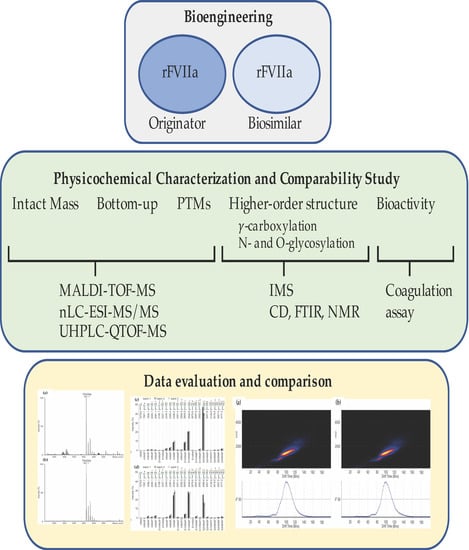
1. Introduction
One of the major classes of therapeutical biopharmaceuticals are blood factors. They represent six percent of the approved biotherapeuticals up to 2014 [
1]. Many of them are used to treat hemophilia, a hereditary (chromosome X) blood-coagulation disorder, due to a deficiency of the clotting factors VIII (hemophilia A) and IX (hemophilia B), respectively [
2]. Activated factor VII (FVIIa) initiates the extrinsic coagulation pathway and thereby activates factor IX and factor X to bind to tissue factor on the surface of cells that were exposed to circulating blood due to an injury. The application of pharmacologic doses of FVIIa results in the binding of sufficient amounts of FVIIa to activated platelets and subsequent activation of factor X, further passing of the tenase complex and finally an inducing of thrombin burst [
3].
Eptacog alfa is a vitamin K-dependent recombinant FVIIa (rFVIIa) produced by genetic engineering from baby hamster kidney (BHK) cells. The cleavage of Arg152–Ile153 bond of the single peptide chain of 406 residues (about 50 kDa) in FVII leads to its activation. The resulting FVIIa consists of a light chain (LC) of 152 amino acids (about 20 kDa) and a heavy chain (HC) of 254 amino acids (about 30 kDa), linked to each other by a single disulfide bond (Cys135–Cys262). During bioengineering rFVIIa undergoes many post-translational modifications (PTMs). The first ten glutamic acids of the N-terminal moiety are γ-carboxylated, Asn145 and Asn322 are N-glycosylated, and Ser52 and Ser60 are O-glycosylated. The N-glycosylation plays important roles in protein folding of FVII, while O-glycosylation is more meaningful for the biological activity of FVII. It was suggested that structural elements found in the O-glycans are important for the association of FVIIa with tissue factor [
4,
5]. γ-Carboxylation is described to be sensitive to limitations in the cellular post-translational protein modification machinery in the secretion pathway of recombinant vitamin K dependent coagulation factors [
6].
Eptacog alfa with its trade name NovoSeven
® (Novo Nordisk, Bagsværd, Denmark) was regulatory licensed in 1996 (EU), 1999 (USA) and 2000 (Japan) to treat congenital and acquired hemophilia [
7]. AryoSeven™ is a biosimilar version of NovoSeven
®, which has been recently proved to be comparable in terms of clinical efficacy [
8]. It has been designed by bioengineering to meet criteria of biosimilarity and clinical data are already available to show similar efficacy in humans [
8]. In general, biosimilars represent a subclass of biotherapeutics with high structural and clinical similarities compared to the already marketed inventor biodrug [
9]. According to the European Medicines Agency’s (EMA) biosimilar application pathway criteria or equivalent criteria from other countries, similarity of two biologicals needs to be demonstrated. Critical quality attributes (CQA) that may impact pharmacological response need to be investigated and specifications need to be set [
10]. Since the variability of biological production processes may alter the product quality, batch-to-batch changes of CQA over an extended manufacturing period need to be thoroughly controlled. Consequently, the preset CQA specifications of the originator have to be realized in an acceptable range by engineering of the bioprocess for originator as well as for biosimilar production [
11]. Several state-of-the-art analytics need to be applied to evaluate the requirement of International Conference on Harmonization (ICH) Q6B and Q5E specifications. They request an in-depth characterization and comparability of multiple CQA, concerning the physical, chemical and biological characteristics for at least three batches [
12,
13,
14]. Since glycosylation is highly sensitive to manufacturing changes, EMA and Food and Drug Administration (FDA) request extensive glycoanalytics, including glycan pattern and site-specific glycosylation analysis for glycoprotein-based pharmaceutics approval. Alteration of glycosylation profiles and modifications in a biosimilar can hamper the approval as a follow-on biodrug due to the known impact on activity and pharmacokinetics [
15,
16,
17]. Bioengineering of a follow-on rFVIIa biopharmaceutical was therefore performed to obtain a product that meets criteria for approval as biosimilar. A head-to-head biosimilarity study was applied for NovoSeven
® (originator) and AryoSeven™ (follow-on product). Physicochemical properties were analyzed based on mass spectrometry, including intact mass, PTMs and higher order structure (HOS), and other biophysical methods. This was complemented by a potency assay for coagulation to investigate potential influence of CQA variations on bioactivity.
Eptacog alfa with its trade name NovoSeven
® (Novo Nordisk, Bagsværd, Denmark) was regulatory licensed in 1996 (EU), 1999 (USA) and 2000 (Japan) to treat congenital and acquired hemophilia [
7]. AryoSeven™ is a biosimilar version of NovoSeven
®, which has been recently proved to be comparable in terms of clinical efficacy [
8]. It has been designed by bioengineering to meet criteria of biosimilarity and clinical data are already available to show similar efficacy in humans [
8]. In general, biosimilars represent a subclass of biotherapeutics with high structural and clinical similarities compared to the already marketed inventor biodrug [
9]. According to the European Medicines Agency’s (EMA) biosimilar application pathway criteria or equivalent criteria from other countries, similarity of two biologicals needs to be demonstrated. Critical quality attributes (CQA) that may impact pharmacological response need to be investigated and specifications need to be set [
10]. Since the variability of biological production processes may alter the product quality, batch-to-batch changes of CQA over an extended manufacturing period need to be thoroughly controlled. Consequently, the preset CQA specifications of the originator have to be realized in an acceptable range by engineering of the bioprocess for originator as well as for biosimilar production [
11]. Several state-of-the-art analytics need to be applied to evaluate the requirement of International Conference on Harmonization (ICH) Q6B and Q5E specifications. They request an in-depth characterization and comparability of multiple CQA, concerning the physical, chemical and biological characteristics for at least three batches [
12,
13,
14]. Since glycosylation is highly sensitive to manufacturing changes, EMA and Food and Drug Administration (FDA) request extensive glycoanalytics, including glycan pattern and site-specific glycosylation analysis for glycoprotein-based pharmaceutics approval. Alteration of glycosylation profiles and modifications in a biosimilar can hamper the approval as a follow-on biodrug due to the known impact on activity and pharmacokinetics [
15,
16,
17]. Bioengineering of a follow-on rFVIIa biopharmaceutical was therefore performed to obtain a product that meets criteria for approval as biosimilar. A head-to-head biosimilarity study was applied for NovoSeven
® (originator) and AryoSeven™ (follow-on product). Physicochemical properties were analyzed based on mass spectrometry, including intact mass, PTMs and higher order structure (HOS), and other biophysical methods. This was complemented by a potency assay for coagulation to investigate potential influence of CQA variations on bioactivity.
Eptacog alfa with its trade name NovoSeven
® (Novo Nordisk, Bagsværd, Denmark) was regulatory licensed in 1996 (EU), 1999 (USA) and 2000 (Japan) to treat congenital and acquired hemophilia [
7]. AryoSeven™ is a biosimilar version of NovoSeven
®, which has been recently proved to be comparable in terms of clinical efficacy [
8]. It has been designed by bioengineering to meet criteria of biosimilarity and clinical data are already available to show similar efficacy in humans [
8]. In general, biosimilars represent a subclass of biotherapeutics with high structural and clinical similarities compared to the already marketed inventor biodrug [
9]. According to the European Medicines Agency’s (EMA) biosimilar application pathway criteria or equivalent criteria from other countries, similarity of two biologicals needs to be demonstrated. Critical quality attributes (CQA) that may impact pharmacological response need to be investigated and specifications need to be set [
10]. Since the variability of biological production processes may alter the product quality, batch-to-batch changes of CQA over an extended manufacturing period need to be thoroughly controlled. Consequently, the preset CQA specifications of the originator have to be realized in an acceptable range by engineering of the bioprocess for originator as well as for biosimilar production [
11]. Several state-of-the-art analytics need to be applied to evaluate the requirement of International Conference on Harmonization (ICH) Q6B and Q5E specifications. They request an in-depth characterization and comparability of multiple CQA, concerning the physical, chemical and biological characteristics for at least three batches [
12,
13,
14]. Since glycosylation is highly sensitive to manufacturing changes, EMA and Food and Drug Administration (FDA) request extensive glycoanalytics, including glycan pattern and site-specific glycosylation analysis for glycoprotein-based pharmaceutics approval. Alteration of glycosylation profiles and modifications in a biosimilar can hamper the approval as a follow-on biodrug due to the known impact on activity and pharmacokinetics [
15,
16,
17]. Bioengineering of a follow-on rFVIIa biopharmaceutical was therefore performed to obtain a product that meets criteria for approval as biosimilar. A head-to-head biosimilarity study was applied for NovoSeven
® (originator) and AryoSeven™ (follow-on product). Physicochemical properties were analyzed based on mass spectrometry, including intact mass, PTMs and higher order structure (HOS), and other biophysical methods. This was complemented by a potency assay for coagulation to investigate potential influence of CQA variations on bioactivity.
2. Materials and Methods
2.1. Chemicals and Consumables
All purchased chemicals were of highest purity. Diammonium hydrogen citrate, dithiothreitol (DTT), iodoacetamide (IAA), D2O, dextran ladder, super-dihydroxybenzoic acid (sDHB), sodium hydroxide, methyl iodide, ammonium bicarbonate, MS grade acetonitrile (ACN), formic acid (FA), and trifluoroacetic acid (TFA) were purchased from Sigma-Aldrich (Steinheim, Germany). Dimethyl sulfoxide, sodium iodide, 2-propanol and chloroform were purchased from Carl Roth (Karlsruhe, Germany). Ultrapure water was obtained from a Milli-Q water purifier (Millipore Corporation, Medford, MA, USA). Sequencing grade trypsin and PNGase F were purchased from Roche Applied Science (Mannheim, Germany). ZIC-HILIC kit was purchased from Merck (Darmstadt, Germany), Amicon Ultra-0.5 mL centrifugal filters from Merck (Darmstadt, Germany), Empore® solid phase extraction (SPE) used for C18 stage tips from Sigma-Aldrich, and carbograph extract clean columns from Alltech (Deerfield, IL, USA). The National Institute for Biological Standards and Control standard ampoule (NIBSC, UK) was obtained and used as a reference standard. Factor VII deficient plasma (Hemosil, NY, USA), calibration plasma (Hemosil) and thromboplastin (Hemosil) were purchased in lyophilized form and reconstituted according to the manufacturer’s instructions. Factor diluent and reference emulsion were obtained from Hemosil as well.
The originator “NovoSeven® 1.2 mg/mL” (Lot-No: CU60561, ES6S824, and ES6T726) was obtained from Novo Nordisk (Bagsværd, Denmark). The biosimilar “AryoSeven™ 1.2 mg/mL” (Lot-No: 9401043-a, 9401044-a, and 9401045-a) was received from AryoGen Pharmed (Tehran, Iran). Both biologicals were delivered as a lyophilized powder for reconstitution in a vial.
2.2. Instrumentation
Glycopeptide analysis using liquid chromatography accurate mass spectrometry (UHPLC-QTOF-MS) was performed on a Waters ACQUITY UPLC system coupled to a SYNAPT HDMS G2-S mass spectrometer (Waters; Manchester, UK) with a resolution of 18,000 and equipped with a BEH C18 (2.1 × 100 mm, 1.7 µm particle size, Waters, Manchester, UK) using H2O:FA (99.9:0.1, v:v) as mobile phase A and ACN:FA (99.9:0.1, v:v) as mobile phase B. The flow rate was set to 200 µL/min and a linear gradient from 5% B to 35% B in 35 min, 35% B to 50% B in 10 min followed by an isocratic flow at 80% B for 5 min was applied. QTOF mass calibration was performed using the singly charged ions produced by a 2 μg/μL sodium iodide solution in 2-propanol:water (50:50, v:v). Samples were ionized in positive electrospray ionization (ESI+) mode. The capillary voltage was at 2500 V, cone voltage at 50 V, and acquisition range was m/z 250–2000. In elevated energy scan mass spectrometry (MSE) experiments acquisition range was m/z 50–2000, trap collision energy 4 V and transfer collision energy from 15 to 45 V.
Ion mobility spectrometry (IMS) analyses were carried out using analogous conditions as described for UHPLC-QTOF-MS, with additional usage of the IMS option (UHPLC-IMS). IMS settings were as following: capillary voltage 2800 V, wave velocity 2000 m/s, wave height 1.0 V, transfer wave velocity 1968 m/s and transfer wave height 1.8 V. Data interpretation was performed using MassLynx 4.1 and Driftscope 2.1 (Waters; Manchester, UK).
Matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF-MS) of N- and O-glycans was performed on an Ultraflextreme mass spectrometer (Bruker Daltonics; Bremen, Germany) equipped with a smartbeam-IITM laser and a TOF/TOF MS facility (LIFTTM technology). Aliquots (0.5 µL) of the sample were mixed directly on a ground steel MALDI target plate (Bruker Daltonics) in a 1:1 ratio (v/v) with 10 mg/mL sDHB matrix dissolved in ACN:H2O (10:90, v:v) and allowed to dry at ambient temperature. Spectra were recorded from m/z 700 to 5000 (N-glycans) or m/z 200 to 3000 (O-glycans) in the reflectron positive ion mode. Acquisition was proceeded at 2000 Hz for 4000 shots (200 shots per step) using a random-walk algorithm and the ions were accelerated with 25 kV. External calibration for glycans was performed using a dextran ladder (Sigma). Data analysis was performed in FlexControl 3.4 (Bruker Daltonics) and spectra were evaluated using the GlycoPeakfinder (EuroCarbDB, European Bioinformatics Institute, Heidelberg, Germany) software. Assigned glycan structures were built with the GlycoWorkbench software (1.1 version, EuroCarbDB).
Intact mass of biotherapeutics was assessed by MALDI-TOF-MS. Protein standard II (Bruker Daltonics) was used as an external calibration standard. Aliquots (2 µL) of the sample solution were mixed with 2 μL of a 2% TFA solution. The matrix solution (2 µL) was added and the mixture was pipetted up and down until the crystallization starts. An aliquot (1 μL) of the crystal suspension was spotted and thoroughly mixed on the target. Ions were analyzed in the linear mode after acceleration at 20 kV and the laser energy was set to 50%.
Nanoflow liquid chromatography electrospray ionization tandem mass spectrometry (nLC-ESI-MS/MS) analyses were performed on a Dionex Ultimate 3000 nanoLC (Thermo Fisher Scientific; Bremen, Germany) coupled to a LTQ Orbitrap Velos mass spectrometer (Thermo Fisher Scientific) using nESI+ ionization. Chromatographic separation was achieved on an in-house manufactured 250 mm fritless silica microcolumn with an inner diameter of 100 μm. Columns were packed with ReproSil-Pur C18-AQ 3 μm resin (Maisch GmbH; Ammerbuch-Entringen, Germany). A linear gradient from 5% B to 60% B in 90 min at flow rate of 350 nL/min for mobile phase A (H2O:FA, 99.9:0.1, v:v) and mobile phase B (ACN:FA, 99.9:0.1, v:v) was applied. Full scan MS spectra (from m/z 300–1700) as well as product ion spectra after fragmentation of the twenty most intense ions by collision-induced dissociation (CID) were recorded. Protein sequence identification and PTM analysis were performed using MaxQuant, version 1.3.0.5 (Max-Planck-Institute of Biochemistry; Martinsried, Germany).
2.3. Sample Processing
2.3.1. Intact Mass Determination
rFVIIa samples were buffer exchanged in 30 kDa MWCO Amicon ultra centrifugal filter units and diluted to 1 μg/μL in H2O:FA (9.9:0.1, v:v). Then, 2,5-dihydroxy actetophenone (2,5-DHAP) matrix (7.6 mg) was dissolved in 375 µL ethanol mixed with 125 µL of a solution containing 18 mg/mL of diammonium hydrogen citrate (DAC) dissolved in water and used for the analysis of native rFVIIa.
2.3.2. N- and O-Glycan Analysis
Aliquots (50 μg) of biologicals were dissolved in 100 μL of 50 mM ammonium bicarbonate (ABC) buffer. Reduction was performed with DTT (20 mM in 50 mM ABC buffer) within 45 min at 60 °C and alkylation with 20 mM of IAA within 30 min at room temperature in the dark. Subsequently, samples were digested with 2 U PNGase F enzyme in 50 µL ABC buffer for 4 h at 37°C to release total N-glycans. In contrast, the O-linked carbohydrates were cleaved using β-elimination performed as follows: Aliquots of 50 μg of protein were mixed with 10 µL of 200 mM DTT and the resulting mixture was incubated for 60 min at 60 °C. The solution was allowed to cool down at RT, after which 10 μL of a 200 mM IAA solution in water were added and incubated at RT for 45 min in the dark. Subsequently, 50 µL of aqueous ammonia (25% v:v) were added to the mixture and incubated at 50 °C overnight. Samples were dried under vacuum. Purification was achieved using a Carbograph SPE cartridge equilibrated with 3 × 400 µL of ACN:H
2O:TFA (80:19.9:0.1, v:v:v), followed by H
2O:TFA (99.9:0.1, v:v). Samples were acidified with 1% TFA to a final pH value < 4 and applied onto the column. Columns were washed with 3 × 400 µL of H
2O:TFA (99.9:0.1, v:v) in order to desalt N-glycans. Glycans were then eluted with 3 × 400 µL of ACN:H
2O:TFA (25:74.9:0.1, v:v:v). Eluates were dried under vacuum. The dried N-glycans were permethylated in dimethyl sulfoxide using sodium hydroxide and methyl iodide as described in literature [
18]. The mixtures were subsequently incubated at room temperature for 1 h. Samples were mixed vigorously, then the aqueous phase was discarded. The organic phase was washed five times with water and then evaporated to dryness. The permethylated N-glycans were dissolved in 10 µL of ACN:H
2O (75:25, v:v) and aliquots were analyzed by MALDI-TOF-MS.
2.3.3. Peptide Mapping
Aliquots (50 μg) of biologicals were dissolved in 100 μL of 50 mM ABC buffer. Reduction was performed with DTT (20 mM in 50 mM ABC buffer) for 45 min at 37 °C and alkylation with 20 mM of IAA for 30 min at room temperature in the dark. Subsequently, samples were digested with 2 U PNGase F enzyme in 50 µL ABC buffer for 4 h at 37 °C. Deglycosylated rFVIIa samples were digested with trypsin (1 μg trypsin, 1:50 enzyme to protein ratio in 50 mM ABC buffer) and desalted using C18 stage tips. C18 tips were equilibrated with 100 µL MeOH, 190 µL of ACN:H2O:FA (5:94.9:0.1, v:v:v), followed by 120 µL ACN:H2O:TFA (5:92:3, v:v:v). Samples were applied and C18 tips were washed with 200 µL of ACN:H2O:FA (5:94.9:0.1, v:v:v). Peptides were then eluted with 200 µL of ACN:H2O:FA (80:19.9:0.1, v:v:v). Eluates were dried under vacuum. Purified peptide samples were dissolved in 15 µL of ACN:H2O:FA (5:94.9:0.1, v:v:v) and subjected to protein sequencing and PTMs analysis using nLC-ESI-MS/MS. Data were compared to DrugBank online database containing both HC and LC sequences of rFVIIa.
2.3.4. Glycopeptide Analysis
Aliquots of the samples containing 50 μg of rFVIIa were dissolved in 100 μL of ABC buffer. Reduction was performed with DTT (20 mM in 50 mM ABC buffer) within 45 min at 60 °C and alkylation with 20 mM IAA within 30 min at room temperature in the dark. Trypsin (1 μg, 1:50 enzyme to protein ratio) was added and incubation was carried out over night at 37 °C. Prior to analysis, separation of the glycopeptides from hydrophobic peptides was performed using ZIC-HILIC ProteoExtract kit (Merck, Darmstadt, Germany) as described by the manufacturer. The resulting supernatant containing glycopeptides was analyzed by UHPLC-QTOF-MS.
2.3.5. Analysis of Higher Order Structure
rFVIIa samples were buffer exchanged into ABC buffer with a 30 kDa Amicon filter (Merck, Germany). rFVIIa samples reconstituted in 50 μL ABC buffer were delivered to the UHPLC-IMS and analyzed as described above.
Secondary structures of innovator and biosimilar were compared by circular dichroism (CD) using a JASCO J-810 CD spectrometer (Jasco, Easton, MD, USA) equipped with 0.1 cm path length CD Suprasil cuvettes (Hellma Analytics, Müllheim, Germany). The analyses were carried out at 293 K. A band width of 2 nm and a scanning speed of 100 nm/min were used. Three scans were performed for each sample three times (n = 3), and the resulting spectra were averaged. Fourier transform infrared spectroscopy (FTIR) measurements were performed using a PerkinElmer Spectrum 100 FTIR spectrometer. The measurement time was set to 50 s with a resolution of 4 cm−1.
One-dimensional proton NMR spectra were recorded on a Avance III 700 MHz NMR system (Bruker Daltonics) with Topspin 2.1 software. The formulation buffer of the biopharmaceuticals was changed to water using a 30 kDa Amicon filter. Heavy water was used to suppress the residual water signal. The measurement of samples at concentrations of 0.25 mM were performed with a spectral width of 20 ppm and 8k scans at 293 K.
2.3.6. Coagulation Assay
FVIIa activity was analyzed by automatic coagulation analyzer, ACL 7000 (Instrumentation Laboratory, Bedford, MA, USA). The ACL 7000 methodology is based on the change of light scatter associated with the formation of a fibrin clot. After calibration of the instrument, the total content of NIBSC standard ampoule, containing the 2nd International Standard for FVII concentrate, was reconstituted and serial dilutions of standard and sample were prepared according to the manufacturers’ instructions. Clotting times for each dilution were analyzed by the PLA 2.0 Bioassay software (Stegmann Systems, Rodgau, Germany). Average results of the three purchased NovoSeven® originator lots (Lot-No: CU60561, ES6S824, ES6T726) were used as a reference value.
4. Discussion
Intact masses were found to be comparable for NovoSeven
® and AryoSeven™. The mass shifts observed for the biosimilar are most likely from variances in PTMs, such as deamidation [
19], oxidation or glycosylation, which are closer evaluated after digestion of the protein. During the oxidation process, the sulfur atom at methionine is oxidized into a sulfoxide (mass increase of 16 Da) and subsequently into a sulfone form (mass increase of 32 Da) [
20]. The process of deamidation occurs mainly at Asn residues either under acidic conditions or neutral and basic conditions leading to Asp and/or isoAsp with a mass increase of 1 Da [
21,
22]. A similar low degree of oxidation and deamidation was detected in both biotherapeutics, indicating that these modifications only have very low impact on the bioactivity.
γ-Carboxylation was analyzed, since it was reported for monitoring of the limitations in the cellular post-translational protein modification machinery in the secretion pathway of recombinant vitamin K dependent coagulation factors [
6,
23]. The γ-carboxyglutamic acid-rich (GLA) domain in rFVII contains multiple glutamate residues (at E6, E7, E14, E16, E19, E20, E25, E26, E29, and E35) that have been post-translationally modified by vitamin K-dependent carboxylation to form γ-carboxyglutamate (Gla) [
24,
25,
26]. γ-Carboxylated peptides were found to be comparable in NovoSeven
® and AryoSeven™ since fully γ-carboxylated peptide containing 10-times Gla was found in both biologics. Hence, this PTM is in line with previous studies [
23].
Changes in glycosylation are often caused by alterations in the bioprocess used for production of biologicals [
27,
28]. In particular, O-glycosylation site identification is a challenging task, because Eptacog alfa bears O-fucosylation at position Ser60 and O-glucose or O-glucose-(xylose)
1,2 motifs at Ser52 [
29]. Since the glycosidic bond of O-fucose to Ser is highly labile, it causes complex CID mass spectra [
30]. However, within this study the O- and N-glycopeptide mass spectra of follow-on biological (AryoSeven™) and reference product (NovoSeven
®) were comparable and enabled to confirm each O- and N-glycosylation sites (Ser52, Ser60, Asn145 and Asn322). Thus, by carefully designing the bioengineering process it was possible to obtain biosimilarity also in this CQA. GalNAc-bearing N-glycan structures were found to be much more abundant in AryoSeven™, which is often correlated with an overall lower sialylation [
23]. The latter one was also observed in the analyzed samples. The follow-on biologic possesses mainly the non-sialylated structures ending with Gal and GalNAc (galacto- and agalacto-type), respectively. Both structures bind to the asialoglycoprotein receptor with high affinity. This may accelerate clearance of rFVIIa from blood circulation and lead to a shortened half-life of a drug [
31].
CD and FTIR were used to assess multiple geometries such as α-helices, polyproline helices, β-sheets and random coil, which impact the far ultraviolet CD spectrum and IR bands [
13]. CD spectral analysis in the far UV (200–260 nm) exhibit information about the α-helix, β-sheet and random coil, which can resume the conformation state of the molecule. Near UV (260–350 nm) spectra represent the tertiary structure of proteins [
32]. The CD data (
Figure 6) revealed an absorbance minimum in the wavelength range of 190–230 nm, representing a predominant α-helical structure in both biotherapeutics [
33]. Similar site and shape of the amide bands (between 1750 and 750 cm
−1) in FTIR spectra confirmed the results from CD [
13,
32]. Minor differences in CD spectra arise from the variation of the calcium amount in the formulation buffer of the biologics [
33], and did not reflect structural differences of the two proteins.
NMR spectroscopy can produce a spectral fingerprint of biologicals in solution, providing structural and hydrodynamic data for assessing biopharmaceuticals’ comparability [
32,
34]. NMR data were also found to be comparable for NovoSeven
® and AryoSeven™.
Using IMS as a powerful visualization technique under denaturing conditions supported the biosimilarity study [
35,
36]. The separation of ion species with different size, charge and shape was performed in a drift cell filled with a buffer carrier gas (N
2) in the presence of an electric field, where the speed of an ion species plays a critical parameter [
37]. The data from IMS were highly comparable.
The potency of both rFVIIa biologicals was assigned by adding a thromboplastin reagent that contains tissue factor and calcium to Factor VII-deficient plasma and measuring the clotting time. The prothrombin time (PT) varies with reagent and coagulometer, but typically ranges between 10 and 14 s [
38]. The bioactivity of the biosimilar is almost identical to the originator NovoSeven
®.