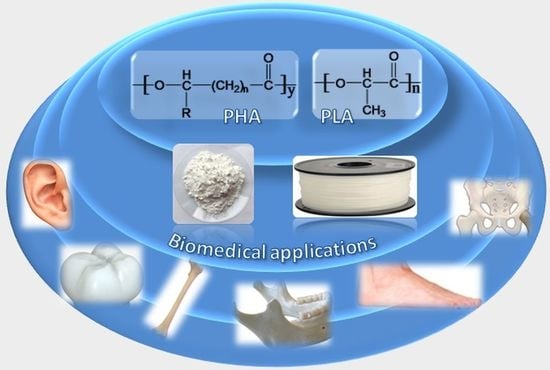
Recent Advances in 3D Printing of Aliphatic Polyesters
Abstract
:1. Introduction
2. Short Overview of the Main 3D-Printing Techniques
2.1. Selective Laser Sintering
2.2. Fused Deposition Modeling
3. Aliphatic Polyesters for Additive Manufacturing
3.1. Poly(Lactic Acid)
3.1.1. 3D Printing of PLA through Fused Deposition Modeling
3.1.2. 3D Printing of PLA Composites through Fused Deposition Modeling
3.1.3. 3D Printing of PLA and PLA Composites through SLS
3.1.4. Other Directions in 3D Printing of PLA Based Materials
3.2. Polyhydroxyalkanoates
3.2.1. PHA Filaments for Fused Deposition Modeling
3.2.2. PHA Structures Obtained by Selective Laser Sintering
3.2.2.1. SLS Applied to Pure PHB
3.2.2.2. SLS Applied to PHA Nanocomposites
4. Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wüst, S.; Müller, R.; Hofmann, S. Controlled positioning of cells in biomaterials—Approaches towards 3D tissue printing. J. Funct. Biomater. 2011, 2, 119–154. [Google Scholar] [CrossRef] [PubMed]
- Balletti, C.; Ballarin, M.; Guerra, F. 3D printing: State of the art and future perspectives. J. Cult. Herit. 2017, 26, 172–182. [Google Scholar] [CrossRef]
- Lee, J.-Y.; An, J.; Chua, C.K. Fundamentals and applications of 3D printing for novel materials. Appl. Mater. Today 2017, 7, 120–133. [Google Scholar] [CrossRef]
- Wohlers, T. Wohlers Report 2016; WOHLERS Associates: Fort Collins, CO, USA, 2016. [Google Scholar]
- Kamei, K.-I.; Mashimo, Y.; Koyama, Y.; Fockenberg, C.; Nakashima, M.; Nakajima, M.; Li, J.; Chen, Y. 3D printing of soft lithography mold for rapid production of polydimethylsiloxane-based microfluidic devices for cell stimulation with concentration gradients. Biomed. Microdevices 2015, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Shahali, H.; Jaggessar, A.; Yarlagadda, P.K.D.V. Recent advances in manufacturing and surface modification of titanium orthopaedic applications. Procedia Eng. 2017, 174, 1067–1076. [Google Scholar] [CrossRef]
- Patel, D.K.; Sakhaei, A.H.; Layani, M.; Zhang, B.; Ge, Q.; Magdassi, S. Highly stretchable and UV curable elastomers for digital light processing based 3D printing. Adv. Mater. 2017, 29, 1606000. [Google Scholar] [CrossRef] [PubMed]
- Muth, J.T.; Vogt, D.M.; Truby, R.L.; Mengüç, Y.; Kolesky, D.B.; Wood, R.J.; Lewis, J.A. Embedded 3D printing of strain sensors within highly stretchable elastomers. Adv. Mater. 2014, 26, 6307–6312. [Google Scholar] [CrossRef] [PubMed]
- Bâlc, N.; Vilău, C. Design for additive manufacturing, to produce assembled products, by SLS. MATEC Web Conf. 2017, 121, 04002. [Google Scholar] [CrossRef]
- Additive Manufacturing in Archeology. Available online: http://3dprintingcenter.net/2017/06/16/additive-manufacturing-in-archeology/ (accessed on 3 November 2017).
- David, O.T.; Szuhanek, C.; Tuce, R.A.; David, A.P.; Leretter, M. Polylactic acid 3D printed drill guide for dental implants using CBCT. Rev. Chim.-Bucharest 2017, 68, 341–342. [Google Scholar]
- Wang, M.; Favi, P.; Cheng, X.; Golshan, N.H.; Ziemer, K.S.; Keidar, M.; Webster, T.J. Cold atmospheric plasma (CAP) surface nanomodified 3D printed polylactic acid (PLA) scaffolds for bone regeneration. Acta Biomater. 2016, 46, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Panaitescu, D.M.; Frone, A.N.; Chiulan, I. Nanostructured biocomposites from aliphatic polyesters and bacterial cellulose. Ind. Crops Prod. 2016, 93, 251–266. [Google Scholar] [CrossRef]
- Mondschein, R.J.; Kanitkar, A.; Williams, C.B.; Verbridge, S.S.; Long, T.E. Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds. Biomaterials 2017, 140, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.-H.; Hsu, S. Polymeric-based 3D printing for tissue engineering. J. Med. Biol. Eng. 2015, 35, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Kruth, J.P.; Wang, X.; Laoui, T.; Froyen, L. Lasers and materials in selective laser sintering. Assem. Autom. 2003, 23, 357–371. [Google Scholar] [CrossRef]
- 3D Printing Material: Alumide. Available online: https://www.sculpteo.com/en/materials/alumide-material/ (accessed on 3 November 2017).
- Türk, D.-A.; Kussmaul, R.; Zogg, M.; Klahn, C.; Leutenecker-Twelsiek, B.; Meboldt, M. Composites part production with additive manufacturing technologies. Procedia CIRP 2017, 66, 306–311. [Google Scholar] [CrossRef]
- Schmid, M.; Amado, A.; Wegener, K. Polymer powders for selective laser sintering (sls). AIP Conf. Proc. 2015, 1664, 160009. [Google Scholar]
- Dieste, J.A.; Fernández, A.; Roba, D.; Gonzalvo, B.; Lucas, P. Automatic grinding and polishing using spherical robot. Procedia Eng. 2013, 63, 938–946. [Google Scholar] [CrossRef]
- He, Y.; Xue, G.; Fu, J. Fabrication of low cost soft tissue prostheses with the desktop 3D printer. Sci. Rep.-UK 2014, 4, 6973. [Google Scholar] [CrossRef] [PubMed]
- Takagishi, K.; Umezu, S. Development of the improving process for the 3D printed structure. Sci. Rep.-UK 2017, 7, 39852. [Google Scholar] [CrossRef] [PubMed]
- Panaitescu, D.; Frone, A.N.; Chiulan, I. Green Composites with Cellulose Nanoreinforcements. In Handbook of Composites from Renewable Materials; Thakur, V.K., Thakur, M.K., Kessler, M.R., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2017; Volume 7, pp. 299–338. [Google Scholar]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Ye, W.; Wu, Z.; Geng, P.; Wang, Y.; Zhao, J. Influence of layer thickness, raster angle, deformation temperature and recovery temperature on the shape-memory effect of 3D-printed polylactic acid samples. Materials 2017, 10, 970. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (pla) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Abdulkhani, A.; Hosseinzadeh, J.; Ashori, A.; Dadashi, S.; Takzare, Z. Preparation and characterization of modified cellulose nanofibers reinforced polylactic acid nano composite. Polym. Test. 2014, 35, 73–79. [Google Scholar] [CrossRef]
- Gu, J.; Catchmark, J.M. Polylactic acid composites incorporating casein functionalized cellulose nanowhiskers. J. Biol. Eng. 2013, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Oksman, K.; Mathew, A.P.; Bondeson, D.; Kvien, I. Manufacturing process of cellulose whiskers/polylactic acid nanocomposites. Compos. Sci. Technol. 2006, 66, 2776–2784. [Google Scholar] [CrossRef]
- Ambrosio-Martın, J.; Fabra, M.J.; Lopez-Rubio, A.; Lagaron, J.M. Melt polycondensation to improve the dispersion of bacterial cellulose into polylactide via melt compounding: Enhanced barrier and mechanical properties. Cellulose 2015, 22, 1201–1226. [Google Scholar] [CrossRef]
- Frone, A.N.; Panaitescu, D.; Chiulan, I.; Nicolae, C.A.; Vuluga, Z.; Vitelaru, C.; Damian, C.M. The effect of cellulose nanofibers on the crystallinity and nanostructure of poly(lactic acid) composites. J. Mater. Sci. 2016, 51, 9771–9791. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Song, W.; Yee, K.; Lee, K.Y.; Tagarielli, V.L. Measurements of the mechanical response of unidirectional 3D-printed pla. Mater. Des. 2017, 123, 154–164. [Google Scholar] [CrossRef]
- Letcher, T.; Waytashek, M. Material property testing of 3D-printed specimen in PLA on an entry-level 3D printer. Adv. Manuf. 2014, 2A, IMECE2014-39379. [Google Scholar]
- Chacón, J.M.; Caminero, M.A.; García-Plaza, E.; Núñez, P.J. Additive manufacturing of pla structures using fused deposition modelling: Effect of process parameters on mechanical properties and their optimal selection. Mater. Design 2017, 124, 143–157. [Google Scholar] [CrossRef]
- Guo, R.; Lu, S.; Page, J.M.; Merkel, A.R.; Basu, S.; Sterling, J.A.; Guelcher, S.A. Fabrication of 3D scaffolds with precisely controlled substrate modulus and pore size by templated-fused deposition modeling to direct osteogenic differentiation. Adv. Healthc. Mater. 2015, 4, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Pedro, F.C.; Cédryck, V.; Jeremy, B.; Mohit, C.; Manuela, E.G.; Rui, L.R.; Christina, T.; Dietmar, W.H. Biofabrication of customized bone grafts by combination of additive manufacturing and bioreactor knowhow. Biofabrication 2014, 6, 035006. [Google Scholar] [Green Version]
- Almeida, C.R.; Serra, T.; Oliveira, M.I.; Planell, J.A.; Barbosa, M.A.; Navarro, M. Impact of 3-d printed pla- and chitosan-based scaffolds on human monocyte/macrophage responses: Unraveling the effect of 3-d structures on inflammation. Acta Biomater. 2014, 10, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Wurm, M.C.; Möst, T.; Bergauer, B.; Rietzel, D.; Neukam, F.W.; Cifuentes, S.C.; Wilmowsky, C.V. In-vitro evaluation of polylactic acid (PLA) manufactured by fused deposition modeling. J. Biol. Eng. 2017, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Guduric, V.; Metz, C.; Siadous, R.; Bareille, R.; Levato, R.; Engel, E.; Fricain, J.-C.; Devillard, R.; Luzanin, O.; Catros, S. Layer-by-layer bioassembly of cellularized polylactic acid porous membranes for bone tissue engineering. J. Mater. Sci. Mater. Med. 2017, 28, 78. [Google Scholar] [CrossRef] [PubMed]
- Chiulan, I.; Mihaela Panaitescu, D.; Nicoleta Frone, A.; Teodorescu, M.; Andi Nicolae, C.; Căşărică, A.; Tofan, V.; Sălăgeanu, A. Biocompatible polyhydroxyalkanoates/bacterial cellulose composites: Preparation, characterization, and in vitro evaluation. J. Biomed. Mater. Res. A 2016, 104, 2576–2584. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Ahn, H.-J.; Lian, C.; Lee, K.-H.; Lee, C.-H. Design and optimization of prosthetic foot by using polylactic acid 3D printing. J. Mech. Sci. Technol. 2017, 31, 2393–2398. [Google Scholar] [CrossRef]
- Flores, R.L.; Liss, H.; Raffaelli, S.; Humayun, A.; Khouri, K.S.; Coelho, P.G.; Witek, L. The technique for 3D printing patient-specific models for auricular reconstruction. J. Cranio Maxill. Surg. 2017, 45, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, T. Polylactic acid-coated cable. Fujikura Tech. Rev. 2011, 40, 39–45. [Google Scholar]
- Dichtl, C.; Sippel, P.; Krohns, S. Dielectric properties of 3D printed polylactic acid. Adv. Mater. Sci. Eng. 2017, 2017, 10. [Google Scholar] [CrossRef]
- Prashantha, K.; Roger, F. Multifunctional properties of 3D printed poly(lactic acid)/graphene nanocomposites by fused deposition modeling. J. Macromol. Sci. A 2017, 54, 24–29. [Google Scholar] [CrossRef]
- Niaza, K.V.; Senatov, F.S.; Stepashkin, A.; Anisimova, N.Y.; Kiselevsky, M.V. Long-term creep and impact strength of biocompatible 3D-printed PLA-based scaffolds. Nano Hybrids Compos. 2017, 13, 15–20. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Gervaso, F.; Scalera, F.; Montagna, F.; Sannino, A.; Maffezzoli, A. The feasibility of printing polylactic acid–nanohydroxyapatite composites using a low-cost fused deposition modeling 3D printer. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Ferreira, R.T.L.; Amatte, I.C.; Dutra, T.A.; Bürger, D. Experimental characterization and micrography of 3D printed PLA and PLA reinforced with short carbon fibers. Compos. B-Eng. 2017, 124, 88–100. [Google Scholar] [CrossRef]
- Zhuang, Y.; Song, W.; Ning, G.; Sun, X.; Sun, Z.; Xu, G.; Zhang, B.; Chen, Y.; Tao, S. 3D–printing of materials with anisotropic heat distribution using conductive polylactic acid composites. Mater. Des. 2017, 126, 135–140. [Google Scholar] [CrossRef]
- Chen, Q.; Mangadlao, J.D.; Wallat, J.; De Leon, A.; Pokorski, J.K.; Advincula, R.C. 3D printing biocompatible polyurethane/poly(lactic acid)/graphene oxide nanocomposites: Anisotropic properties. ACS Appl. Mater. Interfaces 2017, 9, 4015–4023. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.Y.; Lee, S.H.; Wang, M.; Cheung, W.L. Selective Laser Sintering of Tissue Engineering Scaffolds Using Poly(L-Lactide) Microspheres. Key Eng. Mater. 2007, 334–335, 1225–1228. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Lee, S.H.; Wang, M.; Cheung, W.L.; Ip, W.Y. Selective laser sintering of porous tissue engineering scaffolds from poly(L-lactide)/carbonated hydroxyapatite nanocomposite microspheres. J. Mater. Sci.-Mater. Med. 2008, 19, 2535–2540. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.Y.; Duan, B.; Wang, M.; Cheung, W.L. Crystallization Kinetics of Poly(l-Lactide)/Carbonated Hydroxyapatite Nanocomposite Microspheres. J. Appl. Polym. Sci. 2009, 113, 4100–4115. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Wang, M.; Cheung, W.L.; Ip, W.Y. Selective Laser Sintering of Poly(l-Lactide)/Carbonated Hydroxyapatite Nanocomposite Porous Scaffolds for Bone Tissue Engineering. In Tissue Engineering; Eberli, D., Ed.; InTech: Vukovar, Croatia, 2010; pp. 179–204. [Google Scholar]
- Duan, B.; Wang, M.; Zhou, W.Y.; Cheung, W.L.; Li, Z.Y.; Lu, W.W. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater. 2010, 6, 4495–4505. [Google Scholar] [CrossRef] [PubMed]
- Filgueira, D.; Holmen, S.; Melbø, J.K.; Moldes, D.; Echtermeyer, A.T.; Chinga-Carrasco, G. Enzymatic-assisted modification of TMP fibers for improving the interfacial adhesion with PLA for 3D printing. ACS Sustain. Chem. Eng. 2017, 5, 9338–9346. [Google Scholar] [CrossRef]
- Tian, X.; Liu, T.; Wang, Q.; Dilmurat, A.; Li, D.; Ziegmann, G. Recycling and remanufacturing of 3D printed continuous carbon fiber reinforced PLA composites. J Clean. Prod. 2017, 142, 1609–1618. [Google Scholar] [CrossRef]
- Ten, E.; Turtle, J.; Bahr, D.; Jiang, L.; Wolcott, M. Thermal and mechanical properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/cellulose nanowhiskers composites. Polymer 2010, 51, 2652–2660. [Google Scholar] [CrossRef]
- Pinto, C.E.D.S.; Arizaga, G.G.C.; Wypych, F.; Ramos, L.P.; Satyanarayana, K.G. Studies of the effect of molding pressure and incorporation of sugarcane bagasse fibers on the structure andproperties of poly (hydroxy butyrate). Compos. A-Appl. Sci. Manuf. 2009, 40, 573–582. [Google Scholar] [CrossRef]
- Cai, Z.; Ynag, G.; Kim, J. Biocompatible nanocomposites prepared by impregnating bacterial cellulose nanofibrils into poly(3-hydroxybutyrate). Curr. Appl. Phys. 2011, 11, 247–249. [Google Scholar]
- Jiang, L.; Morelius, E.; Zhang, J.; Wolcott, M. Study of the poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/cellulose nanowhisker composites prepared by solution casting and melt processing. J. Compos. Mater. 2008, 42, 2629–2645. [Google Scholar] [CrossRef]
- Wu, C.-S.; Liao, H.; Cai, Y.-X. Characterisation, biodegradability and application of palm fibre-reinforced polyhydroxyalkanoate composites. Polym. Degrad. Stabil. 2017, 140, 55–63. [Google Scholar] [CrossRef]
- Wu, C.-S.; Liao, H. Fabrication, characterization, and application of polyester/wood flour composites. J. Polym. Eng. 2017, 37, 689–698. [Google Scholar] [CrossRef]
- Wu, C.-S.; Liao, H. Interface design of environmentally friendly carbon nanotube-filled polyester composites: Fabrication, characterisation, functionality and application. Express Polym. Lett. 2017, 11, 187–198. [Google Scholar] [CrossRef]
- Oliveira, M.F.; Maia, I.A.; Noritomi, P.Y.; Nargi, G.C.; Silva, J.V.L.; Ferreira, B.M.P.; Duek, E.A.R. Construção de Scaffolds para engenharia tecidual utilizando prototipagem rápida. Matéria 2007, 12, 373–382. [Google Scholar] [CrossRef]
- Pereira, T.F.; Oliveira, M.F.; Maia, I.A.; Silva, J.V.L.; Costa, M.F.; Thire, R.M.S.M. 3D Printing of Poly(3-hydroxybutyrate) Porous Structures Using Selective Laser Sintering. Macromol Symp. 2012, 319, 64–73. [Google Scholar] [CrossRef]
- Duan, B.; Wang, M. Customized Ca–P/PHBV nanocomposite scaffolds for bone tissue engineering: design, fabrication, surface modification and sustained release of growth factor. J. Roy. Soc. Interface 2010, 7, S615–S629. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Wang, M. Encapsulation and release of biomolecules from Ca-P/PHBV nanocomposite microspheres and three-dimensional scaffolds fabricated by selective laser sintering. Polym. Degrad. Stabil. 2010, 95, 1655–1664. [Google Scholar] [CrossRef]
- Duan, B.; Cheung, W.L.; Wang, M. Optimized fabrication of Ca–P/PHBV nanocomposite scaffolds via selective laser sintering for bone tissue engineering. Biofabrication 2011, 3, 015001. [Google Scholar] [CrossRef] [PubMed]







| Properties | Tg, °C | Tm, °C | Tensile Strength, MPa | Young’s Modulus, GPa | References |
|---|---|---|---|---|---|
| PLA (Bio-flex®F 6510) solution casting from chloroform | 57.5 | 156.3 | 15.2 | 1.17 | [28] |
| PLA (Nature Works™ 4032D) solution casting from DMF | - | - | 32.8 | 2.5 | [29] |
| PLA (Nature Works™ 4031D) extrusion | - | - | 40.9 | 2.9 | [30] |
| PLA film extrusion grade (Nature Works™) | 55.3 | 151.3 | 40.0 | 1.4 | [31] |
| PLA (Nature Works™ 4032D) Melt compounding | 60.0 | 167.0 | 40.0 | 2.7 | [32] |
| Properties | Tg, °C | Tm, °C | Tensile Strength, MPa | Young’s Modulus, GPa | Reference |
|---|---|---|---|---|---|
| PHB (Biocycle)—Ccompression molding | 164/174 | 43 | 3.5 | [60] | |
| PHB—solution casting from chloroform | 28 | 2.1 | [61] | ||
| PHBV 12 mol% HV (Metabolix Inc)—solvent casting from DMF | 140 | 17 | [59] | ||
| PHBV 12 mol% HV (Metabolix Inc)—solvent casting from DMF | ~0 | 140/154 | 14 | 0.8 | [62] |
| Technique | Material | Results | Application | Reference |
|---|---|---|---|---|
| FDM | PLA | Controllable porosity and pore size by controlling the extrusion and 3D-printing parameters | quantifying anisotropic responses of PLA parts | [33] |
| FDM | PLA | The 3D-printed samples supports the growth of human fetal osteoblast | Bone reconstruction | [39] |
| FDM | PLA | The 3D-printed model with optimized design displayed a reduction with 62% of the weight as compared to the initial model | Prosthetic foot | [42] |
| FDM | PLA | Accurate anatomic aspect, reduced amount of raw material, inexpensive final product | Artificial ear | [43] |
| FDM | PLA, PLA/ionic liquid (IL) | The addition of IL led to enhanced conductivity | Electronic devices | [45] |
| FDM | PLA/HA | Good dispersion of the HA in the PLA matrix; increased viscosity and compressive modulus for the composites with 15 wt.% HA | Molar tooth | [48] |
| FDM | PLA, PLA/graphene | Enhanced electrical resistivity and mechanical strength | Electronics | [46] |
| FDM | PLA | The increased surface roughness and hydrophilicity conducted to cells attachment and proliferation | Bone regeneration | [12] |
| FDM | TPU/PLA/GO | 0.5 wt.% GO led to the highest tensile modulus and cell proliferation | Tissue engineering scaffolds | [51] |
| FDM | PHA, PHA-g-MA, PHA/palm fibers, PHA-g-MA/ wood flower | Silane treatment of the palm fibers enhanced the adhesion with the polymer matrix; increased mechanical properties and higher degradation rate of the treated composites as compared to pure PHA and untreated composites; Increased tensile strength and antibacterial activity for PHA-g-MA/ wood flower | [63,64] | |
| SLS | PHB | Fidel replication of the 3D-printed structure with the design model; no thermal degradation of the PHB observed after 3D printing | Tissue engineering | [66,67] |
| SLS | PHBV/Ca-P | The addition of the inorganic filler led to improved cell proliferation; the SLS process didn’t influenced the bioactivity of the incorporated model protein | Bone tissue | [56,68,69] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiulan, I.; Frone, A.N.; Brandabur, C.; Panaitescu, D.M. Recent Advances in 3D Printing of Aliphatic Polyesters. Bioengineering 2018, 5, 2. https://doi.org/10.3390/bioengineering5010002
Chiulan I, Frone AN, Brandabur C, Panaitescu DM. Recent Advances in 3D Printing of Aliphatic Polyesters. Bioengineering. 2018; 5(1):2. https://doi.org/10.3390/bioengineering5010002
Chicago/Turabian StyleChiulan, Ioana, Adriana Nicoleta Frone, Călin Brandabur, and Denis Mihaela Panaitescu. 2018. "Recent Advances in 3D Printing of Aliphatic Polyesters" Bioengineering 5, no. 1: 2. https://doi.org/10.3390/bioengineering5010002
APA StyleChiulan, I., Frone, A. N., Brandabur, C., & Panaitescu, D. M. (2018). Recent Advances in 3D Printing of Aliphatic Polyesters. Bioengineering, 5(1), 2. https://doi.org/10.3390/bioengineering5010002