A Review on Bioconversion of Agro-Industrial Wastes to Industrially Important Enzymes
Abstract
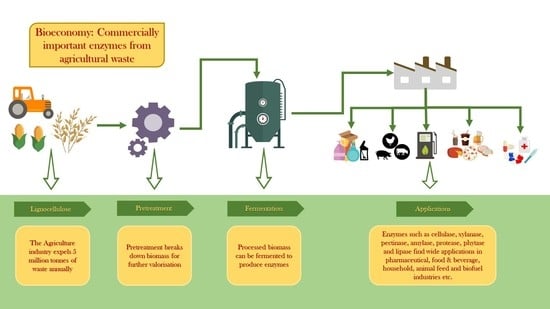
:1. Introduction
2. Agro-Industry Wastes
3. Valorisation of Agro-Industry Wastes
4. Industrial Enzymes
4.1. Agro-Industry Wastes as Substrate
4.2. Production of Enzymes at Industrial Scale
4.2.1. Enzymes that Act on Polysaccharides
α-amylase
Amyloglucosidase
Cellulase
Xylanase
Inulinase
Hemicellulases
Mannanase
Lactase
β-glucanase
Invertase
Pectinase
4.2.2. Enzymes that Act on Proteins
Protease
Transglutaminase
4.2.3. Other Industrially Important Enzymes
Lipases
Phytase
Laccase
4.2.4. Cellulosomes: The Future Prospect of Cellulytic Enzymes
4.2.5. Control of a Fermentation Processes
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bharathiraja, S.; Suriya, J.; Krishnan, M.; Manivasagan, P.; Kim, S.-K. Production of Enzymes From Agricultural Wastes and Their Potential Industrial Applications. Adv. Food Nutr. Res. 2017, 80, 125–148. [Google Scholar] [PubMed]
- Ravindran, R.; Jaiswal, A.K. Exploitation of Food Industry Waste for High-Value Products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Lignocellulosic Biorefineries in Europe: Current State and Prospects. Trends Biotechnol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Searle, S.M.C. Availability of Cellulosic Residues and Wastes in the EU; ICCT: Washington, DC, USA, 2013. [Google Scholar]
- Solomon, B.D.; Barnes, J.R.; Halvorsen, K.E. Grain and cellulosic ethanol: History, economics, and energy policy. Biomass Bioenergy 2007, 31, 416–425. [Google Scholar] [CrossRef]
- Guan, W.; Shi, S.; Tu, M.; Lee, Y.Y. Acetone–butanol–ethanol production from Kraft paper mill sludge by simultaneous saccharification and fermentation. Bioresour. Technol. 2016, 200, 713–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravindran, R.; Jaiswal, A. Microbial Enzyme Production Using Lignocellulosic Food Industry Wastes as Feedstock: A Review. Bioengineering 2016, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Veana, F.; Martínez-Hernández, J.L.; Aguilar, C.N.; Rodríguez-Herrera, R.; Michelena, G. Utilization of molasses and sugar cane bagasse for production of fungal invertase in solid state fermentation using Aspergillus niger GH1. Braz. J. Microbiol. 2014, 45, 373–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, A.; Selvakumar, P.; Ashakumary, L. Glucoamylase production by Aspergillus niger on rice bran is improved by adding nitrogen sources. World J. Microbiol. Biotechnol. 1994, 10, 348–349. [Google Scholar] [CrossRef] [PubMed]
- Onilude, A.A.; Fadaunsi, I.F.; Garuba, E.O. Inulinase production by Saccharomyces sp. in solid state fermentation using wheat bran as substrate. Ann. Microbiol. 2012, 62, 843–848. [Google Scholar] [CrossRef]
- Ravindran, R.; Desmond, C.; Jaiswal, S.; Jaiswal, A.K. Optimisation of organosolv pretreatment for the extraction of polyphenols from spent coffee waste and subsequent recovery of fermentable sugars. Bioresour. Technol. Rep. 2018, 3, 7–14. [Google Scholar] [CrossRef]
- Francis, F.; Sabu, A.; Nampoothiri, K.M.; Ramachandran, S.; Ghosh, S.; Szakacs, G.; Pandey, A. Use of response surface methodology for optimizing process parameters for the production of α-amylase by Aspergillus oryzae. Biochem. Eng. J. 2003, 15, 107–115. [Google Scholar] [CrossRef]
- Adeniran, H.A.; Abiose, S.H.; Ogunsua, A.O. Production of Fungal β-amylase and Amyloglucosidase on Some Nigerian Agricultural Residues. Food Bioprocess Technol. 2010, 3, 693–698. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Brar, S.K.; Verma, M. Potential of apple pomace as a solid substrate for fungal cellulase and hemicellulase bioproduction through solid-state fermentation. Ind. Crops Prod. 2012, 38, 6–13. [Google Scholar] [CrossRef]
- Leite, P.; Salgado, J.M.; Venâncio, A.; Domínguez, J.M.; Belo, I. Ultrasounds pretreatment of olive pomace to improve xylanase and cellulase production by solid-state fermentation. Bioresour. Technol. 2016, 214, 737–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biz, A.; Finkler, A.T.J.; Pitol, L.O.; Medina, B.S.; Krieger, N.; Mitchell, D.A. Production of pectinases by solid-state fermentation of a mixture of citrus waste and sugarcane bagasse in a pilot-scale packed-bed bioreactor. Biochem. Eng. J. 2016, 111, 54–62. [Google Scholar] [CrossRef]
- BCC Research. Global Markets for Enzymes in Industrial Applications; BCC Research: Wellesley, MA, USA, 2014. [Google Scholar]
- Novozymes. Novozymes: Quality Environmentally-Friendly Enzymes; Novozymes: Bagsvaerd, Denmark, 2018. [Google Scholar]
- Ravindran, R.; Jaiswal, A.K. A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: Challenges and opportunities. Bioresour. Technol. 2016, 199, 92–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging Technologies for the Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Salim, A.A.; Grbavčić, S.; Šekuljica, N.; Stefanović, A.; Jakovetić Tanasković, S.; Luković, N.; Knežević-Jugović, Z. Production of enzymes by a newly isolated Bacillus sp. TMF-1 in solid state fermentation on agricultural by-products: The evaluation of substrate pretreatment methods. Bioresour. Technol. 2017, 228, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Fernández Núñez, E.G.; Barchi, A.C.; Ito, S.; Escaramboni, B.; Herculano, R.D.; Mayer, C.R.M.; de Oliva Neto, P. Artificial intelligence approach for high level production of amylase using Rhizopus microsporus var. oligosporus and different agro-industrial wastes. J. Chem. Technol. Biotechnol. 2017, 92, 684–692. [Google Scholar] [CrossRef]
- Sahnoun, M.; Kriaa, M.; Elgharbi, F.; Ayadi, D.-Z.; Bejar, S.; Kammoun, R. Aspergillus oryzae S2 alpha-amylase production under solid state fermentation: Optimization of culture conditions. Int. J. Biol. Macromol. 2015, 75, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Sundarram, A.; Murthy, T.P.K. α-Amylase Production and Applications: A Review. J. Appl. Environ. Microbiol. 2014, 2, 166–175. [Google Scholar] [CrossRef]
- Prakash, B.; Vidyasagar, M.; Madhukumar, M.S.; Muralikrishna, G.; Sreeramulu, K. Production, purification, and characterization of two extremely halotolerant, thermostable, and alkali-stable α-amylases from Chromohalobacter sp. TVSP 101. Process Biochem. 2009, 44, 210–215. [Google Scholar] [CrossRef]
- Roohi; Kuddus, M. Bio-statistical approach for optimization of cold-active α-amylase production by novel psychrotolerant M. foliorum GA2 in solid state fermentation. Biocatal. Agric. Biotechnol. 2014, 3, 175–181. [Google Scholar] [CrossRef]
- Sen, S.K.; Dora, T.K.; Bandyopadhyay, B.; Das Mohapatra, P.K.; Raut, S.; Sen, S.K.; Dora, T.K.; Bandyopadhyay, B.; Mohapatra, P.K.D.; Raut, S. Thermostable alpha-amylase enzyme production from hot spring isolates Alcaligenes faecalis SSB17—Statistical optimization. Biocatal. Agric. Biotechnol. 2014, 3, 218–226. [Google Scholar] [CrossRef]
- Krishna, C.; Chandrasekaran, M. Banana waste as substrate for α-amylase production by Bacillus subtilis (CBTK 106) under solid-state fermentation. Appl. Microbiol. Biotechnol. 1996, 46, 106–111. [Google Scholar] [CrossRef]
- Rajagopalan, G.; Krishnan, C. α-Amylase production from catabolite derepressed Bacillus subtilis KCC103 utilizing sugarcane bagasse hydrolysate. Bioresour. Technol. 2008, 99, 3044–3050. [Google Scholar] [CrossRef] [PubMed]
- James, J.A.; Lee, B.H. Glucoamylases: Microbial sources, industrial applications and molecular biology? A review. J. Food Biochem. 1997, 21, 1–52. [Google Scholar] [CrossRef]
- Espinosa-Ramírez, J.; Pérez-Carrillo, E.; Serna-Saldívar, S.O. Maltose and glucose utilization during fermentation of barley and sorghum lager beers as affected by β-amylase or amyloglucosidase addition. J. Cereal Sci. 2014, 60, 602–609. [Google Scholar] [CrossRef]
- Kumar, P.; Satyanarayana, T. Microbial glucoamylases: Characteristics and applications. Crit. Rev. Biotechnol. 2009, 29, 225–255. [Google Scholar] [CrossRef] [PubMed]
- Diler, G.; Chevallier, S.; Pöhlmann, I.; Guyon, C.; Guilloux, M.; Le-Bail, A. Assessment of amyloglucosidase activity during production and storage of laminated pie dough. Impact on raw dough properties and sweetness after baking. J. Cereal Sci. 2015, 61, 63–70. [Google Scholar] [CrossRef]
- Singh, H.; Soni, S.K. Production of starch-gel digesting amyloglucosidase by Aspergillus oryzae HS-3 in solid state fermentation. Process Biochem. 2001, 37, 453–459. [Google Scholar] [CrossRef]
- Shin, H.K.; Kong, J.Y.; Lee, J.D.; Lee, T.H. Syntheses of hydroxybenzyl-α-glucosides by amyloglucosidase-catalyzed transglycosylation. Biotechnol. Lett. 2000, 22, 321–325. [Google Scholar] [CrossRef]
- Pandey, A. Improvements in solid-state fermentation for glucoamylase production. Biol. Wastes 1990, 34, 11–19. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. Microbial cellulases: Engineering, production and applications. Renew. Sustain. Energy Rev. 2014, 33, 188–203. [Google Scholar] [CrossRef]
- Singhania, R.R.; Saini, J.K.; Saini, R.; Adsul, M.; Mathur, A.; Gupta, R.; Tuli, D.K. Bioethanol production from wheat straw via enzymatic route employing Penicillium janthinellum cellulases. Bioresour. Technol. 2014, 169, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.L.; Margeot, A.; Blanquet, S.; Berrin, J.-G. Use of Cellulases from Trichoderma reesei in the Twenty-First Century—Part I: Current Industrial Uses and Future Applications in the Production of Second Ethanol Generation. Biotechnol. Biol. Trichoderma 2014, 245–261. [Google Scholar] [CrossRef]
- Pakarinen, A.; Haven, M.; Djajadi, D.; Várnai, A.; Puranen, T.; Viikari, L. Cellulases without carbohydrate-binding modules in high consistency ethanol production process. Biotechnol. Biofuels 2014, 7, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doi, R.H.; Kosugi, A. Cellulosomes: Plant-cell-wall-degrading enzyme complexes. Nat. Rev. Microbiol. 2004, 2, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Várnai, A.; Mäkelä, M.R.; Djajadi, D.T.; Rahikainen, J.; Hatakka, A.; Viikari, L. Carbohydrate-Binding Modules of Fungal Cellulases. Adv. Appl. Microbiol. 2014, 88, 103–165. [Google Scholar] [PubMed]
- Klein-Marcuschamer, D.; Oleskowicz-Popiel, P.; Simmons, B.A.; Blanch, H.W. The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol. Bioeng. 2012, 109, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Hai-Yan Sun, H.; Li, J.; Zhao, P.; Peng, M. Banana peel: A novel substrate for cellulase production under solid-state fermentation. Afr. J. Biotechnol. 2011, 10, 17887–17890. [Google Scholar] [CrossRef]
- Saravanan, P.; Muthuvelayudham, R.; Viruthagiri, T. Application of Statistical Design for the Production of Cellulase by Trichoderma reesei Using Mango Peel. Enzyme Res. 2012, 2012, 157643. [Google Scholar] [CrossRef] [PubMed]
- Sim, T.S.; Oh, J.C.S. Spent brewery grains as substrate for the production of cellulases by Trichoderma reesei QM9414. J. Ind. Microbiol. 1990, 5, 153–158. [Google Scholar] [CrossRef]
- Harris, A.D.; Ramalingam, C. Xylanases and its Application in Food Industry: A Review. J. Exp. Sci. 2010, 1, 1–11. [Google Scholar] [CrossRef]
- Knob, A.; Beitel, S.M.; Fortkamp, D.; Terrasan, C.R.F.; Almeida, A.F. de Production, Purification, and Characterization of a Major Penicillium glabrum Xylanase Using Brewer’s Spent Grain as Substrate. Biomed. Res. Int. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Polizeli, M.L.T.M.; Rizzatti, A.C.S.; Monti, R.; Terenzi, H.F.; Jorge, J.A.; Amorim, D.S. Xylanases from fungi: Properties and industrial applications. Appl. Microbiol. Biotechnol. 2005, 67, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Das, S.P.; Ravindran, R.; Ahmed, S.; Das, D.; Goyal, D.; Fontes, C.M.G.A.; Goyal, A. Bioethanol Production Involving Recombinant, C. thermocellum Hydrolytic Hemicellulase and Fermentative Microbes. Appl. Biochem. Biotechnol. 2012, 167, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Goswami, G.; Pathak, R. Microbial xylanases and their biomedical applications: A review. Int. J. Basic Clin. Pharmacol. 2013, 2, 237. [Google Scholar] [CrossRef]
- Lowe, S.E.; Theodorou, M.K.; Trinci, A.P. Cellulases and xylanase of an anaerobic rumen fungus grown on wheat straw, wheat straw holocellulose, cellulose, and xylan. Appl. Environ. Microbiol. 1987, 53, 1216–1223. [Google Scholar] [PubMed]
- Gawande, P.V.; Kamat, M.Y. Production of Aspergillus xylanase by lignocellulosic waste fermentation and its application. J. Appl. Microbiol. 1999, 87, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Botella, C.; Diaz, A.; de Ory, I.; Webb, C.; Blandino, A. Xylanase and pectinase production by Aspergillus awamori on grape pomace in solid state fermentation. Process Biochem. 2007, 42, 98–101. [Google Scholar] [CrossRef]
- Seyis, I.; Aksoz, N. Xylanase Production from Trichoderma harzianum 1073 D3 with Alternative Carbon and Nitrogen Sources. Food Technol. Biotechnol. 2005, 43, 37–40. [Google Scholar]
- Vandamme, E.J.; Derycke, D.G. Microbial inulinases: Fermentation process, properties, and applications. Adv. Appl. Microbiol. 1983, 29, 139–176. [Google Scholar] [PubMed]
- Zittan, L. Enzymatic Hydrolysis of Inulin—An Alternative Way to Fructose Production. Starch Stärke 1981, 33, 373–377. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yamini, D.; Ambika, V.; Sravya Sowdamini, N. Trends in inulinase production—A review. Crit. Rev. Biotechnol. 2009, 29, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Chi, Z.; Zhang, T.; Liu, G.; Yue, L. Inulinase-expressing microorganisms and applications of inulinases. Appl. Microbiol. Biotechnol. 2009, 82, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Kaur, N.; Singh, R. Fructose and inulinase production from waste Cichorium intybus roots. Biol. Wastes 1989, 29, 73–77. [Google Scholar] [CrossRef]
- Dilipkumar, M.; Rajasimman, M.; Rajamohan, N. Utilization of copra waste for the solid state fermentatative production of inulinase in batch and packed bed reactors. Carbohydr. Polym. 2014, 102, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Chesini, M.; Neila, L.P.; Fratebianchi de la Parra, D.; Rojas, N.L.; Contreras Esquivel, J.C.; Cavalitto, S.F.; Ghiringhelli, P.D.; Hours, R.A. Aspergillus kawachii produces an inulinase in cultures with yacon (Smallanthus sonchifolius) as substrate. Electron. J. Biotechnol. 2013, 16. [Google Scholar] [CrossRef] [Green Version]
- Mazutti, M.; Bender, P.; Treichel, H.; Luccio, M. Di Optimization of inulinase production by solid-state fermentation using sugarcane bagasse as substrate. Enzyme Microb. Technol. 2006, 39, 56–59. [Google Scholar] [CrossRef]
- Oosterveld, A.; Beldman, G.; Henk, A.S.; Voragen, G.J.A. Characterization of arabinose and ferulic acid rich pectic polysaccharides and hemicelluloses from sugar beet pulp. Carbohydr. Res. 2000, 328, 185–197. [Google Scholar] [CrossRef]
- Obeng, E.M.; Adam, S.N.N.; Budiman, C.; Ongkudon, C.M.; Maas, R.; Jose, J. Lignocellulases: A review of emerging and developing enzymes, systems, and practices. Bioresour. Bioprocess. 2017, 4, 16. [Google Scholar] [CrossRef]
- Guan, W.; Xu, G.; Duan, J.; Shi, S. Acetone–Butanol–Ethanol Production from Fermentation of Hot-Water-Extracted Hemicellulose Hydrolysate of Pulping Woods. Ind. Eng. Chem. Res. 2018, 57, 775–783. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Puri, N.; Sharma, P.; Gupta, N. Mannanases: Microbial sources, production, properties and potential biotechnological applications. Appl. Microbiol. Biotechnol. 2012, 93, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- McCleary, B.V.; Matheson, N.K. Action patterns and substrate-binding requirements of β-d-mannanase with mannosaccharides and mannan-type polysaccharides. Carbohydr. Res. 1983, 119, 191–219. [Google Scholar] [CrossRef]
- Gomes, J.; Terler, K.; Kratzer, R.; Kainz, E.; Steiner, W. Production of thermostable β-mannosidase by a strain of Thermoascus aurantiacus: Isolation, partial purification and characterization of the enzyme. Enzyme Microb. Technol. 2007, 40, 969–975. [Google Scholar] [CrossRef]
- Mamma, D.; Hatzinikolaou, D.G.; Christakopoulos, P. Biochemical and catalytic properties of two intracellular β-glucosidases from the fungus Penicillium decumbens active on flavonoid glucosides. J. Mol. Catal. B Enzym. 2004, 27, 183–190. [Google Scholar] [CrossRef]
- Dhawan, S.; Kaur, J. Microbial Mannanases: An Overview of Production and Applications. Crit. Rev. Biotechnol. 2007, 27, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.H.; Davidson, K.; Rixon, J.E.; Halstead, J.R.; Fransen, M.P.; Gilbert, H.J.; Hazlewood, G.P. A comparison of enzyme-aided bleaching of softwood paper pulp using combinations of xylanase, mannanase and alpha-galactosidase. Appl. Microbiol. Biotechnol. 2000, 53, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Christgau, S.; Andersen, L.; Kauppinen, S.; Heldt-Hansen, H.; Dalboege, H. Enzyme Exhibiting Mannanase Activity. Patent WO1994025576A1, 10 November 1994. [Google Scholar]
- Cuperus, R.A.; Herweijer, M.A.; Van Ooijen, A.J.; Van Schouwen, D.J. Cleaning Compositions Containing Plant Cell Wall Degrading Enzymes and Their Use in Cleaning Methods. U.S. Patent US6602842B2, 5 August 2003. [Google Scholar]
- Naganagouda, K.; Salimath, P.V.; Mulimani, V.H. Purification and characterization of endo-beta-1,4 mannanase from Aspergillus niger gr for application in food processing industry. J. Microbiol. Biotechnol. 2009, 19, 1184–1190. [Google Scholar] [PubMed]
- Yin, J.-S.; Liang, Q.-L.; Li, D.-M.; Sun, Z.-T. Optimization of production conditions for β-mannanase using apple pomace as raw material in solid-state fermentation. Ann. Microbiol. 2013, 63, 101–108. [Google Scholar] [CrossRef]
- Olaniyi, O.O.; Osunla, C.A.; Olaleye, O.O. Exploration of different species of orange peels for mannanase production. E3 J. Biotechnol. Pharm. Res. 2014, 5, 12–17. [Google Scholar]
- Rashid, J.I.A.; Samat, N.; Yusoff, W.M.W. Studies on Extraction of Mannanase Enzyme by Aspergillus terreus SUK-1 from Fermented Palm Kernel Cake. Pak. J. Biol. Sci. 2013, 16, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.M.; Lima, V.A.; Giloni-Lima, P.C.; Knob, A. Passion fruit peel as novel substrate for enhanced β-glucosidases production by Penicillium verruculosum: Potential of the crude extract for biomass hydrolysis. Biomass Bioenergy 2015, 72, 216–226. [Google Scholar] [CrossRef]
- Onilude, A.A.; Festus Fadahunsi, I.; Antia, E.; Garuba, E.O.; Inuwa, M.; Afaru, J. Characterization of Crude Alkaline β-mannosidase produced by Bacillus sp. 3A Isolated from Degraded Palm Kernel Cake. AU J. Technol. 2012, 15, 152–158. [Google Scholar]
- Duan, X.; Sun, X.; Wu, J. Optimization of fermentation conditions of recombinant Pichia pastoris that can produce β-galactosidase. Genom. Appl. Biol. 2014, 33, 1288–1293. [Google Scholar]
- Sadler, M.J.; Michèle, J. Foods, Nutrients and Food Ingredients with Authorised EU Health Claims; Elsevier Science: Amsterdam, The Netherlands, 2014; Volume 1, ISBN 9780857098481. [Google Scholar]
- Bonekamp, F.J.; Oosterom, J. On the safety of Kluyveromyces lactis? A review. Appl. Microbiol. Biotechnol. 1994, 41, 1–3. [Google Scholar] [CrossRef]
- Mahoney, R.R.; Nickerson, T.A.; Whitaker, J.R. Selection of Strain, Growth Conditions, and Extraction Procedures for Optimum Production of Lactase from Kluyveromyces fragilis. J. Dairy Sci. 1975, 58, 1620–1629. [Google Scholar] [CrossRef]
- Neri, D.F.; Balcão, V.M.; Costa, R.S.; CAP Rocha, I.; MFC Ferreira, E.; Torres, D.P.; Rodrigues, L.R.; Carvalho, L.B., Jr.; Teixeira, J.A. Galacto-oligosaccharides production during lactose hydrolysis by free Aspergillus oryzae b-galactosidase and immobilized on magnetic polysiloxane-polyvinyl alcohol. Food Chem. 2009, 115, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Papayannakos, N.; Markas, G.; Kekos, D. Studies on modelling and simulation of lactose hydrolysis by free and immobilized β-galactosidase from Aspergillus niger. Chem. Eng. J. 1993, 52, B1–B12. [Google Scholar] [CrossRef]
- de Bales, S.A.; Castillo, F.J. Production of Lactase by Candida pseudotropicalis Grown in Whey. Appl. Environ. Microbiol. 1979, 37, 1201–1205. [Google Scholar] [PubMed]
- Seyis, I.; Aksoz, N. Production of lactase by Trichoderma sp. Food Technol. Biotechnol. 2004, 42, 121–124. [Google Scholar]
- Macris, B.J.; Markakis, P. Characterization of Extracellular beta-d-Galactosidase from Fusarium moniliforme Grown in Whey. Appl. Environ. Microbiol. 1981, 41, 956–958. [Google Scholar] [PubMed]
- Akolkar, S.K.; Sajgure, A.; Lele, S.S. Lactase Production from Lactobacillus acidophilus. World J. Microbiol. Biotechnol. 2005, 21, 1119–1122. [Google Scholar] [CrossRef]
- Mustranta, A.; Karvonen, E.; Ojamo, H.; Linko, M. Production of mold lactase. Biotechnol. Lett. 1981, 3, 333–338. [Google Scholar] [CrossRef]
- Celestino, K.; Cunha, R.B.; Felix, C.R. Characterization of a beta-glucanase produced by Rhizopus microsporus var. microsporus, and its potential for application in the brewing industry. BMC Biochem. 2006, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-J.; He, G.-Q.; Chen, Q.-H.; Zhang, X.-Y.; Ali, M.A. Medium optimization for the production of thermal stable β-glucanase by Bacillus subtilis ZJF-1A5 using response surface methodology. Bioresour. Technol. 2004, 93, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Villettaz, J.-C.; Steiner, D.; Trogus, H. The Use of a Beta Glucanase as an Enzyme in Wine Clarification and Filtration. Am. J. Enol. Vitic. 1984, 35, 253–256. [Google Scholar]
- Yang, S.Q.; Xiong, H.; Yang, H.Y.; Yan, Q.J.; Jiang, Z.Q. High-level production of β-1,3-1,4-glucanase by Rhizomucor miehei under solid-state fermentation and its potential application in the brewing industry. J. Appl. Microbiol. 2015, 118, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.; Anwar, Z.; Afroz, A. Characterization of Exo 1, 4-β glucanase produced from Trichoderma Viridi through solid-state bio-processing of orange peel waste. Adv. Biosci. Biotechnol. 2012, 3, 580–584. [Google Scholar] [CrossRef]
- Christakopoulos, P.; Kekos, D.; Kolisis, F.N.; Macris, B.J. Controlling simultaneous production of endoglucanase and beta-glucosidase by Fusarium oxysporum in submerged culture. Biotechnol. Lett. 1995, 17, 883–888. [Google Scholar] [CrossRef]
- Sachslehner, A.; Nidetzky, B.; Kulbe, K.D.; Haltrich, D. Induction of Mannanase, Xylanase, and Endoglucanase Activities in Sclerotium rolfsii. Appl. Environ. Microbiol. 1998, 64, 594–600. [Google Scholar] [PubMed]
- Zampieri, D.; Guerra, L.; Camassola, M.; Dillon, A.J.P. Secretion of endoglucanases and β-glucosidases by Penicillium echinulatum 9A02S1 in presence of different carbon sources. Ind. Crops Prod. 2013, 50, 882–886. [Google Scholar] [CrossRef]
- Neumann, N.P.; Lampen, J.O. Purification and properties of yeast invertase. Biochemistry 1967, 6, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Kulshrestha, S.; Tyagi, P.; Sindhi, V.; Yadavilli, S. Invertase and its applications—A brief review. JOPR J. Pharm. Res. 2013, 7, 792–797. [Google Scholar] [CrossRef]
- Hang, Y.D.; Woodams, E.E. β-Fructofuranosidase production by Aspergillus species from apple pomace. LWT Food Sci. Technol. 1995, 28, 340–342. [Google Scholar] [CrossRef]
- Rashad, M.M.; Nooman, M.U. Production, Purification and Characterization of Extracellular Invertase from Saccharomyses Cerevisiae NRRL Y-12632 by Solid-State Fermentation of Red Carrot Residue. Aust. J. Basic Appl. Sci. 2009, 3, 1910–1919. [Google Scholar]
- Alegre, A.C.P.; Polizeli, M.d.L.T.d.M.; Terenzi, H.F.; Jorge, J.A.; Guimarães, L.H.S. Production of thermostable invertases by Aspergillus caespitosus under submerged or solid state fermentation using agroindustrial residues as carbon source. Braz. J. Microbiol. 2009, 40, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Uma, C.; Gomathi, D.; Ravikumar, G.; Kalaiselvi, M.; Palaniswamy, M. Production and properties of invertase from a Cladosporium cladosporioides in SmF using pomegranate peel waste as substrate. Asian Pac. J. Trop. Biomed. 2012, 2, S605–S611. [Google Scholar] [CrossRef]
- Sakai, T.; Sakamoto, T.; Hallaert, J.; Vandamme, E.J. Pectin, pectinase and protopectinase: Production, properties, and applications. Adv. Appl. Microbiol. 1993, 39, 213–294. [Google Scholar] [PubMed]
- Servili, M.; Begliomini, A.L.; Montedoro, G.; Petruccioli, M.; Federici, F. Utilisation of a yeast pectinase in olive oil extraction and red wine making processes. J. Sci. Food Agric. 1992, 58, 253–260. [Google Scholar] [CrossRef]
- Hours, R.A.; Voget, C.E.; Ertola, R.J. Some factors affecting pectinase production from apple pomace in solid-state cultures. Biol. Wastes 1988, 24, 147–157. [Google Scholar] [CrossRef]
- Raj Kashyap, D.; Kumar Soni, S.; Tewari, R. Enhanced production of pectinase by Bacillus sp. DT7 using solid state fermentation. Bioresour. Technol. 2003, 88, 251–254. [Google Scholar] [CrossRef]
- Silva, D.; Tokuioshi, K.; da Silva Martins, E.; Da Silva, R.; Gomes, E. Production of pectinase by solid-state fermentation with Penicillium viridicatum RFC3. Process Biochem. 2005, 40, 2885–2889. [Google Scholar] [CrossRef]
- Almeida, C.; Brányik, T.; Moradas-Ferreira, P.; Teixeira, J. Continuous production of pectinase by immobilized yeast cells on spent grains. J. Biosci. Bioeng. 2003, 96, 513–518. [Google Scholar] [CrossRef]
- Patil, S.R.; Dayanand, A. Production of pectinase from deseeded sunflower head by Aspergillus niger in submerged and solid-state conditions. Bioresour. Technol. 2006, 97, 2054–2058. [Google Scholar] [CrossRef] [PubMed]
- Riddhi Sawant; Saraswathy Nagendran Protease: An enzyme with multiple Industrial Applications. World J. Pharm. Pharm. Sci. 2014, 3, 568–579.
- Gupta, R.; Beg, Q.; Lorenz, P. Bacterial alkaline proteases: Molecular approaches and industrial applications. Appl. Microbiol. Biotechnol. 2002, 59, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Pillai, P.; Mandge, S.; Archana, G. Statistical optimization of production and tannery applications of a keratinolytic serine protease from Bacillus subtilis P13. Process Biochem. 2011, 46, 1110–1117. [Google Scholar] [CrossRef]
- Radha, S.; Nithya, V.J.; Himakiran Babu, R.; Sridevi, A.; Prasad, N.; Narasimha, G. Production and optimization of acid protease by Aspergillus spp under submerged fermentation. Arch. Appl. Sci. Res. 2011, 3, 155–163. [Google Scholar]
- do Nascimento, R.P.; Junior, N.A.; Coelho, R.R.R. Brewer’s spent grain and corn steep liquor as alternative culture medium substrates for proteinase production by Streptomyces malaysiensis AMT-3. Braz. J. Microbiol. 2011, 42, 1384–1389. [Google Scholar] [CrossRef] [PubMed]
- Chancharoonpong, C.; Hsieh, P.-C.; Sheu, S.-C. Enzyme Production and Growth of Aspergillus oryzae S. on Soybean Koji Fermentation. APCBEE Procedia 2012, 2, 57–61. [Google Scholar] [CrossRef]
- Belmessikh, A.; Boukhalfa, H.; Mechakra-Maza, A.; Gheribi-Aoulmi, Z.; Amrane, A. Statistical optimization of culture medium for neutral protease production by Aspergillus oryzae. Comparative study between solid and submerged fermentations on tomato pomace. J. Taiwan Inst. Chem. Eng. 2013, 44, 377–385. [Google Scholar] [CrossRef]
- Veerabhadrappa, M.B.; Shivakumar, S.B.; Devappa, S. Solid-state fermentation of Jatropha seed cake for optimization of lipase, protease and detoxification of anti-nutrients in Jatropha seed cake using Aspergillus versicolor CJS-98. J. Biosci. Bioeng. 2014, 117, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Misiewicz, A. Microbial transglutaminase and its application in the food industry. A review. Folia Microbiol. 2014, 59, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Motoki, M.; Seguro, K. Transglutaminase and its use for food processing. Trends Food Sci. Technol. 1998, 9, 204–210. [Google Scholar] [CrossRef]
- Xian, L.; Wang, F.; Luo, X.; Feng, Y.-L.; Feng, J.-X. Purification and Characterization of a Highly Efficient Calcium-Independent α-Amylase from Talaromyces pinophilus 1–95. PLoS ONE 2015, 10, e0121531. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.N.; Bech, L.; Halkier, T.; Kauppinen, M.S.; Okada, M.N.N.B.L.; Rasmussen, G.; Sandal, T. Transglutaminases from Oomycetes. Patent EP0871712B1, 27 August 2003. [Google Scholar]
- Noda, S.; Miyazaki, T.; Tanaka, T.; Chiaki, O.; Kondo, A. High-level production of mature active-form Streptomyces mobaraensis transglutaminase via pro-transglutaminase processing using Streptomyces lividans as a host. Biochem. Eng. J. 2013, 74, 76–80. [Google Scholar] [CrossRef]
- Cortez, J.; Bonner, P.L.; Griffin, M. Application of transglutaminases in the modification of wool textiles. Enzyme Microb. Technol. 2004, 34, 64–72. [Google Scholar] [CrossRef]
- de Souza, C.F.V.; Rodrigues, R.C.; Heck, J.X.; Ayub, M.A.Z. Optimization of transglutaminase extraction produced by Bacillus circulans BL32 on solid-state cultivation. J. Chem. Technol. Biotechnol. 2008, 83, 1306–1313. [Google Scholar] [CrossRef]
- Téllez-Luis, S.J.; González-Cabriales, J.J.; Ramírez, J.A.; Vázquez, M. Production of Transglutaminase by Streptoverticillium ladakanum NRRL-3191 Using Glycerol as Carbon Source. Food Technol. Biotechnol. 2004, 42, 75–81. [Google Scholar]
- Aravindan, R.; Anbumathi, P.; Viruthagiri, T. Lipase applications in food industry. Indian J. Biotechnol. 2007, 6, 141–158. [Google Scholar]
- Fernandez-Lafuente, R. Lipase from Thermomyces lanuginosus: Uses and prospects as an industrial biocatalyst. J. Mol. Catal. B Enzym. 2010, 62, 197–212. [Google Scholar] [CrossRef]
- Mohammadi, M.; Habibi, Z.; Dezvarei, S.; Yousefi, M.; Samadi, S.; Ashjari, M. Improvement of the stability and selectivity of Rhizomucor miehei lipase immobilized on silica nanoparticles: Selective hydrolysis of fish oil using immobilized preparations. Process Biochem. 2014, 49, 1314–1323. [Google Scholar] [CrossRef]
- Prasad, M.P.; Manjunath, K. Comparative study on biodegradation of lipid-rich wastewater using lipase producing bacterial species. Indian J. Biotechnol. 2011, 10, 121–124. [Google Scholar]
- Salihu, A.; Bala, M.; Alam, M.Z. Lipase production by Aspergillus niger using sheanut cake: An optimization study. J. Taibah Univ. Sci. 2016, 10, 850–859. [Google Scholar] [CrossRef]
- Kanmani, P.; Kumaresan, K.; Aravind, J. Utilization of coconut oil mill waste as a substrate for optimized lipase production, oil biodegradation and enzyme purification studies in Staphylococcus pasteuri. Electron. J. Biotechnol. 2015, 18, 20–28. [Google Scholar] [CrossRef]
- Suzuki, U.; Yoshimura, K.; Takaishi, M. About the enzyme “phytase” which splits “anhydro-oxy-methylene diphosphoric acid”. Coll. Agric. Bull. Tokyo Imp. Univ. 1907, 7, 495–512. [Google Scholar] [CrossRef]
- Joshi, J.B. Phytase-A Key to Unlock Phytate Complex. Int. J. Pure App. Biosci. 2014, 2, 304–313. [Google Scholar]
- Selle, P.H.; Ravindran, V. Microbial phytase in poultry nutrition. Anim. Feed Sci. Technol. 2007, 135, 1–41. [Google Scholar] [CrossRef]
- Papagianni, M.; Nokes, S.E.; Filer, K. Production of phytase by Aspergillus niger in submerged and solid-state fermentation. Process Biochem. 1999, 35, 397–402. [Google Scholar] [CrossRef]
- Bajaj, B.K.; Wani, M.A. Enhanced phytase production from Nocardia sp. MB 36 using agro-residues as substrates: Potential application for animal feed production. Eng. Life Sci. 2011, 11, 620–628. [Google Scholar] [CrossRef]
- Mittal, A.; Singh, G.; Goyal, V.; Yadav, A.; Aggarwal, N.K. Production of phytase by acido-thermophilic strain of Klebsiella sp. DB-3FJ711774.1 using orange peel flour under submerged fermentation. Innov. Rom. Food Biotechnol. 2012, 10, 18–27. [Google Scholar]
- El-Batal, A.I.; ElKenawy, N.M.; Yassin, A.S.; Amin, M.A. Laccase production by Pleurotus ostreatus and its application in synthesis of gold nanoparticles. Biotechnol. Rep. 2015, 5, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H. LXIII.—Chemistry of lacquer (Urushi). Part I. Communication from the Chemical Society of Tokio. J. Chem. Soc. Trans. 1883, 43, 472–486. [Google Scholar] [CrossRef]
- Mate, D.M.; Alcalde, M. Laccase engineering: From rational design to directed evolution. Biotechnol. Adv. 2015, 33, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Kumar, S. Cyanobacterial biomass as N-supplement to agro-waste for hyper-production of laccase from Pleurotus ostreatus in solid state fermentation. Process Biochem. 2007, 42, 681–685. [Google Scholar] [CrossRef]
- Rodríguez Couto, S.; Toca Herrera, J.L. Industrial and biotechnological applications of laccases: A review. Biotechnol. Adv. 2006, 24, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Shraddha; Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial Sources, Production, Purification, and Potential Biotechnological Applications. Enzyme Res. 2011, 2011, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, M.; Moldes, D.; Rodríguez Couto, S.; Sanromán, A. Improving laccase production by employing different lignocellulosic wastes in submerged cultures of Trametes versicolor. Bioresour. Technol. 2002, 82, 109–113. [Google Scholar] [CrossRef]
- Couto, S.R.; Sanromán, M.Á. Effect of two wastes from groundnut processing on laccase production and dye decolourisation ability. J. Food Eng. 2006, 73, 388–393. [Google Scholar] [CrossRef]
- Osma, J.F.; Toca Herrera, J.L.; Rodríguez Couto, S. Banana skin: A novel waste for laccase production by Trametes pubescens under solid-state conditions. Application to synthetic dye decolouration. Dye. Pigment. 2007, 75, 32–37. [Google Scholar] [CrossRef]
- García-Alvarez, B.; Melero, R.; Dias, F.M.; Prates, J.A.; Fontes, C.M.; Smith, S.P.; Romão, M.J.; Carvalho, A.L.; Llorca, O. Molecular architecture and structural transitions of a Clostridium thermocellum mini-cellulosome. J. Mol. Biol. 2011, 407, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Union, E. Boosting Lignocellulose Biomass Deconstruction with Designer Cellulosomes for Industrial Applications; European Commission: Brussels, Belgium, 2017; Available online: https://cordis.europa.eu/project/rcn/110786_en.html (accessed on 22 October 2018).
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Erickson, L.E.; Selga, S.E.; Viesturs, U.E. Application of mass and energy balance regularities to product formation. Biotechnol. Bioeng. 1978, 20, 1623–1638. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Chen, H. Correlations of medium physical properties and process performance in solid-state fermentation. Chem. Eng. Sci. 2017, 165, 65–73. [Google Scholar] [CrossRef]
| Agro-Industry Residue | Carbohydrates | Crude Fibre | Ash | Pectin | Fat | Protein | Lignin | Ref. |
|---|---|---|---|---|---|---|---|---|
| Sugarcane Bagasse | 66.48 ± 2.68 | - | 8.80 ± 0.02 | - | - | 2.3 | 17.79 ± 0.62 | [8] |
| Rice Bran | 14.1 ± 1.1 | 26.9 | 3.4–8.1 | - | 30.4 ± 0.9 | 38.2 ± 2.3 | 25.63 | [9] |
| Wheat Bran | 56.8 | 33.4–63.0 | 3.9–8.10 | 3.5–3.9 | 13.2–18.4 | 5.6 | [10] | |
| Spent Coffee Waste | 55.53 ± 0.85 | 60.46 ± 2.2 | 1.30 ± 0.10 | - | 2.29 ± 0.30 | 17.44 ± 0.10 | 23.90 ± 0.30 | [11] |
| Brewer’s spent grain | 79.9 ± 0.5 | 3.3 ± 0.1 | 7.9 ± 0.1 | - | 0.0 ± 0.0 | 2.4 ± 0.2 | 30.48 ± 0.8 | [12] |
| Cassava peel | 75.5 ± 1.2 | 11.2 ± 0.6 | 2.4 ± 0.2 | - | 3.1 ± 0.1 | 1.7 ± 0.1 | 1.92 ± 0.07 | [13] |
| Apple Pomace | 48.0–62.0 | - | 4.7–51.1 | - | - | 3.9–5.7 | 23.5 | [14] |
| Crude Olive Pomace | 34.8 ± 0.9 | - | 6.6 ± 0.5 | - | 16.65 ± 0.09 | 0.4 ± 1.0 | 43.2 ± 0.5 | [15] |
| Banana peel | 79.0 ± 0.5 | 9.3 ± 0.1 | 2.7 ± 0.0 | - | 3.0 ± 0.2 | 0.6 ± 0.1 | 6.4–9.6 | [13] |
| Citrus peel | 30 | - | 1.7 | 14.4 | - | 7.9 | 1.0 | [16] |
| Brand Name | Product | Quantity | Price (in €) |
|---|---|---|---|
| 1,4-α-d-Glucan glucohydrolase AMG 300L™ Exo-1,4-α-glucosidase Glucoamylase | Amyloglucosidase from Aspergillus niger ≥260 U/mL, aqueous solution | 50 mL | 127.00 |
| 1,4-α-d-Glucan-glucanohydrolase Fungamyl® 800 L | α-Amylase from Aspergillus oryzae aqueous solution, ≥800 FAU/g | 50 mL | 95.00 |
| 1,6-α-d-Glucan 6-glucanohydrolase Dextranase Plus L | Dextranase from Chaetomium erraticum | 50 mL | 85.50 |
| D-xylose ketol-isomerase Sweetzyme® IT Extra | Glucose Isomerase from Streptomyces murinus ≥350 U/g | 50 G | 248.00 |
| Carezyme 1000L® | Cellulase from Aspergillus sp. aqueous solution | 50 mL | 92.50 |
| Lactase Lactozyme® 2600 L | β-Galactosidase from Kluyveromyces lactis ≥2600 units/g | 50 mL | 96.50 |
| Pectinex Ultra Clear® | Pectinase from Aspergillus aculeatus | 50 mL | 71.00 |
| Pentopan Mono BG® | Xylanase powder, ≥2500 units/g, recombinant, expressed in Aspergillus oryzae | 50 G | 297.00 |
| Promozyme® D2 Pullulanase microbial | Pullulanase microbial | 50 mL | 78.50 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravindran, R.; Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. A Review on Bioconversion of Agro-Industrial Wastes to Industrially Important Enzymes. Bioengineering 2018, 5, 93. https://doi.org/10.3390/bioengineering5040093
Ravindran R, Hassan SS, Williams GA, Jaiswal AK. A Review on Bioconversion of Agro-Industrial Wastes to Industrially Important Enzymes. Bioengineering. 2018; 5(4):93. https://doi.org/10.3390/bioengineering5040093
Chicago/Turabian StyleRavindran, Rajeev, Shady S. Hassan, Gwilym A. Williams, and Amit K. Jaiswal. 2018. "A Review on Bioconversion of Agro-Industrial Wastes to Industrially Important Enzymes" Bioengineering 5, no. 4: 93. https://doi.org/10.3390/bioengineering5040093
APA StyleRavindran, R., Hassan, S. S., Williams, G. A., & Jaiswal, A. K. (2018). A Review on Bioconversion of Agro-Industrial Wastes to Industrially Important Enzymes. Bioengineering, 5(4), 93. https://doi.org/10.3390/bioengineering5040093