Advances in Magnetic Noble Metal/Iron-Based Oxide Hybrid Nanoparticles as Biomedical Devices
Abstract
:1. Introduction
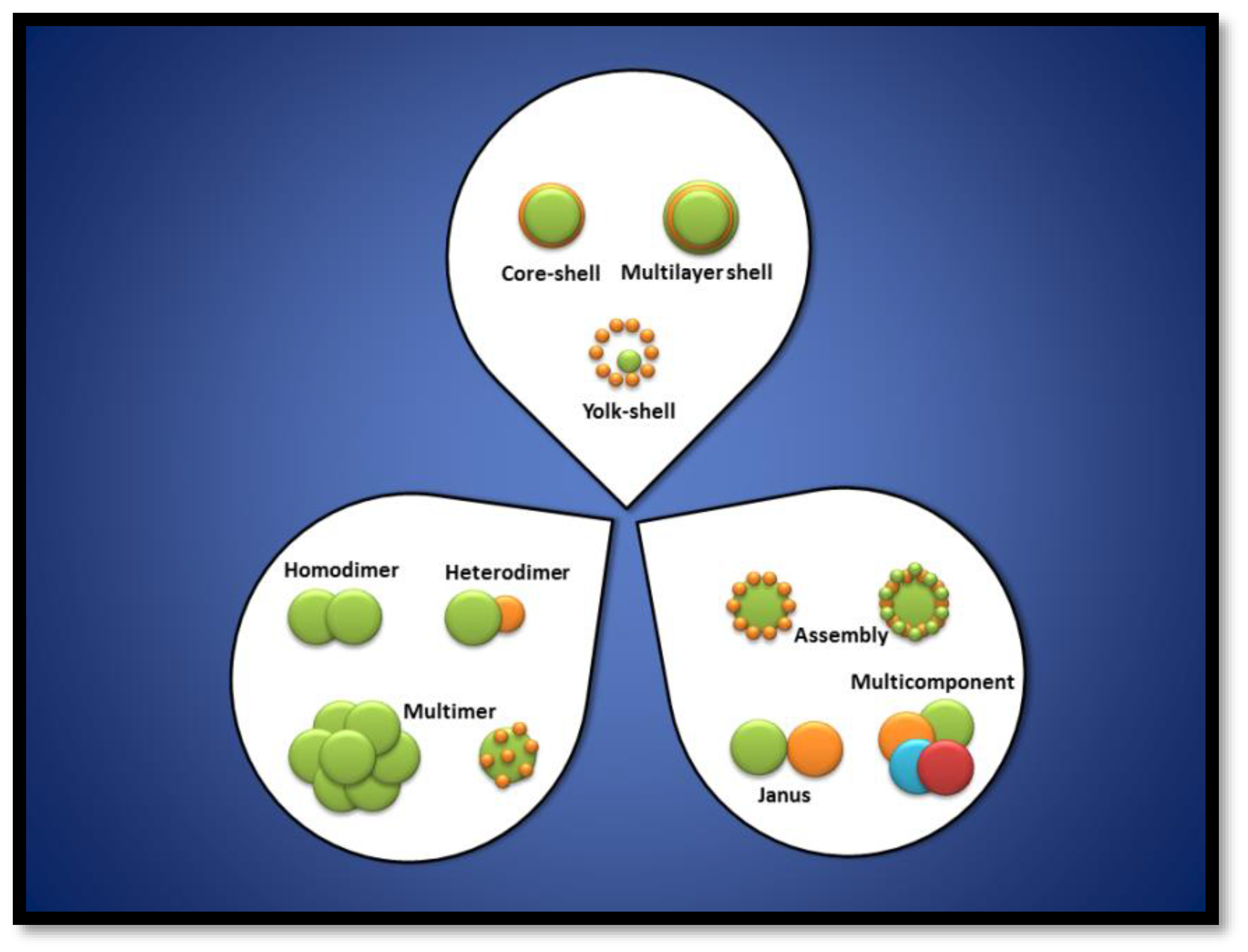
2. Magnetic Noble Metal Nanoparticles (NPs)
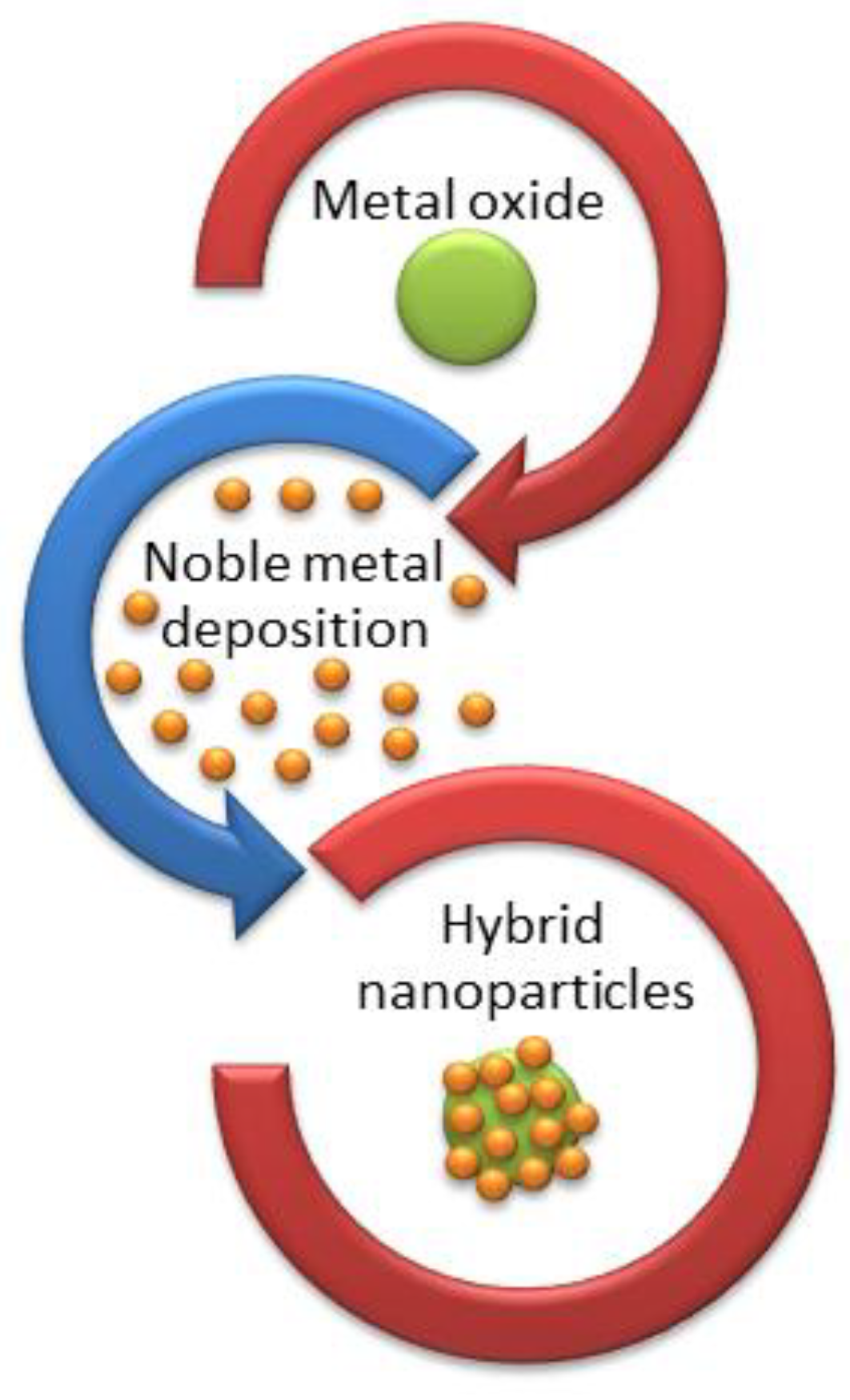
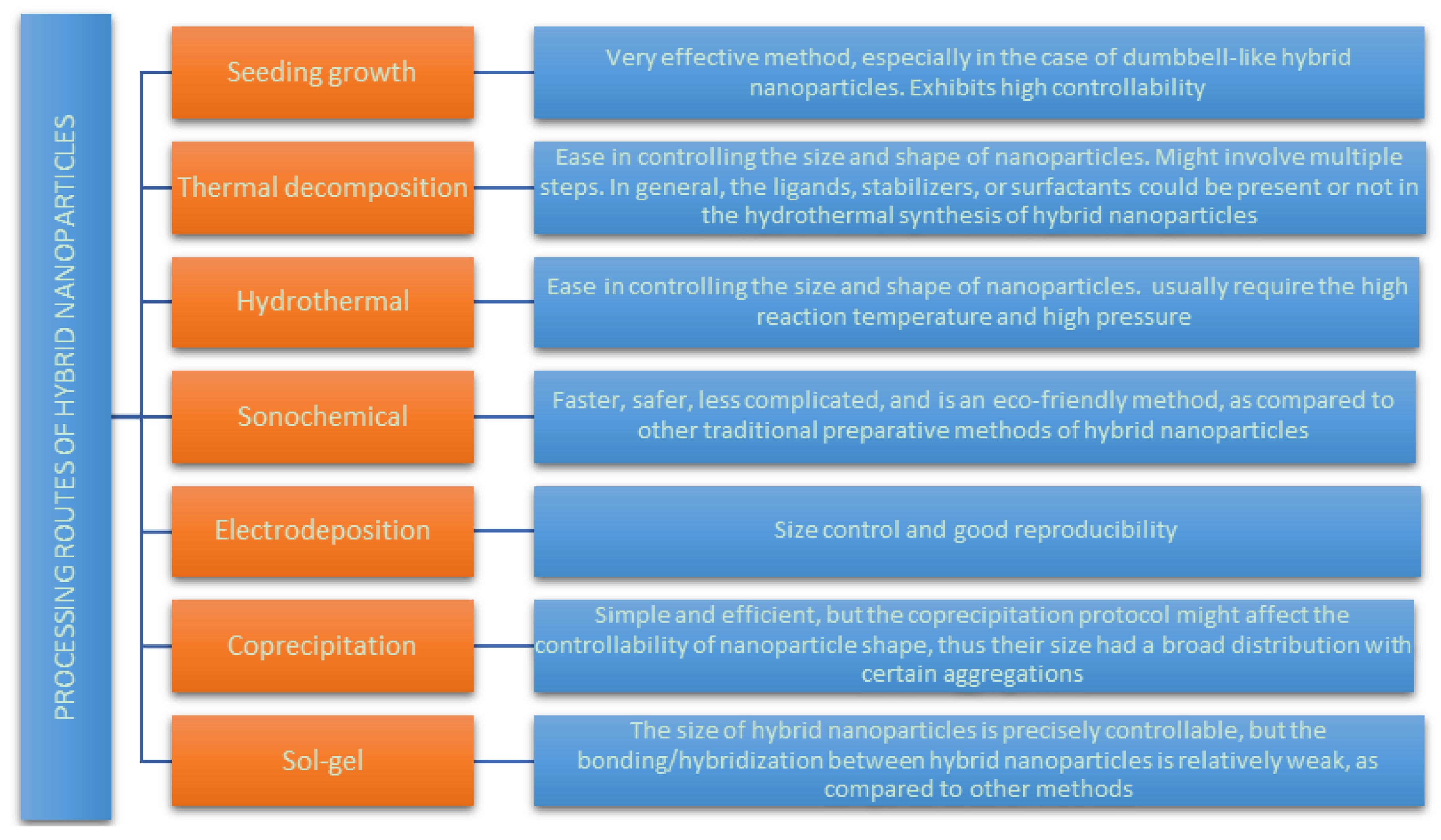
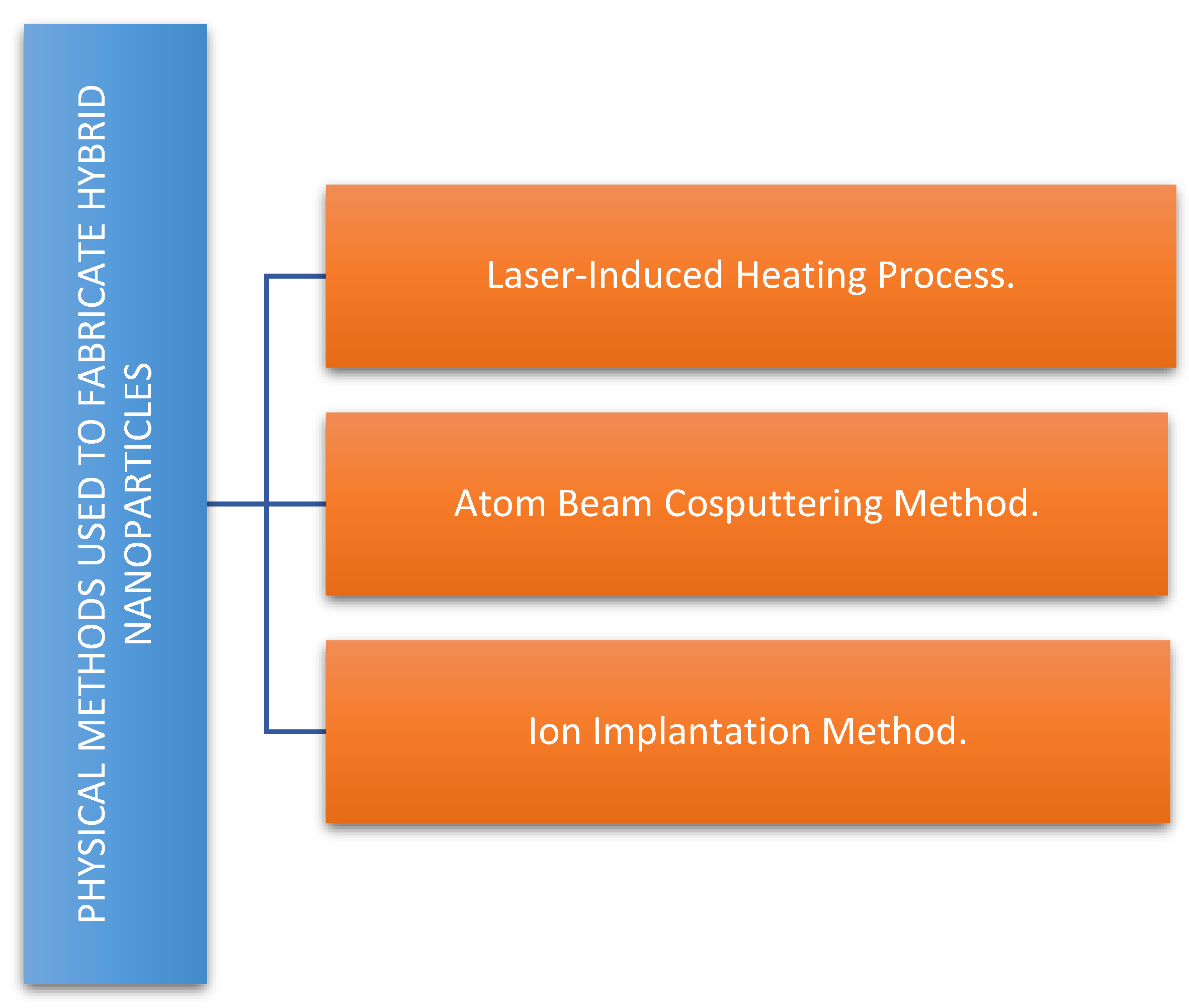
2.1. Methods for the Synthesis of Magnetic Noble Metal Nanoparticles
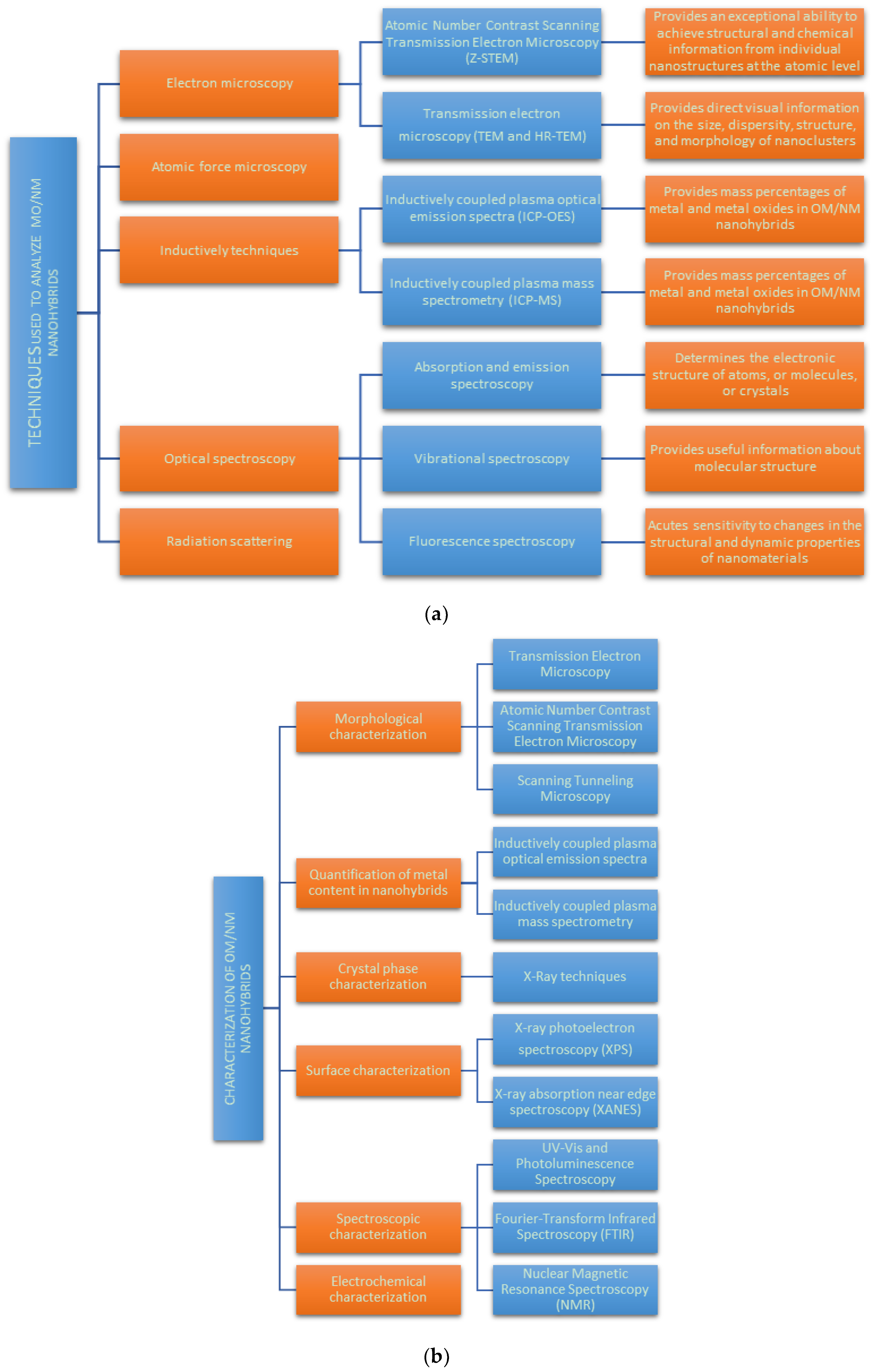
2.2. Nanoscale Characterization of Magnetic Noble Metal Nanoparticles
2.3. Applications of Magnetic Noble Metal Nanoparticles in Biomedicine
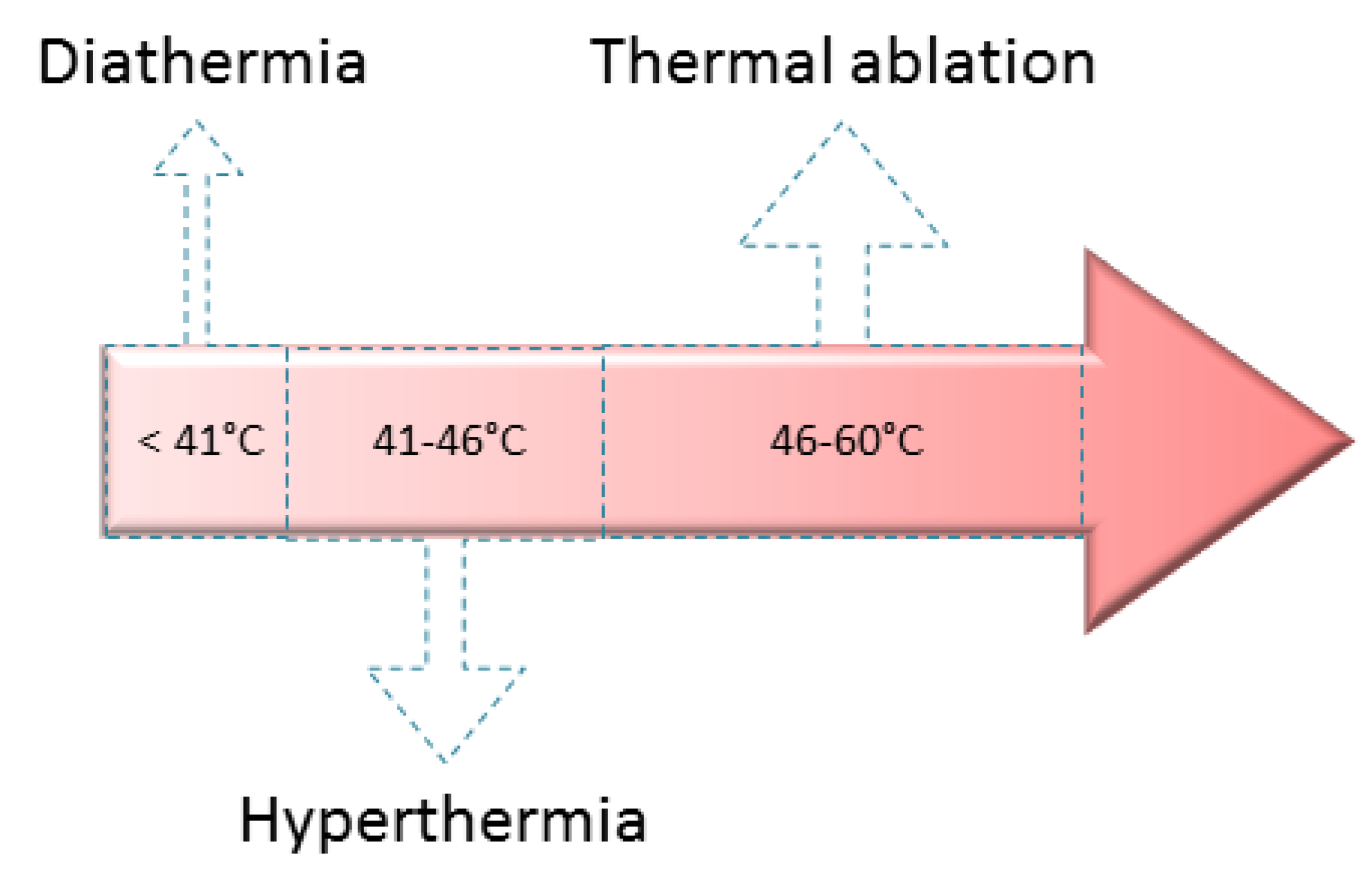
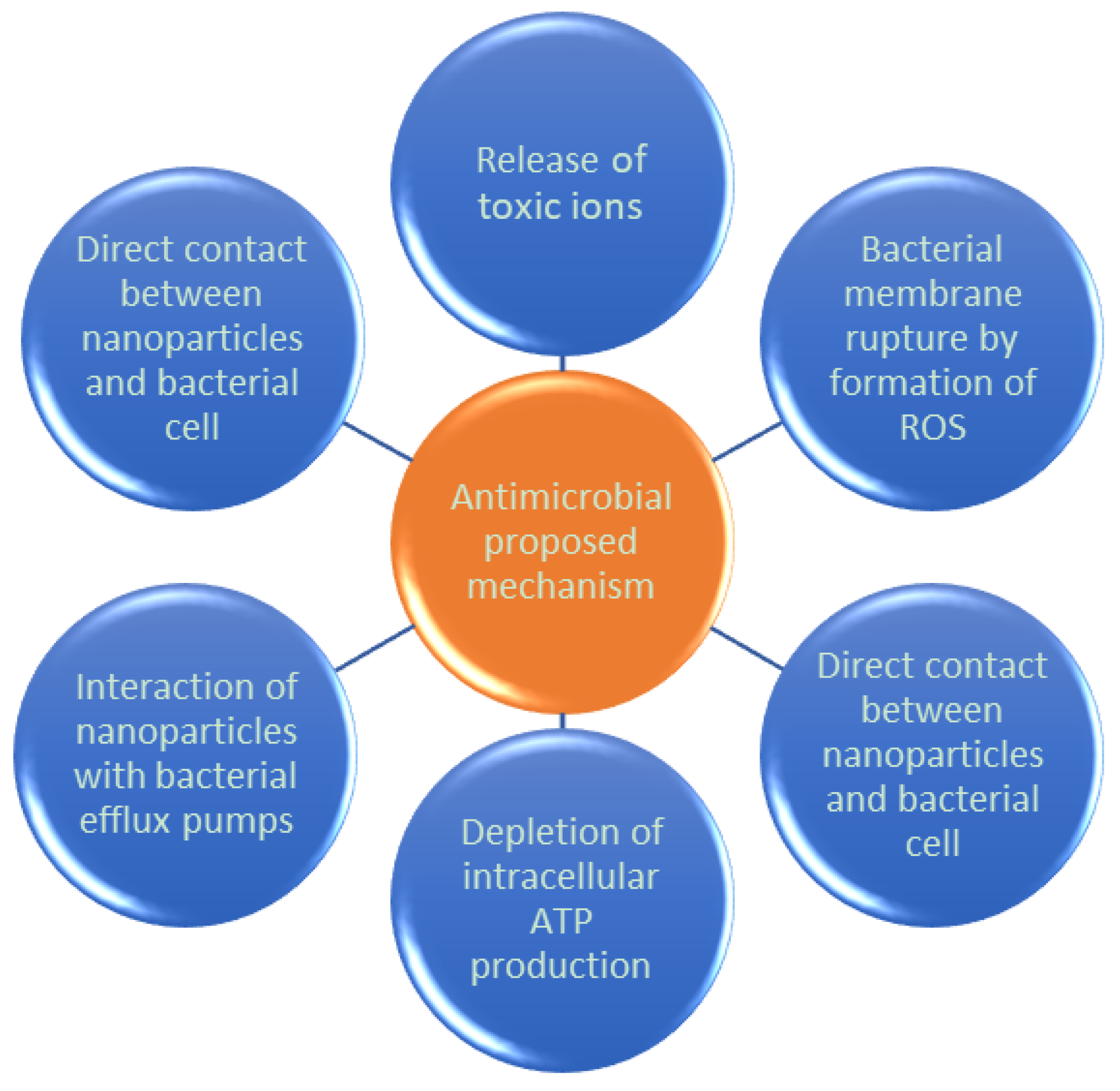
2.3.1. Medical Treatments
2.3.2. Medical Diagnosis
2.3.3. Theranostic Agents
3. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marks, L.D. Experimental studies of small particle structures. Rep. Prog. Phys. 1994, 57, 603–649. [Google Scholar] [CrossRef]
- Salabas, E.L.; Schuth, F.; Lu, A.H.; Lu, A. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar]
- Gao, Y.; Hilgendorff, M.; Irsen, S.; Spasova, M.; Farle, M.; Giersig, M.; Pazos-Perez, N.; Perez-Juste, J.; Liz-Marzan, L.M.; Pazos-Perez, N.; et al. Magnetic—Noble Metal Nanocomposites with Morphology-Dependent Optical Response. Chem. Mater. 2007, 19, 4415–4422. [Google Scholar]
- Vlamidis, Y.; Voliani, V. Bringing Again Noble Metal Nanoparticles to the Forefront of Cancer Therapy. Front. Bioeng. Biotechnol. 2018, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2014, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Monopoli, M.P.; Åberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Walkey, C.D.; Olsen, J.B.; Guo, H.; Emili, A.; Chan, W.C.W. Secreted Biomolecules Alter the Biological Identity and Cellular Interactions of Nanoparticles. ACS Nano 2014, 8, 5515–5526. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.P.; Birla, S.; Yadav, A.; Dos Santos, C.A. Strategic role of selected noble metal nanoparticles in medicine. Crit. Rev. Microbiol. 2016, 42, 696–719. [Google Scholar] [CrossRef]
- Lee, W.; Kim, K.J.; Lee, D.G. A novel mechanism for the antibacterial effect of silver nanoparticles on Escherichia coli. BioMetals 2014, 27, 1191–1201. [Google Scholar] [CrossRef]
- Chen, H.; Kou, X.; Yang, Z.; Ni, W.; Wang, J. Shape-and Size-Dependent Refractive Index Sensitivity of Gold Nanoparticles. Langmuir 2008, 24, 5233–5237. [Google Scholar] [CrossRef] [PubMed]
- Malathi, S.; Balakumaran, M.D.; Kalaichelvan, P.T.; Balasubramanian, S. Green Synthesis of Gold Nanoparticles For controlled Delivery. Adv. Mater. Lett. 2013, 4, 933–940. [Google Scholar] [CrossRef]
- Castillo, R.R.; Vallet-Regí, M. Functional Mesoporous Silica Nanocomposites: Biomedical Applications and Biosafety. Int. J. Mol. Sci. 2019, 20, 929. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Xu, H.; Li, Y.; Gu, H.; Wei, M. Influence of the physical and chemical properties of magnetic nanoparticles on their performance in a chemiluminescence immunoassay. Clin. Biochem. 2014, 47, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Majidi, S.; Zeinali Sehrig, F.; Farkhani, S.M. Current methods for synthesis of magnetic nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Nijhawan, G.; Nijhawan, S.S.; Sethi, M. Hyperthermia Treatments. In Noble Metal-Metal Oxide Hybrid Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 241–263. [Google Scholar]
- Liu, X.Q.; Iocozzia, J.; Wang, Y.; Cui, X.; Chen, Y.H.; Zhao, S.Q.; Li, Z.; Lin, Z.Q. Noble metal–metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017, 10, 402–434. [Google Scholar] [CrossRef]
- Walker, J.M.; Zaleski, J.M. A simple route to diverse noble metal-decorated iron oxide nanoparticles for catalysis. Nanoscale 2016, 8, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.P.; Pandikumar, A.; Huang, N.M.; Lim, H.N. Facile synthesis of Au@TiO2 nanocomposite and its application as a photoanode in dye-sensitized solar cells. RSC Adv. 2015, 5, 44398–44407. [Google Scholar] [CrossRef]
- Damato, T.C.; De Oliveira, C.C.S.; Camargo, P.H.C.; Ando, R.A. A Facile Approach to TiO2 Colloidal Spheres Decorated with Au Nanoparticles Displaying Well-Defined Sizes and Uniform Dispersion. Langmuir 2013, 29, 1642–1649. [Google Scholar] [CrossRef]
- Pearson, A.; Bhargava, S.K.; Bansal, V. UV-Switchable Polyoxometalate Sandwiched between TiO2and Metal Nanoparticles for Enhanced Visible and Solar Light Photococatalysis. Langmuir 2011, 27, 9245–9252. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, W.; Shen, T.; Zhao, Z.; Feng, H.; Cui, F. One-step synthesis of noble metal/oxide nanocomposites with tunable size of noble metal particles and their size-dependent catalytic activity. RSC Adv. 2014, 4, 30624–30629. [Google Scholar] [CrossRef]
- Karthikeyan, B.; Govindhan, R.; Amutheesan, M. Chemical Methods for Synthesis of Hybrid Nanoparticles. In Noble Metal-Metal Oxide Hybrid Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 179–188. [Google Scholar] [CrossRef]
- Kowlgi, K.N.K.; Koper, G.J.M.; Picken, S.J.; Lafont, U.; Zhang, L.; Norder, B. Synthesis of Magnetic Noble Metal (Nano) Particles. Langmuir 2011, 27, 7783–7787. [Google Scholar] [CrossRef] [PubMed]
- Sanders, W.C. Nanoscale Characterization. In Basic Principles of Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 123–147. [Google Scholar]
- Abel, K.A.; Boyer, J.C.; Van Veggel, F.C.J.M. Hard Proof of the NaYF4/NaGdF4 Nanocrystal Core/Shell Structure. J. Am. Chem. Soc. 2009, 131, 14644–14645. [Google Scholar] [CrossRef]
- Newville, M. Fundamentals of XAFS. Rev. Mineral. Geochem. 2014, 78, 33–74. [Google Scholar] [CrossRef]
- Chan, S.C.; Barteau, M.A. Preparation of Highly Uniform Ag/TiO2 and Au/TiO2 Supported Nanoparticle Catalysts by Photodeposition. Langmuir 2005, 21, 5588–5595. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Bera, S.; Bysakh, S.; Basu, R.N. Highly Active Multimetallic Palladium Nanoalloys Embedded in Conducting Polymer as Anode Catalyst for Electrooxidation of Ethanol. ACS Appl. Mater. Interfaces 2017, 9, 33775–33790. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Arciniegas, L.; Alvarez, V.A.; Gonzalez, J.S. Phantom gels towards medicine improvement: Uses for magnetic device tests and enhancements on magnetic-dependent clinical techniques. In Materials for Biomedical Engineering: Nanomaterials-Based Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 429–450. [Google Scholar] [CrossRef]
- Bazrafshan, B.; Hübner, F.; Farshid, P.; Hammerstingl, R.; Paul, J.; Vogel, V.; Mäntele, W.; Vogl, T.J. Temperature imaging of laser-induced thermotherapy (LITT) by MRI: Evaluation of different sequences in phantom. Lasers Med. Sci. 2014, 29, 173–183. [Google Scholar] [CrossRef]
- Józefczak, A.; Kaczmarek, K.; Hornowski, T.; Kubovčíková, M.; Rozynek, Z.; Timko, M.; Skumiel, A. Magnetic nanoparticles for enhancing the effectiveness of ultrasonic hyperthermia. Appl. Phys. Lett. 2016, 108, 263701. [Google Scholar] [CrossRef]
- Lee, E.H.; Kim, C.Y.; Choa, Y.H. Magnetite nanoparticles dispersed within nanoporous aerogels for hyperthermia application. Curr. Appl. Phys. 2012, 12, S47–S52. [Google Scholar] [CrossRef]
- McDonald, M.; Lochhead, S.; Chopra, R.; Bronskill, M.J. Multi-modality tissue-mimicking phantom for thermal therapy. Phys. Med. Biol. 2004, 49, 2767–2778. [Google Scholar] [CrossRef] [PubMed]
- Kampinga, H.H. Cell biological effects of hyperthermia alone or combined with radiation or drugs: A short introduction to newcomers in the field. Int. J. Hyperth. 2006, 22, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Javidi, M.; Heydari, M.; Attar, M.M.; Haghpanahi, M.; Karimi, A.; Navidbakhsh, M.; Amanpour, S. Cylindrical agar gel with fluid flow subjected to an alternating magnetic field during hyperthermia. Int. J. Hyperth. 2014, 31, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Maeda, K.; Moriya, M.; Sakamoto, W.; Yogo, T. In situ synthesis of cobalt ferrite nanoparticle/polymer hybrid from a mixed Fe–Co methacrylate for magnetic hyperthermia. J. Magn. Magn. Mater. 2012, 324, 3158–3164. [Google Scholar] [CrossRef]
- Sohail, A.; Ahmad, Z.; Bég, O.A.; Arshad, S.; Sherin, L. A review on hyperthermia via nanoparticle-mediated therapy. Bull. Cancer 2017, 104, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.S. Nanoparticle-Related Heat Transfer Phenomenon and Its Application in Biomedical Fields. Heat Transf. Eng. 2013, 34, 1171–1179. [Google Scholar] [CrossRef]
- Dutz, S.; Hergt, R. Magnetic particle hyperthermia—A promising tumour therapy? Nanotechnology 2014, 25, 452001. [Google Scholar] [CrossRef]
- Salloum, M.; Ma, R.; Weeks, D.; Zhu, L. Controlling nanoparticle delivery in magnetic nanoparticle hyperthermia for cancer treatment: Experimental study in agarose gel. Int. J. Hyperth. 2008, 24, 337–345. [Google Scholar] [CrossRef]
- Fortin, J.P.; Wilhelm, C.; Servais, J.; Ménager, C.; Bacri, J.C.; Gazeau, F. Size-Sorted Anionic Iron Oxide Nanomagnets as Colloidal Mediators for Magnetic Hyperthermia. J. Am. Chem. Soc. 2007, 129, 2628–2635. [Google Scholar] [CrossRef]
- Mohammad, F.; Balaji, G.; Weber, A.; Uppu, R.M.; Kumar, C.S.S.R. Influence of Gold Nanoshell on Hyperthermia of Superparamagnetic Iron Oxide Nanoparticles. J. Phys. Chem. C 2010, 114, 19194–19201. [Google Scholar] [CrossRef] [Green Version]
- Das, R.; Rinaldi-Montes, N.; Alonso, J.; Amghouz, Z.; Garaio, E.; García, J.A.; Gorria, P.; Blanco, J.A.; Phan, M.H.; Srikanth, H. Boosted Hyperthermia Therapy by Combined AC Magnetic and Photo-Thermal Exposures in Ag/Fe3O4 Nanoflowers. ACS Appl. Mater. Interfaces 2016, 8, 25162–25169. [Google Scholar] [CrossRef] [PubMed]
- Tri, P.N.; Nguyen, T.A.; Nguyen, T.H.; Carriere, P. Antibacterial Behavior of Hybrid Nanoparticles. In Noble Metal-Metal Oxide Hybrid Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 141–155. [Google Scholar]
- Ngo, T.D.; Le, T.M.H.; Nguyen, T.H.; Nguyen, T.V.; Nguyen, T.A.; Le, T.L.; Nguyen, T.T.; Van Tran, T.T.; Le, T.B.T.; Doan, N.H. Antibacterial Nanocomposites Based on Fe3O4-Ag Hybrid Nanoparticles and Natural Rubber-Polyethylene Blends. Int. J. Polym. Sci. 2016, 2016, 7478161. [Google Scholar] [CrossRef]
- Chudasama, B.; Vala, A.K.; Andhariya, N.; Upadhyay, R.V.; Mehta, R.V.; Mehta, R. Enhanced antibacterial activity of bifunctional Fe3O4-Ag core-shell nanostructures. Nano Res. 2009, 2, 955–965. [Google Scholar] [CrossRef]
- Sunasee, R.; Narain, R. Covalent and Noncovalent Bioconjugation Strategies. In Chemistry of Bioconjugates: Synthesis, Characterization, and Biomedical Applications; Wiley: Hoboken, NJ, USA, 2014; pp. 1–75. [Google Scholar]
- Hermanson, G.T. Functional Targets for Bioconjugation. In Bioconjugate Techniques; Academic Press: Cambridge, MA, USA, 2013; pp. 127–228. [Google Scholar]
- Hermanson, G.T. Introduction to Bioconjugation. In Bioconjugate Techniques; Academic Press: Cambridge, MA, USA, 2013; pp. 1–125. [Google Scholar]
- Xu, C.; Wang, B.; Sun, S. Dumbbell-Like Au-Fe3O4 Nanoparticles for Target-Specific Platin Delivery. J. Am. Chem. Soc. 2009, 131, 4216–4217. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.K.S.; Raizaday, A. Inorganic nanobiomaterials for medical imaging. In Nanobiomaterials in Medical Imaging: Applications of Nanobiomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 365–401. [Google Scholar]
- Zhu, J.; Zhang, B.; Tian, J.; Wang, J.; Chong, Y.; Wang, X.; Deng, Y.; Tang, M.; Li, Y.; Ge, C.; et al. Synthesis of heterodimer radionuclide nanoparticles for magnetic resonance and single-photon emission computed tomography dual-modality imaging. Nanoscale 2015, 7, 3392–3395. [Google Scholar] [CrossRef] [PubMed]
- Palczewska, G.; Dong, Z.; Golczak, M.; Hunter, J.J.; Williams, D.R.; Alexander, N.S.; Palczewski, K. Noninvasive two-photon fluorescence microscopy imaging of mouse retina and RPE through the pupil of the eye. Nat. Med. 2014, 20, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Gu, H.; Shao, H.; Devlin, E.; Papaefthymiou, G.C.; Ying, J.Y. Bifunctional Fe3O4-Ag Heterodimer Nanoparticles for Two-Photon Fluorescence Imaging and Magnetic Manipulation. Adv. Mater. 2008, 20, 4403–4407. [Google Scholar] [CrossRef]
- Geraldes, C.F.G.C.; Laurent, S. Classification and basic properties of contrast agents for magnetic resonance imaging. Contrast Media Mol. Imaging 2009, 4, 1–23. [Google Scholar] [CrossRef]
- Nowogrodzki, A. Researchers are pushing non-invasive brain imaging to new limits. Nature 2018, 563, 24–26. [Google Scholar] [CrossRef]
- Schepkin, V.D.; Bejarano, F.C.; Morgan, T.; Gower-Winter, S.; Ozambela, M., Jr.; Levenson, C.W. In vivo magnetic resonance imaging of sodium and diffusion in rat glioma at 21.1 T. Magn. Reson. Med. 2012, 67, 1159–1166. [Google Scholar] [CrossRef]
- Xiao, Y.D.; Paudel, R.; Zhang, Z.S.; Zhou, S.K.; Liu, J.; Ma, C. MRI contrast agents: Classification and application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Ryvolova, M.; Chomoucká, J.; Drbohlavova, J.; Kopel, P.; Babula, P.; Hynek, D.; Adam, V.; Eckschlager, T.; Hubalek, J.; Stiborova, M.; et al. Modern Micro and Nanoparticle-Based Imaging Techniques. Sensors 2012, 12, 14792–14820. [Google Scholar] [CrossRef] [PubMed]
- Long, N.V.; Yang, Y.; Teranishi, T.; Thi, C.M.; Cao, Y.; Nogami, M.; Viet, L.N.; Yong, Y.; Toshiharu, T.; Minh, T.C.; et al. Biomedical Applications of Advanced Multifunctional Magnetic Nanoparticles. J. Nanosci. Nanotechnol. 2015, 15, 10091–10107. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lu, Y.; Li, Y.; Jiang, J.; Cheng, L.; Liu, Z.; Guo, L.; Pan, Y.; Gu, H. Synthesis of Au-Fe3O4 heterostructured nanoparticles for in vivo computed tomography and magnetic resonance dual model imaging. Nanoscale 2014, 6, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Suchý, M.; Bartha, R.; Hudson, R.H.E. “Click” chemistry toward bis (DOTA-derived) heterometallic complexes: Potential bimodal MRI/PET (SPECT) molecular imaging probes. RSC Adv. 2013, 3, 3249–3259. [Google Scholar] [CrossRef]
- Jing, L.; Liang, X.; Deng, Z.; Feng, S.; Li, X.; Huang, M.; Li, C.; Dai, Z. Prussian blue coated gold nanoparticles for simultaneous photoacoustic/CT bimodal imaging and photothermal ablation of cancer. Biomaterials 2014, 35, 5814–5821. [Google Scholar] [CrossRef]
- Wang, G.; Gao, W.; Zhang, X.; Mei, X. Au Nanocage Functionalized with Ultra-small Fe3O4 Nanoparticles for Targeting T1–T2Dual MRI and CT Imaging of Tumor. Sci. Rep. 2016, 6, 28258. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Liu, S.; He, J.; Pan, C.C.; Li, H.; Zhou, Z.Y.; Ding, Y.; Huo, D.; Hu, Y. Synthesis and application of strawberry-like Fe3O4-Au nanoparticles as CT-MR dual-modality contrast agents in accurate detection of the progressive liver disease. Biomaterials 2015, 51, 194–207. [Google Scholar] [CrossRef]
- Li, J.; Zheng, L.; Cai, H.; Sun, W.; Shen, M.; Zhang, G.; Shi, X. Facile One-Pot Synthesis of Fe3O4@Au Composite Nanoparticles for Dual-Mode MR/CT Imaging Applications. ACS Appl. Mater. Interfaces 2013, 5, 10357–10366. [Google Scholar] [CrossRef]
- Cai, H.; Li, K.; Shen, M.; Wen, S.; Luo, Y.; Peng, C.; Zhang, G.; Shi, X. Facile assembly of Fe3O4@Au nanocomposite particles for dual mode magnetic resonance and computed tomography imaging applications. J. Mater. Chem. 2012, 22, 15110–15120. [Google Scholar] [CrossRef]
- Yang, M.; Cheng, K.; Qi, S.; Liu, H.; Jiang, Y.; Jiang, H.; Li, J.; Chen, K.; Zhang, H.; Cheng, Z. Affibody Modified and Radiolabeled Gold-Iron Oxide Hetero-nanostructures for Tumor PET, Optical and MR Imaging. Biomaterials 2013, 34, 2796–2806. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wu, B.; Xing, D. Bio-modified Fe3O4 core/Au shell nanoparticles for targeting and multimodal imaging of cancer cells. J. Mater. Chem. 2012, 22, 470–477. [Google Scholar] [CrossRef]
- Sintov, A.; Velasco-Aguirre, C.; Gallardo-Toledo, E.; Araya, E.; Kogan, M. Metal Nanoparticles as Targeted Carriers Circumventing the Blood-Brain Barrier. Int. Rev. Neurobiol. 2016, 130, 199–227. [Google Scholar] [PubMed]
- Singh, P.; Gupta, B.K.; Prasad, N.K.; Yadav, P.K.; Upadhyay, C. Novel facets of multifunctional Ag@Fe3O4 core-shell nanoparticles for multimodal imaging applications. J. Appl. Phys. 2018, 124, 074901. [Google Scholar] [CrossRef]
- Ivashchenko, O.; Gapiński, J.; Peplińska, B.; Przysiecka, Ł.; Zalewski, T.; Nowaczyk, G.; Jarek, M.; Marcinkowska-Gapińska, A.; Jurga, S. Self-organizing silver and ultrasmall iron oxide nanoparticles prepared with ginger rhizome extract: Characterization, biomedical potential and microstructure analysis of hydrocolloids. Mater. Des. 2017, 133, 307–324. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, K.; Cheng, L.; Zhu, J.; Ma, X.; Xu, H.; Li, Y.; Guo, L.; Gu, H.; Liu, Z. PEGylated FePt@Fe2O3 core-shell magnetic nanoparticles: Potential theranostic applications and in vivo toxicity studies. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.; Prabaharan, M. Theranostic Application of Fe3O4-Au Hybrid Nanoparticles. In Noble Metal-Metal Oxide Hybrid Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 607–623. [Google Scholar]
- Han, Y.; Lei, S.L.; Lu, J.H.; He, Y.; Chen, Z.W.; Ren, L.; Zhou, X. Potential use of SERS-assisted theranostic strategy based on Fe3O4/Au cluster/shell nanocomposites for bio-detection, MRI, and magnetic hyperthermia. Mater. Sci. Eng. C 2016, 64, 199–207. [Google Scholar] [CrossRef]
- Li, C.M.; Chen, T.; Ocsoy, I.; Zhu, G.Z.; Yasun, E.; You, M.X.; Wu, C.C.; Zheng, J.; Song, E.; Huang, C.Z. Gold-Coated Fe3O4 nanoroses with five unique functions for cancer cell targeting, imaging, and therapy. Adv. Funct. Mater. 2014, 24, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, Y.; Yang, J.; Wei, P.; Sun, W.; Shen, M.; Zhang, G.X.; Shi, X.Y. Hyaluronic acid-modified Fe3O4 at Au core/shell nanostars for multimodal imaging and photothermal therapy of tumors. Biomaterials 2015, 38, 10–21. [Google Scholar] [CrossRef]
- Hoskins, C.; Min, Y.; Gueorguieva, M.; McDougall, C.; Volovick, A.; Prentice, P.; Wang, Z.; Melzer, A.; Cuschieri, A.; Wang, L. Hybrid gold-iron oxide nanoparticles as a multifunctional platform for biomedical application. J. Nanobiotechnol. 2012, 10, 27. [Google Scholar] [CrossRef]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [PubMed] [Green Version]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, L.M.; Alvarez, V.A. Advances in Magnetic Noble Metal/Iron-Based Oxide Hybrid Nanoparticles as Biomedical Devices. Bioengineering 2019, 6, 75. https://doi.org/10.3390/bioengineering6030075
Sanchez LM, Alvarez VA. Advances in Magnetic Noble Metal/Iron-Based Oxide Hybrid Nanoparticles as Biomedical Devices. Bioengineering. 2019; 6(3):75. https://doi.org/10.3390/bioengineering6030075
Chicago/Turabian StyleSanchez, Laura M., and Vera A. Alvarez. 2019. "Advances in Magnetic Noble Metal/Iron-Based Oxide Hybrid Nanoparticles as Biomedical Devices" Bioengineering 6, no. 3: 75. https://doi.org/10.3390/bioengineering6030075
APA StyleSanchez, L. M., & Alvarez, V. A. (2019). Advances in Magnetic Noble Metal/Iron-Based Oxide Hybrid Nanoparticles as Biomedical Devices. Bioengineering, 6(3), 75. https://doi.org/10.3390/bioengineering6030075




