Application of NiTi in Assistive and Rehabilitation Devices: A Review
Abstract
:1. Introduction
2. SMAs in Assistive Devices
2.1. Prosthesis
2.1.1. Y. Nakano
2.1.2. K. Laurentis and C. Mavroidis
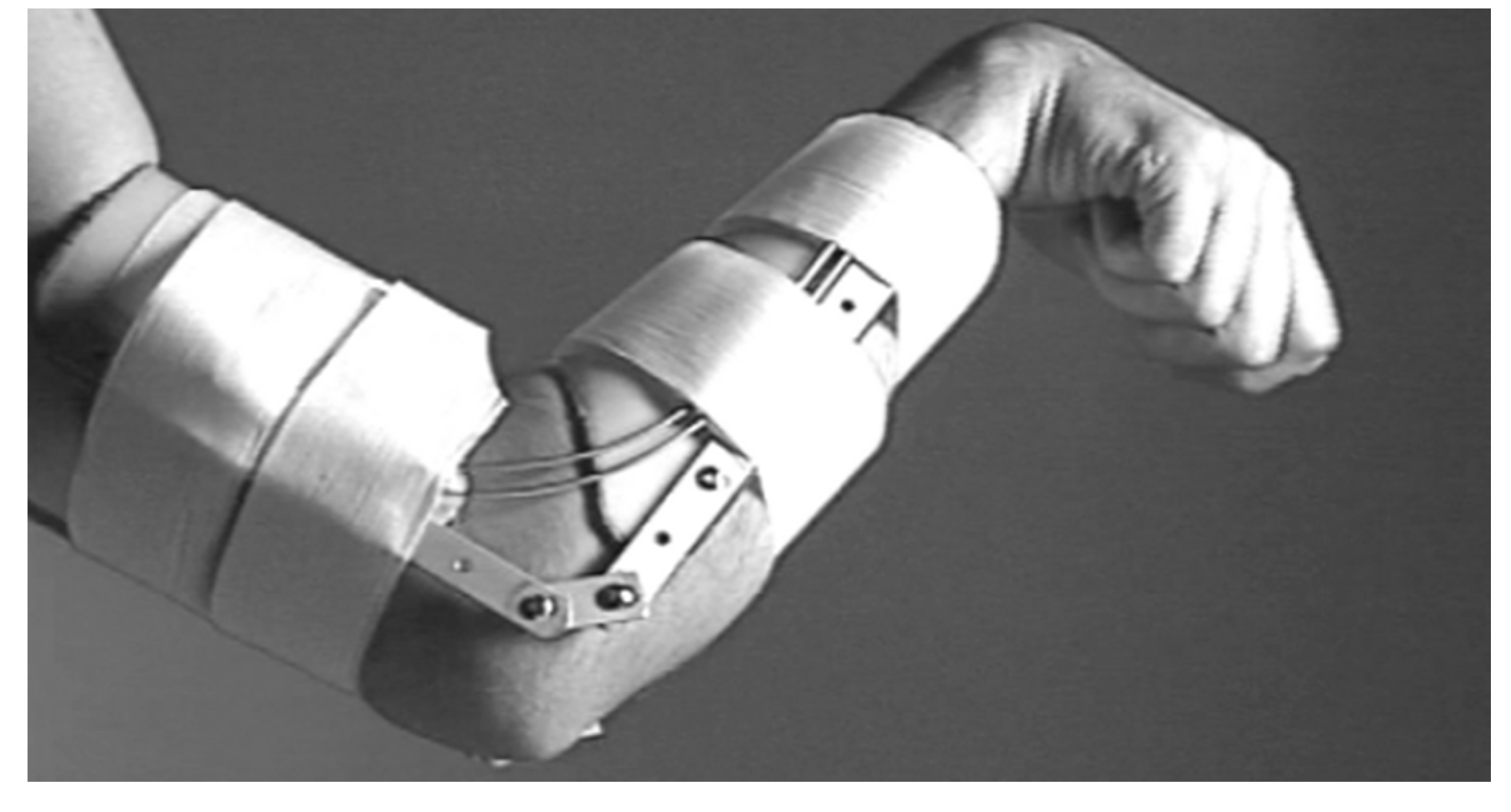
2.1.3. K. Andrianesis and A. Tzes
2.1.4. H. Taniguchi
2.1.5. Jae H. Lee et al.
2.2. Orthosis
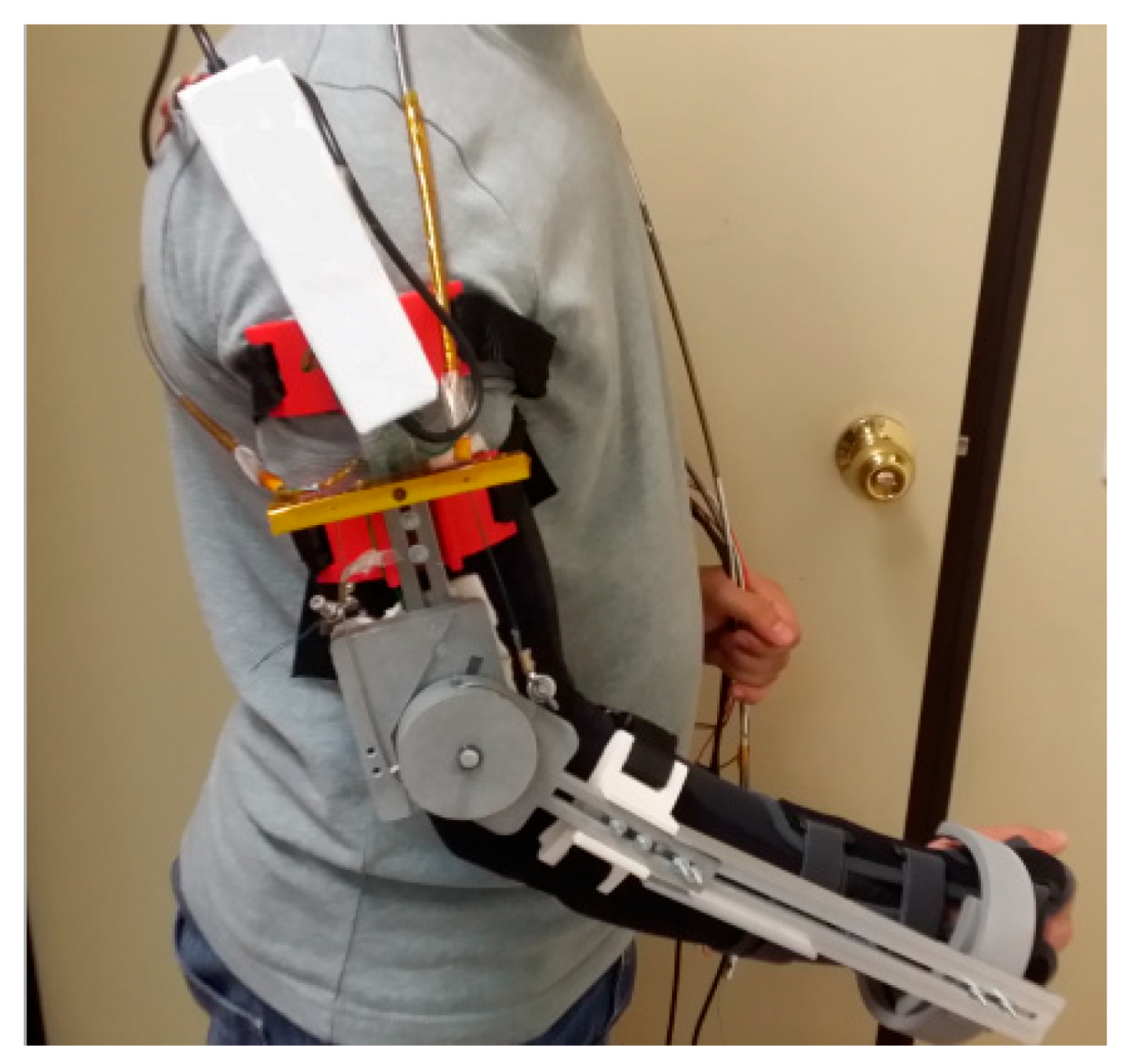
2.2.1. SMA Orthotic Devices for Elbow
Pittaccio et. al.
Copaci et al.
Hope et al.
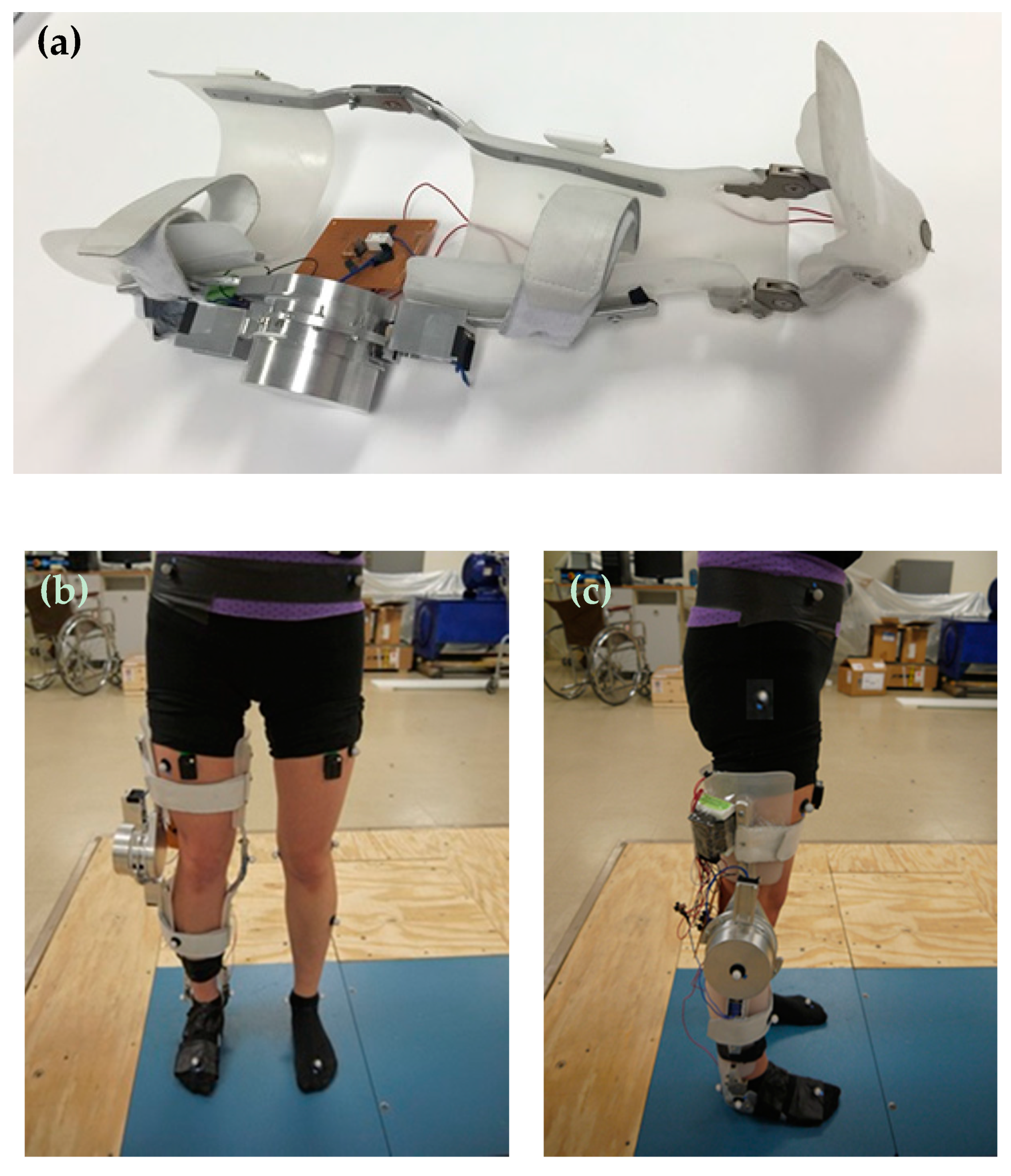
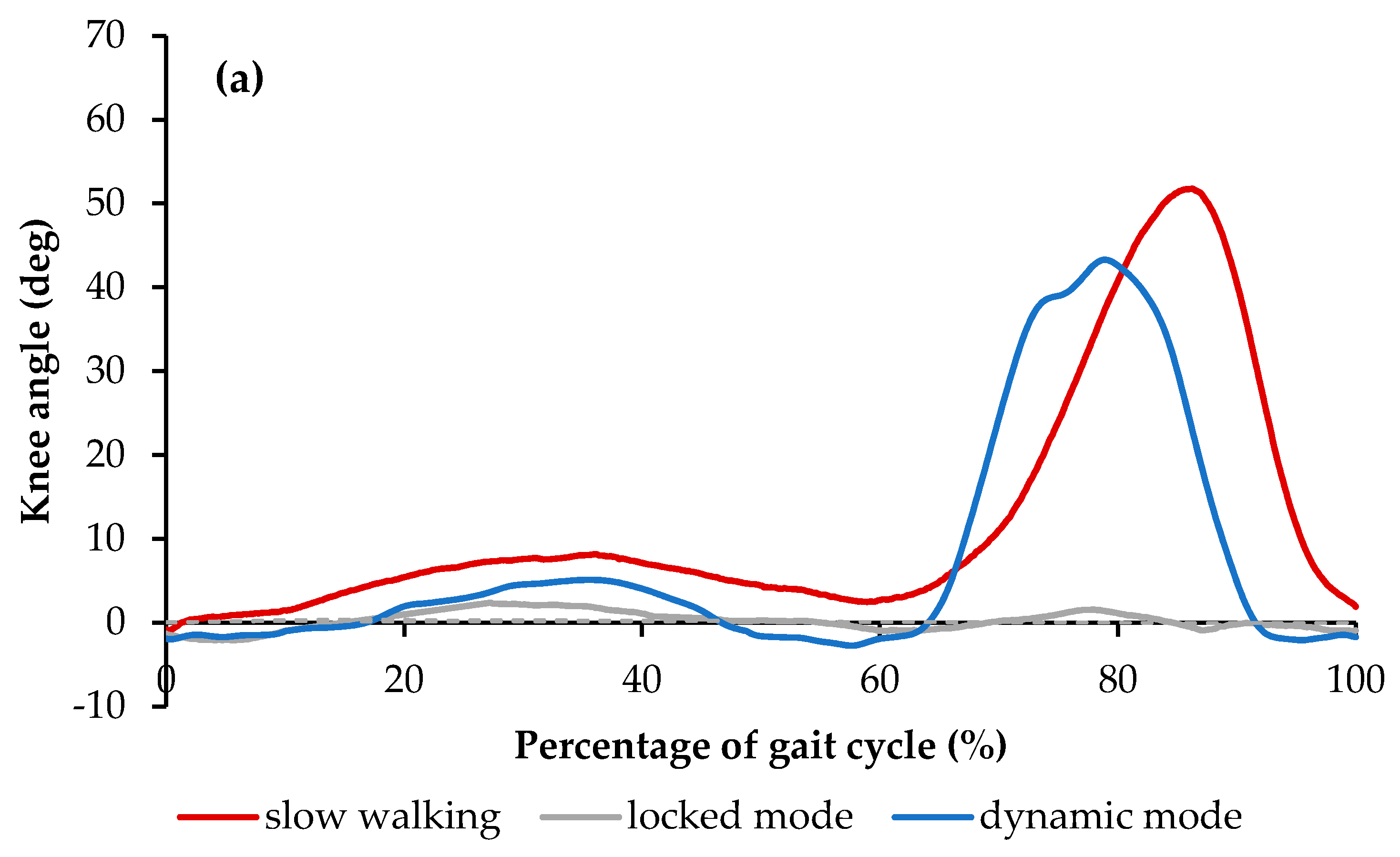
2.2.2. SMA Orthotic Devices for Knee
Feng et al.
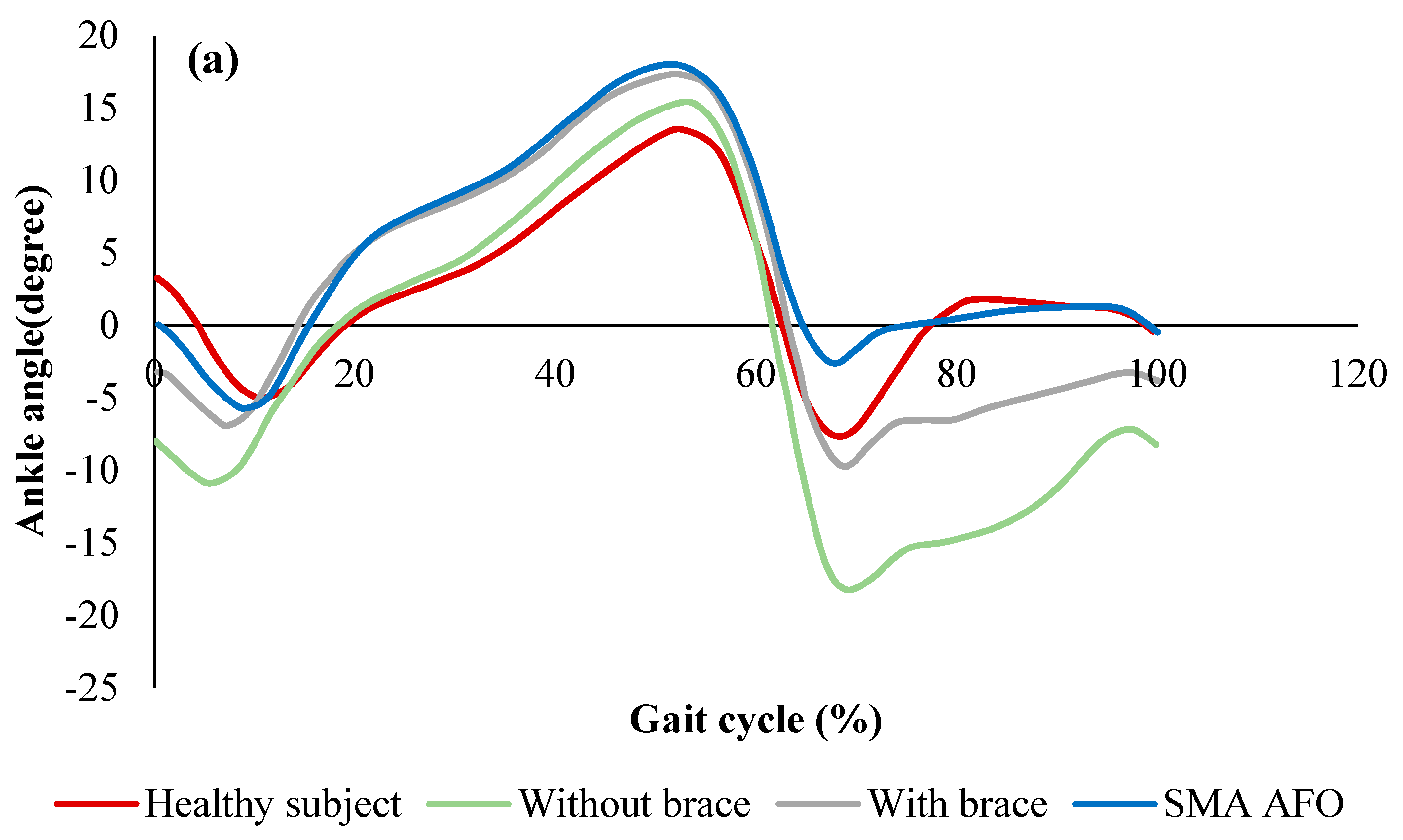
2.2.3. SMA Orthotic Devices for Ankle
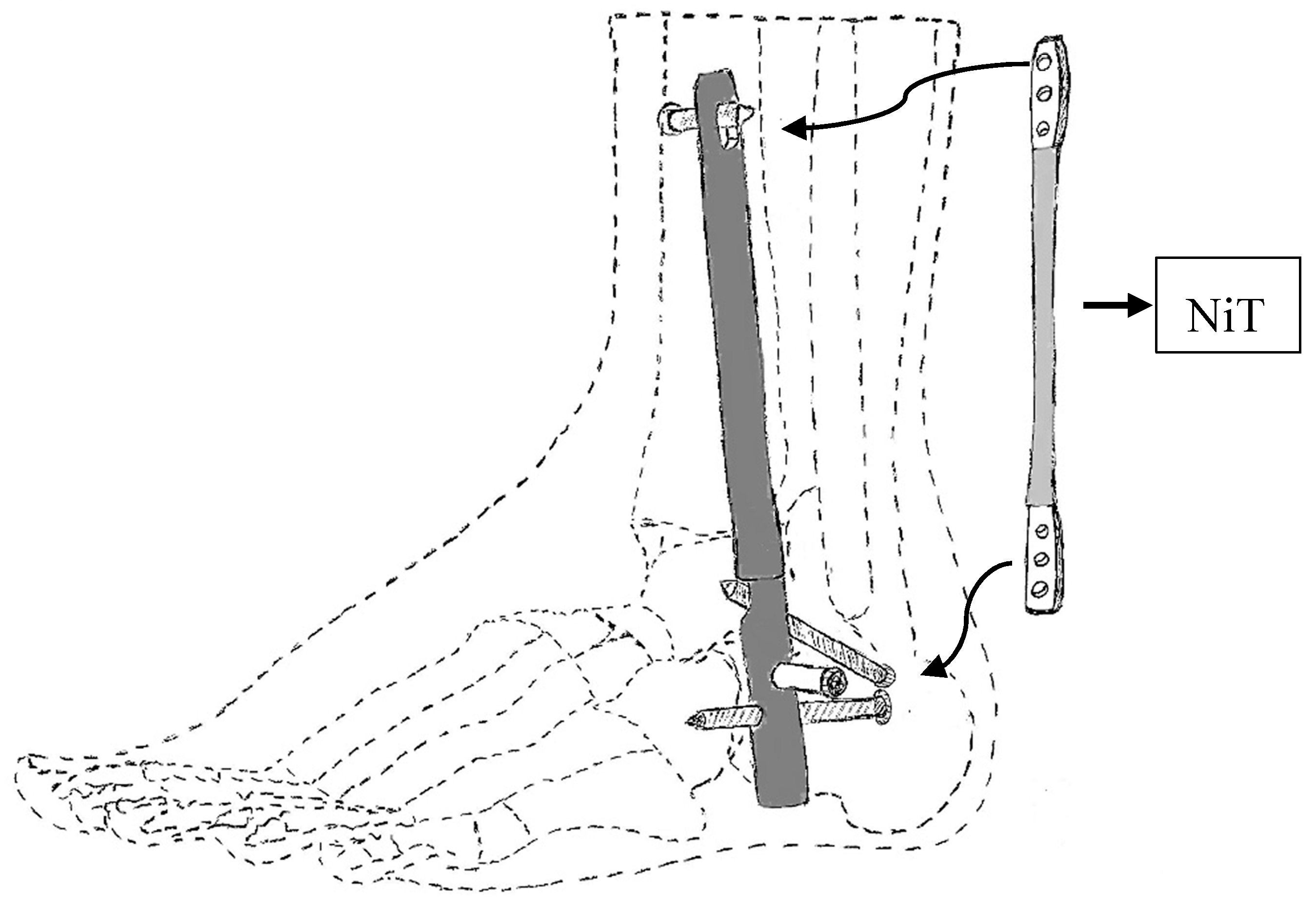
Bhadane-Deshpande et al.
Deberg et al.
Mataee et al.
Amerinatanzi et al.
2.2.4. Orthosis for Correcting Deformities and Abnormal Postures
2.2.5. SMAs in Rehabilitation Devices
Pittaccio et al.
Pittaccio et al.
Krishnan et al.
3. Discussion
3.1. Prosthetics
- SMA wires need time to be cooled; hence the cooling rate greatly affects the efficiency of these type of actuation. There have been different methods like using forced cooling mechanisms or metallic heat sinks to improve the efficiency of the cooling. However, it should be noted that adding these items can influence the controlling issues as well as increasing weight and the need for space.
- One of the most difficulties of employing SMA as an actuator is its control complexity. Large temperature hysteresis, nonlinearities, lack of a reliable feedback signal due to changing SMA parameters are some of the parameters that make it hard to control this device. Many papers have been published on the SMA control methods that can address this issue and applying these methods on prosthesis hands can improve position and force control of these devices. For example, position control and stiffness control methods using strategies like nonlinear or adaptive controls, have been employed to control SMA-based actuators. Due to the lack of an accurate model for SMA based actuators and the presence of various uncertainties in the considered model, some researchers prefer to use non-model-based control methods [62,63]. The control method used in reference [62] is based on the several empirical rules while reference [63] is founded based on two-stage fuzzy controllers.
- Another interesting field of research that can be conducted for enhancing the hand prosthesis performance is to investigate the benefit of employing different kinds of SMA shapes like SMA springs or a bundle of wires. Also, a parametric study is needed on the length, diameter and the transformation temperature of SMA wires and their effect on the actuator performance.
3.2. Orthotics
3.3. Rehabilitation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, P.; Lagoudas, D. Introduction to Shape Memory Alloys. In Shape Memory Alloys; Springer Nature: Basingstoke, UK, 2008; Volume 1, pp. 1–51. [Google Scholar]
- Nematollahi, M.; Mehrabi, R.; Callejas, M.A.; Elahinia, H.; Elahinia, M. A two-way architectural actuator using NiTi SE wire and SME spring. Act. Passiv. Smart Struct. Integr. Syst. XII 2018, 10595, 105952J. [Google Scholar]
- Hartl, D.J.; Lagoudas, D.C.; Lagoudas, D. Aerospace applications of shape memory alloys. Proc. Inst. Mech. Eng. N.a. G: J. Aerosp. Eng. 2007, 221, 535–552. [Google Scholar] [CrossRef] [Green Version]
- Williams, E.A.; Shaw, G.; Elahinia, M. Control of an automotive shape memory alloy mirror actuator. Mechatronics 2010, 20, 527–534. [Google Scholar] [CrossRef]
- Parsai, E.I.; Jahadakbar, A.; Lavvafi, H.; Elahinia, M. A novel and innovative device to retract rectum during radiation therapy of pelvic tumors. J. Appl. Clin. Med. Phys. 2019, 20, 194–199. [Google Scholar] [CrossRef]
- Elahinia, M.H. Shape Memory Alloy Actuators: Design, Fabrication, and Experimental Evaluation; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Lagoudas, D.C. Shape Memory Alloys: Modeling and Engineering Applications; Springer Science & Business Media: Berlin, Germany, 2008. [Google Scholar]
- Saghaian, S.E.; Amerinatanzi, A.; Moghaddam, N.S.; Majumdar, A.; Nematollahi, M.; Saedi, S.; Elahinia, M.; Karaca, H.E. Mechanical and shape memory properties of triply periodic minimal surface (TPMS) NiTi structures fabricated by selective laser melting. Boil. Eng. Med. 2018, 3, 1–7. [Google Scholar]
- Elahinia, M.; Moghaddam, N.S.; Andani, M.T.; Amerinatanzi, A.; Bimber, B.A.; Hamilton, R.F. Fabrication of NiTi through additive manufacturing: A review. Prog. Mater. Sci. 2016, 83, 630–663. [Google Scholar] [CrossRef] [Green Version]
- Walker, J.M.; Haberland, C.; Andani, M.T.; Karaca, H.E.; Dean, D.; Elahinia, M. Process development and characterization of additively manufactured nickel–titanium shape memory parts. J. Intell. Mater. Syst. Struct. 2016, 27, 2653–2660. [Google Scholar] [CrossRef]
- Saedi, S.; Turabi, A.S.; Andani, M.T.; Haberland, C.; Karaca, H.; Elahinia, M. The influence of heat treatment on the thermomechanical response of Ni-rich NiTi alloys manufactured by selective laser melting. J. Alloy. Compd. 2016, 677, 204–210. [Google Scholar] [CrossRef]
- Petrini, L.; Migliavacca, F. Biomedical Applications of Shape Memory Alloys. J. Met. 2011, 2011, 1–15. [Google Scholar] [CrossRef]
- Caldwell, D.G.; Tsagarakis, N. Biomimetic actuators in prosthetic and rehabilitation applications. Technol. Heal. Care 2002, 10, 107–120. [Google Scholar]
- Nakano, Y.; Fujie, M.; Hosada, Y. Hitachi robot Hand. Robot. Age 1984, 6, 18–20. [Google Scholar]
- De Laurentis, K.J.; Mavroidis, C. Mechanical design of a shape memory alloy actuated prosthetic hand. Technol. Heal. Care 2002, 10, 91–106. [Google Scholar]
- Mavroidis, C.; Pfeiffer, C.; DeLaurentis, K.J.; Mosley, M.J. Prosthetic, orthotic, and other rehabilitative robotic assistive devices actuated by smart materials. U.S. Patent 6,379,393, 30 April 2002. [Google Scholar]
- Pfeiffer, C.; DeLaurentis, K.; Mavroidis, C. Shape memory alloy actuated robot prostheses: Initial experiments. In Proceedings of the IEEE International Conference on Robotics and Automation, Detroit, MI, USA, 10–15 May 1999; pp. 2385–2391. [Google Scholar]
- DeLaurentis, K.J.; Mavroidis, C.; Pfeiffer, C. Development of a shape memory alloy actuated robotic hand. In Proceedings of the 7th International Conference on New Actuators (ACTUATOR 2000), Bremen, Germany, 19–21 June 2000; pp. 19–21. [Google Scholar]
- DeLaurentis, K.J.; Won, J.; Alam, M.; Mavroidis, C. Fabrication of Non-Assembly Mechanisms and Robotic Systems Using Rapid Prototyping. J. Mech. 2001, 123, 516–524. [Google Scholar] [Green Version]
- Andrianesis, K.; Tzes, A. Design of an anthropomorphic prosthetic hand driven by Shape Memory Alloy actuators. In Proceedings of the EMBS International Conference on Biomedical Robotics and Biomechatronics, Scottsdale, AZ, USA, 19–22 October 2008; pp. 517–522. [Google Scholar]
- Andrianesis, K.; Tzes, A. Development and control of a multifunctional prosthetic hand with shape memory alloy actuators. J. Intell. Robot. Syst. 2015, 78, 257–289. [Google Scholar] [CrossRef]
- Andrianesis, K.; Koveos, Y.; Nikolakopoulos, G.; Tzes, A. Experimental Study of a Shape Memory Alloy Actuation System for a Novel Prosthetic Hand. In Shape Memory Alloys; Sciyo: Rijeka, Croatia, 2010. [Google Scholar] [Green Version]
- Andrianesis, K.; Tzes, A. Design of an innovative prosthetic hand with compact shape memory alloy actuators. In Proceedings of the 21st Mediterranean Conference on Control and Automation, Chania, Greece, 25–28 June 2013; pp. 697–702. [Google Scholar]
- Taniguchi, H. Flexible Artificial Muscle Actuator Using Coiled Shape Memory Alloy Wires. APCBEE Procedia 2013, 7, 54–59. [Google Scholar] [CrossRef] [Green Version]
- TanDiguchi, H.; Hashimoto, A.; Izuhara, S. Design of a Functional Prosthetic Hand for Children using Novel Shape Memory Alloy Actuators. Int. J. Innov. Eng. Technol. 2014, 2014, 57–63. [Google Scholar]
- Matsubara, S.; Okamoto, S.; Lee, J.H. Prosthetic hand using shape memory alloy type artificial muscle. In Proceedings of the International MultiConference of Engineers and Computer Scientists, Hong Kong, China, 14–16 March 2012; Volume 2. [Google Scholar]
- Lee, J.H.; Okamoto, S.; Matsubara, S. Development of multi-fingered prosthetic hand using shape memory alloy type artificial muscle. Comput. Technol. Appl. 2012, 3, 7. [Google Scholar]
- Viscuso, S.; Pittaccio, S.; Caimmi, M.; Gasperini, G.; Pirovano, S.; Villa, E.; Besseghini, S.; Molteni, F. Pseudoelastic Nitinol-Based Device for Relaxation of Spastic Elbow in Stroke Patients. J. Mater. Eng. Perform. 2009, 18, 805–813. [Google Scholar] [CrossRef]
- Viscuso, S.; Pittaccio, S.; Caimmi, M.; Gasperini, G.; Pirovano, S.; Besseghini, S.; Molteni, F. Pseudoelastic alloy devices for spastic elbow relaxation. In Proceedings of the 3rd Kuala Lumpur International Conference on Biomedical Engineering, Kuala Lumpur, Malaysiam, 11–14 December 2016; Volume 23, pp. 1584–1587. [Google Scholar]
- Pittaccio, S.; Viscuso, S. The use of muscle ‘creep’ as opposed to relaxation in stretching braces: A pseudoelastic device. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Munich, Germany, 7–12 September 2009; pp. 178–181. [Google Scholar]
- Pittaccio, S.; Viscuso, S. Customizable neuro-mechanical model of a hemiplegic elbow interacting with a pseudoelastic dynamic orthosis. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Munich, Germany, 7–12 September 2009; pp. 182–185. [Google Scholar]
- Pittaccio, S.; Garavaglia, L.; Ceriotti, C.; Passaretti, F.; Petrini, L. Applications of Shape Memory Alloys for Neurology and Neuromuscular Rehabilitation. J. Funct. Biomater. 2015, 6, 328–344. [Google Scholar] [CrossRef] [Green Version]
- Pittaccio, S.; Garavaglia, L.; Viscuso, S.; Beretta, E.; Strazzer, S. Implementation, Testing and Pilot Clinical Evaluation of Superelastic Splints that Decrease Joint Stiffness. Ann. Biomed. Eng. 2013, 41, 2003–2017. [Google Scholar] [CrossRef]
- Copaci, D.; Flores, A.; Rueda, F.; Alguacil, I.; Blanco, D.; Moreno, L. Wearable elbow exoskeleton actuated with shape memory alloy. In Converging Clinical and Engineering Research on Neurorehabilitation II; Springer: Berlin, Germany, 2017; pp. 477–481. [Google Scholar]
- Copaci, D.S.; Moreno, L.; Blanco, D. Wearable elbow exoskeleton actuated with shape memory alloy in antagonist movement. In Proceedings of the Joint Workshop on Wearable Robotics and Assistive Devices, International Conference on Intelligent Robots and Systems, Daejeon, Korea, 9–14 October 2016. [Google Scholar]
- Hope, J.; McDaid, A. Development of Wearable Wrist and Forearm Exoskeleton with Shape Memory Alloy Actuators. J. Intell. Robot. Syst. 2017, 86, 397–417. [Google Scholar] [CrossRef]
- Schofield, J.S. Knee ankle foot orthosis. U.S. Patent US20130245524A1, September 2013. [Google Scholar]
- Zissimopoulos, A.; Fatone, S.; Gard, S.A. Biomechanical and energetic effects of a stance-control orthotic knee joint. J. Rehabil. Dev. 2007, 44, 503. [Google Scholar] [CrossRef]
- Rafiaei, M.; Bahramizadeh, M.; Arazpour, M.; Samadian, M.; Hutchins, S.W.; Farahmand, F.; Mardani, M.A. The gait and energy efficiency of stance control knee–ankle–foot orthoses: A literature review. Prosthet. Orthot. Int. 2016, 40, 202–214. [Google Scholar] [CrossRef]
- Yakimovich, T.; Lemaire, E.D.; Kofman, J. Engineering design review of stance-control knee-ankle-foot orthoses. J. Rehabil. Dev. 2009, 46. [Google Scholar]
- Tian, F.; Hefzy, M.S.; Elahinia, M. A Biologically Inspired Knee Actuator for a KAFO. J. Med. Devices 2016, 10, 045001. [Google Scholar] [CrossRef]
- Tian, F.; Hefzy, M.S.; Elahinia, M. State of the Art Review of Knee–Ankle–Foot Orthoses. Ann. Biomed. Eng. 2015, 43, 427–441. [Google Scholar] [CrossRef]
- Tian, F.; Hefzy, M.S.; Elahinia, M. Development of a Dynamic Knee Actuator for a KAFO Using Superelastic Alloys. In Proceedings of the ASME 2014 International Mechanical Engineering Congress and Exposition, Quebec, Canada, 14–20 November 2014. [Google Scholar]
- Tian, F.; Elahinia, M.; Hefzy, M.S. A Dynamic Knee-Ankle-Foot Orthosis With Superelastic Actuators. In Proceedings of the Mechanics and Behavior of Active Materials; Structural Health Monitoring; Bioinspired Smart Materials and Systems; Energy Harvesting, Snowbird, UT, USA, 16–18 September 2013. [Google Scholar]
- Tian, F.; Elahinia, M.; Hefzy, M.S. Design and Evaluation of a Knee Actuator for a Dynamic Knee-Ankle-Foot Orthosis. In Proceedings of the Mechanics and Behavior of Active Materials; Integrated System Design and Implementation; Bioinspired Smart Materials and Systems; Energy Harvesting, Newport, RI, USA, 8–10 September 2014. [Google Scholar]
- Tian, F. A superelastic variable stiffness knee actuator for a knee-ankle-foot orthosis. Ph.D. Thesis, University of Toledo, Toledo, Canada, 2015. [Google Scholar]
- Bhadane, M.Y.; Armstrong, C.; Hefzy, M.S.; Elahinia, M.H. An Automated Testing Assembly for Characterizing Stiffness of an Ankle Foot Orthosis. In Proceedings of the ASME 2011 Conference on Smart Materials, Adaptive Structures and Intelligent Systems, Scottsdale, AZ, USA, 18–21 September 2011; pp. 405–409. [Google Scholar]
- Bhadane, M.Y.; Elahinia, M.H. Stiffness Control of a SMA Actuator. In Proceedings of the ASME 2009 Conference on Smart Materials, Adaptive Structures and Intelligent Systems, Oxnard, CA, USA, 21–23 September 2009; pp. 589–594. [Google Scholar]
- Bhadane-Deshpande, M. Towards a shape memory alloy based variable stiffness ankle foot orthosis. Ph.D. Thesis, University of Toledo, Toledo, Canada, 2012. [Google Scholar]
- Liberty DeBerg; Andani, M.T.; Hosseinipour, M.; Elahinia, M. An SMA Passive Ankle Foot Orthosis: Design, Modeling, and Experimental Evaluation. Smart Mater. 2014, 2014, 1–11. [Google Scholar] [Green Version]
- Deberg, L. A fast actuator using shape memory alloys for an ankle foot orthosis. Master’s thesis, University of Toledo, Toledo, OH, USA, 2012. [Google Scholar]
- dMataee, M.G.; Andani, M.T.; Elahinia, M. Adaptive ankle–foot orthoses based on superelasticity of shape memory alloys. J. Intell. Mater. Syst. Struct. 2015, 26, 639–651. [Google Scholar]
- Gorzinmataee, M. Design and Simulation of a Single-Hinge and Adaptive Ankle Foot Orthoses Based on Superelasticity of Shape Memory Alloys. Ph.D. Thesis, University of Toledo, Toledo, Canada, 2013. [Google Scholar]
- Amerinatanzi, A.; Zamanian, H.; Moghaddam, N.S.; Jahadakbar, A.; Elahinia, M.; Wang, G.-J.; Luo, L. Application of the Superelastic NiTi Spring in Ankle Foot Orthosis (AFO) to Create Normal Ankle Joint Behavior. Bioengineering 2017, 4, 95. [Google Scholar] [CrossRef]
- Desbiens-Blais, F.; Clin, J.; Parent, S.; Labelle, H.; Aubin, C.-. Éric New brace design combining CAD/CAM and biomechanical simulation for the treatment of adolescent idiopathic scoliosis. Clin. Biomech. 2012, 27, 999–1005. [Google Scholar] [CrossRef]
- Palmer, M.; Fonte, M.; Devaney, R. Systems for correcting digital deformities. U.S. Patent US20170056229A1, March 2017. [Google Scholar]
- Wong, C.; Nielsen, J. Plate for sagittal correction of the spine. U.S. Patent US20170181881A1, June 2017. [Google Scholar]
- Cheung, J.P.Y.; Samartzis, D.; Yeung, K.; To, M.; Luk, K.D.K.; Cheung, K.M.C. A randomized double-blinded clinical trial to evaluate the safety and efficacy of a novel superelastic nickel–titanium spinal rod in adolescent idiopathic scoliosis: 5-year follow-up. Eur. Spine J. 2018, 27, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Garlock, A.; Karnes, W.M.; et al. Arthrodesis devices for generating and applying compression within joints. U.S. Patent US20170296241A1, October 2017. [Google Scholar]
- Pittaccio, S.; Viscuso, S. Shape Memory Actuators for Medical Rehabilitation and Neuroscience. In Smart Actuation and Sensing Systems-Recent Advances and Future Challenges; Sciyo: Rijeka, Croatia, 2012. [Google Scholar] [Green Version]
- Krishnan, S.; Nagarajan, T.; Rani, A.M.A.; Ambaraj, W.; Ramiah, R. REHABILITATION FOR FOOT/ANKLE-CONTINUOUS PASSIVE MOTION (CPM) USING SHAPE MEMORY ALLOY (SMA) ACTUATED STEWART PLATFORM. ARPN J. Eng. Appl. Sci. 2016, 11, 22. [Google Scholar]
- Hadi, A.; Akbari, H.; Tarvirdizadeh, B.; Alipour, K. Developing a novel continuum module actuated by shape memory alloys. Sens. Actuators A Phys. 2016, 243, 90–102. [Google Scholar] [CrossRef]
- Hadi, A.; Akbari, H.; Alipour, K.; Tarvirdizadeh, B. Precise position control of shape memory alloy–actuated continuum modules through fuzzy algorithm. Proc. Inst. Mech. Eng. Part I J. Syst. Control Eng. 2018, 232, 121–136. [Google Scholar] [CrossRef]

































| Time | Sit | Stand | Walk | Relax | Ashworth | |
|---|---|---|---|---|---|---|
| A | T0 | 60° | 50° | 65° | 50° | 3 |
| 3 h | 45° | 45° | 60° | 50° | 3 | |
| 24 h | 45° | 50° | 60° | 50° | 3 | |
| 1 w | 35° | 40° | 55° | 40° | 2 | |
| 1 w after | 50° | 60° | 65° | 60° | 2 | |
| B | T0 | 70° | 110° | 100° | 60° | 2 |
| 3 h | 30° | 60° | 70° | 50° | 1+ | |
| 24 h | 30° | 30° | 40° | 30° | 1 | |
| 1 w | 25° | 30° | 40° | 20° | 1 | |
| 1 w after | 65° | 80° | 95° | 60° | 2 |
| Subject | Age (Years) | Sex | Reaction Torque (N.mm) | Joint | AS | Group | ROM (°) |
|---|---|---|---|---|---|---|---|
| S04 | 12 | F | 364.34 | Elbow | 2 | TP | 80 |
| S05 | 19 | M | 2062.76 | Elbow | 1 | PT | 126 |
| S06 | 13 | F | 1741.22 | Elbow | 2 | TP | 124 |
| S13 | 17 | M | 838.78 | Elbow | 1 | TP | 110 |
| S14 | 17 | M | −26.57 | Elbow | 2 | TP | 121 |
| Reference | Weight (kg) | DOF | Applicable or Gripping Force (N) | No. of Fingers | Note |
|---|---|---|---|---|---|
| The Hitachi Hand | 4.49 | 12 | 4 | ||
| K. Laurentis and C. Mavroidis | 1.36 | 20 | 6.67 | 5 | one-step structure without the need for any further assembly |
| K. Andrianesis and A. Tzes | 0.310 | 7 | 12 | 5 | Additively manufactured has the shape and size of the average human hand |
| H. Taniguchi | - | - | 10 | 5 | prosthetic hand for children; Proposing a cooling system |
| Jae H. Lee et al | - | - | 4(+1 fixed) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nematollahi, M.; Baghbaderani, K.S.; Amerinatanzi, A.; Zamanian, H.; Elahinia, M. Application of NiTi in Assistive and Rehabilitation Devices: A Review. Bioengineering 2019, 6, 37. https://doi.org/10.3390/bioengineering6020037
Nematollahi M, Baghbaderani KS, Amerinatanzi A, Zamanian H, Elahinia M. Application of NiTi in Assistive and Rehabilitation Devices: A Review. Bioengineering. 2019; 6(2):37. https://doi.org/10.3390/bioengineering6020037
Chicago/Turabian StyleNematollahi, Mohammadreza, Keyvan Safaei Baghbaderani, Amirhesam Amerinatanzi, Hashem Zamanian, and Mohammad Elahinia. 2019. "Application of NiTi in Assistive and Rehabilitation Devices: A Review" Bioengineering 6, no. 2: 37. https://doi.org/10.3390/bioengineering6020037
APA StyleNematollahi, M., Baghbaderani, K. S., Amerinatanzi, A., Zamanian, H., & Elahinia, M. (2019). Application of NiTi in Assistive and Rehabilitation Devices: A Review. Bioengineering, 6(2), 37. https://doi.org/10.3390/bioengineering6020037






