound amyloid-beta on microglial accumulation. Sci. Rep. 2013, 3, 1823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, H.; Seo, J.H.; Wong, K.H.; Terasaki, Y.; Park, J.; Bong, K.; Arai, K.; Lo, E.H.; Irimia, D. Three-dimensional blood-brain barrier model for in vitro studies of neurovascular pathology. Sci. Rep. 2015, 5, 15222. [Google Scholar] [CrossRef] [PubMed]
- Herland, A.; van der Meer, A.D.; FitzGerald, E.A.; Park, T.E.; Sleeboom, J.J.; Ingber, D.E. Distinct contributions of astrocytes and pericytes to neuroinflammation identified in a 3d human blood-brain barrier on a chip. PLoS ONE 2016, 11, e0150360. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Codreanu, S.G.; Shi, M.; Sherrod, S.D.; Markov, D.A.; Neely, M.D.; Britt, C.M.; Hoilett, O.S.; Reiserer, R.S.; Samson, P.C.; et al. Metabolic consequences of inflammatory disruption of the blood-brain barrier in an organ-on-chip model of the human neurovascular unit. J. Neuroinflamm. 2016, 13, 306. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tong, X.; Yang, F. Bioengineered 3d brain tumor model to elucidate the effects of matrix stiffness on glioblastoma cell behavior using peg-based hydrogels. Mol. Pharm. 2014, 11, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Ananthanarayanan, B.; Kim, Y.; Kumar, S. Elucidating the mechanobiology of malignant brain tumors using a brain matrix-mimetic hyaluronic acid hydrogel platform. Biomaterials 2011, 32, 7913–7923. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.J.; Wilson, M.; Ward, J.H.; Rahman, C.V.; Peet, A.C.; Macarthur, D.C.; Rose, F.R.; Grundy, R.G.; Rahman, R. Recapitulation of tumor heterogeneity and molecular signatures in a 3d brain cancer model with decreased sensitivity to histone deacetylase inhibition. PLoS ONE 2012, 7, e52335. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.H.; Gary, D.; Malone, M.; Dria, S.; Houdayer, T.; Belegu, V.; McDonald, J.W.; Thakor, N. Axon myelination and electrical stimulation in a microfluidic, compartmentalized cell culture platform. Neuromol. Med. 2012, 14, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Koito, H.; Li, J.; Han, A. Microfluidic compartmentalized co-culture platform for cns axon myelination research. Biomed. Microdevices 2009, 11, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.U.; Blasiak, A.; Agrawal, D.R.; Loong, D.T.B.; Thakor, N.V.; All, A.H.; Ho, J.S.; Yang, I.H. Subcellular electrical stimulation of neurons enhances the myelination of axons by oligodendrocytes. PLoS ONE 2017, 12, e0179642. [Google Scholar] [CrossRef] [PubMed]
- Floyd, R.A. Neuroinflammatory processes are important in neurodegenerative diseases: An hypothesis to explain the increased formation of reactive oxygen and nitrogen species as major factors involved in neurodegenerative disease development. Free Radic. Biol. Med. 1999, 26, 1346–1355. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014, 14, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Neman, J.; Termini, J.; Wilczynski, S.; Vaidehi, N.; Choy, C.; Kowolik, C.M.; Li, H.; Hambrecht, A.C.; Roberts, E.; Jandial, R. Human breast cancer metastases to the brain display gabaergic properties in the neural niche. Proc. Natl. Acad. Sci. USA 2014, 111, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Bottaci, L.; Drew, P.J.; Hartley, J.E.; Hadfield, M.B.; Farouk, R.; Lee, P.W.; Macintyre, I.M.; Duthie, G.S.; Monson, J.R. Artificial neural networks applied to outcome prediction for colorectal cancer patients in separate institutions. Lancet 1997, 350, 469–472. [Google Scholar] [CrossRef]
- Forster, J.I.; Koglsberger, S.; Trefois, C.; Boyd, O.; Baumuratov, A.S.; Buck, L.; Balling, R.; Antony, P.M. Characterization of differentiated sh-sy5y as neuronal screening model reveals increased oxidative vulnerability. J. Biomol. Screen 2016, 21, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Encinas, M.; Iglesias, M.; Liu, Y.; Wang, H.; Muhaisen, A.; Cena, V.; Gallego, C.; Comella, J.X. Sequential treatment of sh-sy5y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J. Neurochem. 2000, 75, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Georges, P.C.; Miller, W.J.; Meaney, D.F.; Sawyer, E.S.; Janmey, P.A. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys. J. 2006, 90, 3012–3018. [Google Scholar] [CrossRef] [PubMed]
- De Camilli, P.; Cameron, R.; Greengard, P. Synapsin i (protein i), a nerve terminal-specific phosphoprotein. I. Its general distribution in synapses of the central and peripheral nervous system demonstrated by immunofluorescence in frozen and plastic sections. J. Cell Biol. 1983, 96, 1337–1354. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Koovakkattu, D.; Kesler, S.R. Altered small-world properties of gray matter networks in breast cancer. BMC Neurol. 2012, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, T.; Choi, J.W. Nano-biosensor for monitoring the neural differentiation of stem cells. Nanomaterials 2016, 6, 224. [Google Scholar] [CrossRef] [PubMed]
- Kafi, M.A.; Cho, H.Y.; Choi, J.W. Engineered peptide-based nanobiomaterials for electrochemical cell chip. Nano Converg. 2016, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Yea, C.H.; Chueng, S.T.; Yin, P.T.; Conley, B.; Dardir, K.; Pak, Y.; Jung, G.Y.; Choi, J.W.; Lee, K.B. Large-scale nanoelectrode arrays to monitor the dopaminergic differentiation of human neural stem cells. Adv. Mater. 2015, 27, 6356–6362. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Cho, H.Y.; Kim, S.K.; Jang, H.D.; Choi, J.W. Au-crumpled graphene modified electrode to detect neurotransmitters based on spectroelectrochemical method. Sci. Adv. Mater. 2014, 6, 2577–2581. [Google Scholar] [CrossRef]
- Cho, H.Y.; Eun Bi, K.; Kim, T.H.; Choi, J.W. Fabrication of carbon nanotubes/rgd peptide composites to enhance electrochemical performance of cell chip. J. Biomed. Nanotechnol. 2013, 9, 1398–1402. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, M.; Miyazaki, I.; Ogawa, N. Dopamine- or l-dopa-induced neurotoxicity: The role of dopamine quinone formation and tyrosinase in a model of parkinson’s disease. Neurotox. Res. 2003, 5, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Beninger, R.J. The role of dopamine in locomotor activity and learning. Brain Res. 1983, 287, 173–196. [Google Scholar] [CrossRef]
- Ikemoto, S.; Panksepp, J. The role of nucleus accumbens dopamine in motivated behavior: A unifying interpretation with special reference to reward-seeking. Brain Res. Rev. 1999, 31, 6–41. [Google Scholar] [CrossRef]
- Cho, H.Y.; Kim, T.H.; Kim, S.U.; Choi, J.W. Fabrication of stem cell chip with peptide nanopatterned layer to detect cytotoxicity of environmental toxicants. J. Nanosci. Nanotechnol. 2012, 12, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, K.B.; Choi, J.W. 3d graphene oxide-encapsulated gold nanoparticles to detect neural stem cell differentiation. Biomaterials 2013, 34, 8660–8670. [Google Scholar] [CrossRef] [PubMed]
- Musick, K.; Khatami, D.; Wheeler, B.C. Three-dimensional micro-electrode array for recording dissociated neuronal cultures. Lab Chip 2009, 9, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Dworak, B.J.; Wheeler, B.C. Novel mea platform with pdms microtunnels enables the detection of action potential propagation from isolated axons in culture. Lab Chip 2009, 9, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Enright, H.A.; Felix, S.H.; Fischer, N.O.; Mukerjee, E.V.; Soscia, D.; McNerney, M.; Kulp, K.; Zhang, J.; Page, G.; Miller, P.; et al. Long-term non-invasive interrogation of human dorsal root ganglion neuronal cultures on an integrated microfluidic multielectrode array platform. Analyst 2016, 141, 5346–5357. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pan, L.; Cheng, X.; Berdichevsky, Y. Perfused drop microfluidic device for brain slice culture-based drug discovery. Biomed. Microdevices 2016, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Odawara, A.; Saitoh, Y.; Alhebshi, A.H.; Gotoh, M.; Suzuki, I. Long-term electrophysiological activity and pharmacological response of a human induced pluripotent stem cell-derived neuron and astrocyte co-culture. Biochem. Biophys. Res. Commun. 2014, 443, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Rowe, L.; Almasri, M.; Lee, K.; Fogleman, N.; Brewer, G.J.; Nam, Y.; Wheeler, B.C.; Vukasinovic, J.; Glezer, A.; Frazier, A.B. Active 3-d microscaffold system with fluid perfusion for culturing in vitro neuronal networks. Lab Chip 2007, 7, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Abbott, J.; Ye, T.; Qin, L.; Jorgolli, M.; Gertner, R.S.; Ham, D.; Park, H. Cmos nanoelectrode array for all-electrical intracellular electrophysiological imaging. Nat. Nanotechnol. 2017, 12, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, H.; Himmerlich, M.; Fischer, M.; Demko, L.; Hyttinen, J.; Schober, A. Ltcc-based multi-electrode arrays for 3d in vitro cell cultures. J. Ceram. Sci. Technol. 2015, 6, 315–324. [Google Scholar]






| Condition | Advantage | Limitation | Function | Ref. | ||
|---|---|---|---|---|---|---|
| 2D neural network | Tissue culture plate-based co-culture | Simple structure Easy to use | Randomly established connections with other types of neural cells Overlapping cellular responses | Monitoring cell–cell interaction | [23,24,25] | |
| Horizontally-aligned neural network | Axonal growth direction control Compartmented structure for spatial drug treatment | Not applicable for the deep tissue drug diffusion | Monitoring neuron–glial neuron–neuron interaction | [26,27,28,29] | ||
| Vertically-aligned neural network | In situ collection of cytokines | No physical interaction between different cell layer Long chip preparation time Not able to treat chemical on specific layer of cell Not applicable for the deep tissue drug diffusion | Secreted cytokine based cellular communication | [30] | ||
| 3D neural network models | Hydrogel-based 3D neural network | Individual cell | Monodispersed neural network | Contraction of hydrogel Different axonal growth with different stiffness of hydrogel | 3D neural signal monitoring | [31,32,33] |
| Spheroid | Novel 3D, spontaneously active networks Brain-approximating characteristics | Contraction of hydrogel Limited spheroid size Not able to compartmental control to study cell-specific features | Monitoring the developmental process of brain in vitro | [34,35] | ||
| Gel-free 3D neural network | Spheroid | Mimicking the interaction between different region of brain | Limited spheroid size Easy to spread the cell after attaching on substrate | Visualization of the spatiotemporal morphological changes of single neurons | [36,37] | |
| Scaffold | Compartmented structure formation Mechanical stability Easy to handle High diffusion rate with porous structure | Making monodispesed cell condition in the scaffold | Mimicking the cerebral cortex Brain homeostasis and injury study | [38,39] | ||
| Neurovascular unit models | Horizontally-aligned neurovascular models | Making tight junction structure Ease to see drug permeability change the trans-endothelial electrical resistance (TEER) within a short time Dividing apical and basolateral space to co-culture the endothelium and astrocyte | The discrepancy with in vivo vascular flow and shear stress Not presenting neuronal cells The difference of the connecting material between the cells with the actual membrane Not inducing polarization of vascular endothelium and its interaction to astrocyte | Testing drug permeability reactive oxide species (ROS) generation by hydrogen peroxidase and confirmation of the change TEER value Enhancement of the efflux by upregulating P-gp. Disruption and recovery of the barrier function | [40,41,42] | |
| Vertically-aligned neurovascular models | Mimicking the actual shear stress The interaction between endothelium and astrocyte and/or pericyte Monitoring the TEER value Insertion of the 3D extracellular matrix (ECM) materials into the chip | Not presenting whole neurovascular unit cells. Absorption of the hydrophobic molecules to the channel and membrane The difference of the connecting material between the cells with the actual membrane | Permeability test using fluorescein isothiocyanate (FITC) conjugated small molecule Enhancement of blood–brain barrier (BBB) integrity compares of Transwell system Change the TEER value for several stimuli such as histamine, glutamine and tumor necrosis factor alpha (TNF-α) | [43,44,45,46,47] | ||
| Disease Model | Advantage | Limitation | Function | Ref. | |
|---|---|---|---|---|---|
| Neurodegenerative disease models | Axonal injury models | Easy to mimic the damaged state to axon Induction of axonal growth direction Facilitates biochemical analyses, such as chemical treatment | Discrepancy of the actual neurodegenerative disease Not presenting neurovasculatures and their interactions Not using the 3D ECM materials | Disconnection and regeneration of the axon using simple methods Myelination of the oligodendrocyte along with axonal growth | [91,92,93] |
| Alzheimer’s disease models | Simple to induce the Alzheimer’s disease (AD) model by applying Aβ Monitoring of the cell viability and physiological alteration by applying Aβ | Not emulating the interaction between neuronal cells and vascular cells during AD progression Short maintainence period when comparing Aβ deposition time | Analysis of neuronal cell viability by applying Aβ Microglia migration assay by applying Aβ Mimicry the interstitial flow in the brain | [62,94] | |
| Neuroinflammation models | Appropriated shear stress to the endothelium with neuronal cells Observation of change the BBB permeability and neuronal viability simultaneously with intercellular interaction | Some missing components, such as pericytes, astrocytes, microglia and monocytes Focusing on the BBB integrity, lack of neuronal function and viability | Change the TEER value of the BBB by neuroinflammation Analyze the detailed mechanism and metabolism of the neuroinflammation | [95,96,97] | |
| Metastatic brain tumor model | Simultaneous observation of interaction between cancer and surrounding neuron | Limited size to tumor growth Imaging of target cell No angiogenesis | Monitoring of metastatic cancer spreading/migration Monitoring of interaction between neural network and cancer cells | [98,99,100] | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-H.; Cho, H.-Y.; Choi, J.-W. Microdevice Platform for In Vitro Nervous System and Its Disease Model. Bioengineering 2017, 4, 77. https://doi.org/10.3390/bioengineering4030077
Choi J-H, Cho H-Y, Choi J-W. Microdevice Platform for In Vitro Nervous System and Its Disease Model. Bioengineering. 2017; 4(3):77. https://doi.org/10.3390/bioengineering4030077
Chicago/Turabian StyleChoi, Jin-Ha, Hyeon-Yeol Cho, and Jeong-Woo Choi. 2017. "Microdevice Platform for In Vitro Nervous System and Its Disease Model" Bioengineering 4, no. 3: 77. https://doi.org/10.3390/bioengineering4030077
APA StyleChoi, J.-H., Cho, H.-Y., & Choi, J.-W. (2017). Microdevice Platform for In Vitro Nervous System and Its Disease Model. Bioengineering, 4(3), 77. https://doi.org/10.3390/bioengineering4030077