S-Nitroso-N-Acetyl-D-Penicillamine Modified Hyperbranched Polyamidoamine for High-Capacity Nitric Oxide Storage and Release
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of HPAMAM
2.3. Synthesis of NAP-Thiolactone
2.4. Nitrosation of S-Nitrosothiol Modified HPAMAM
2.5. Nitric Oxide Release
2.6. Material Characterization
3. Results and Discussion
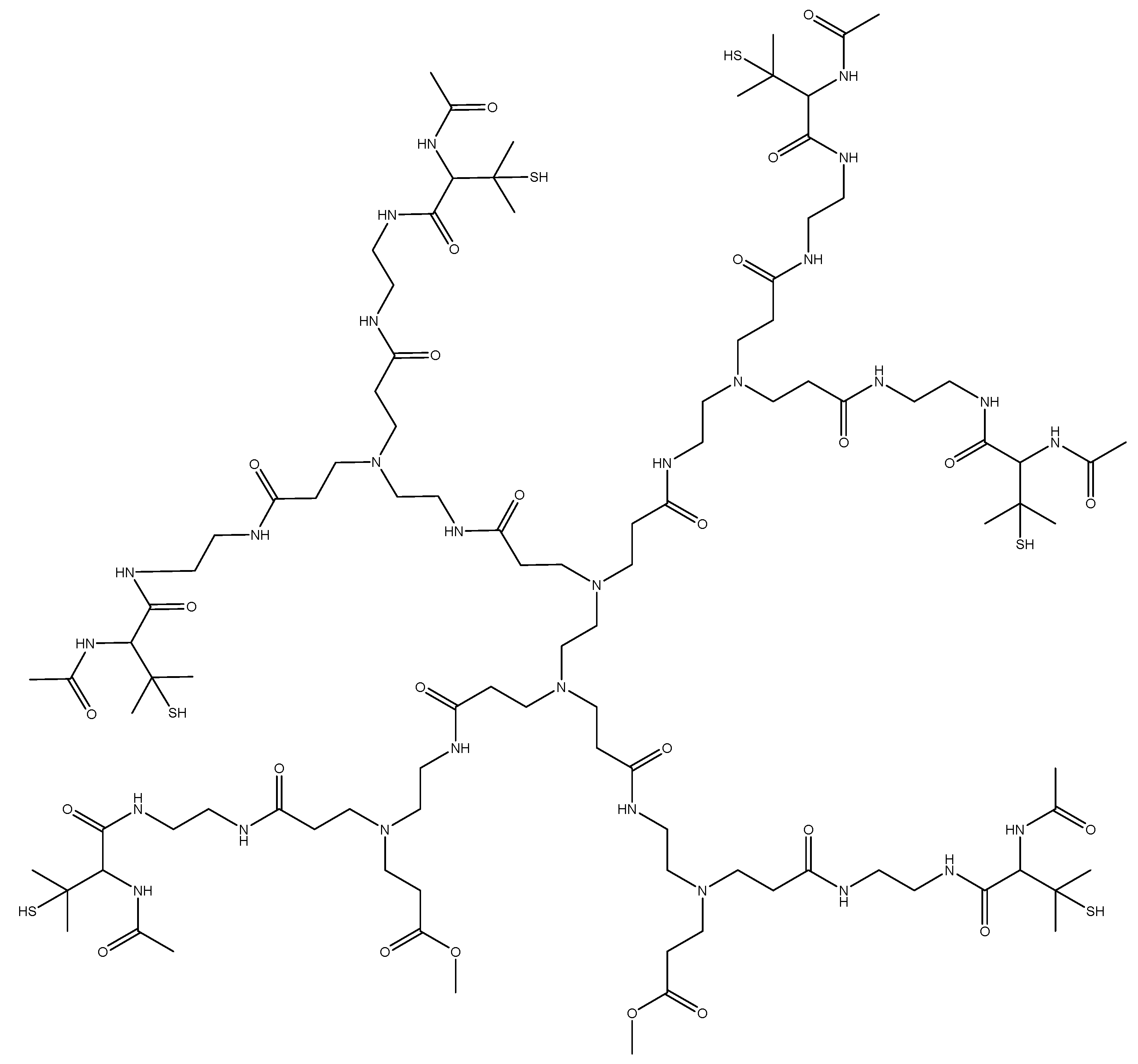
3.1. Synthesis of Generation 2 SNAP-HPAMAM
3.2. NO Release
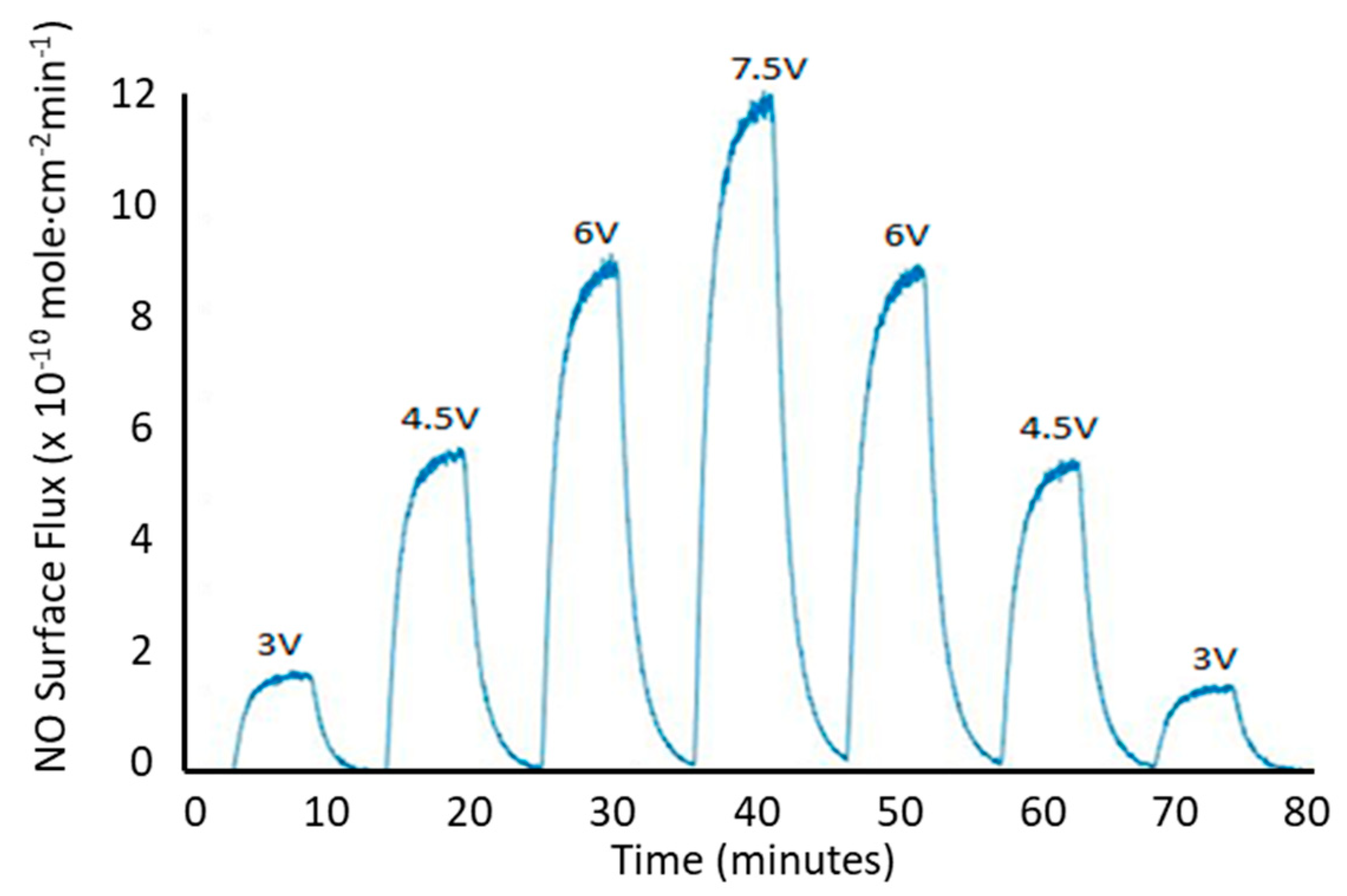
3.2.1. Photoinitiated NO Release
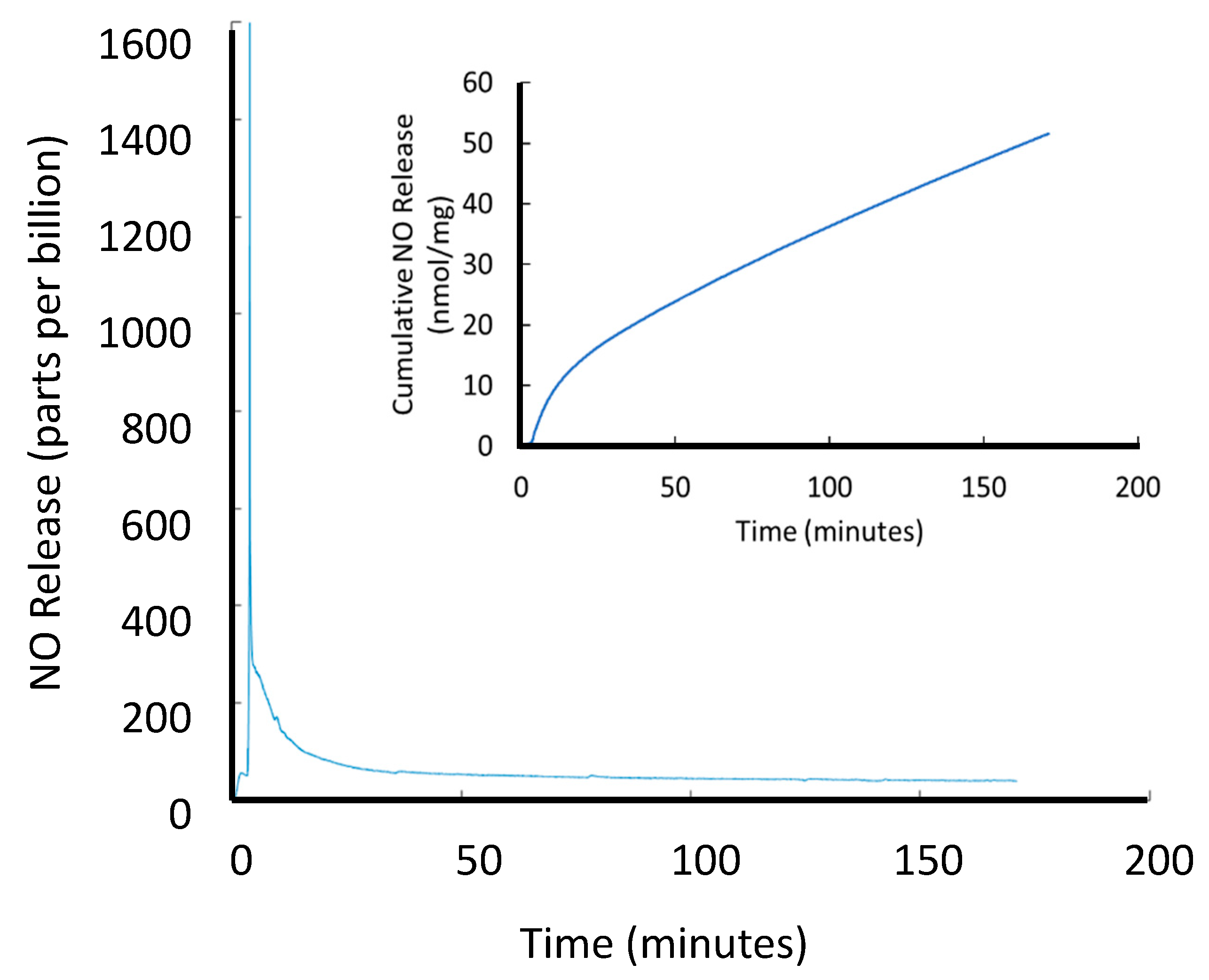
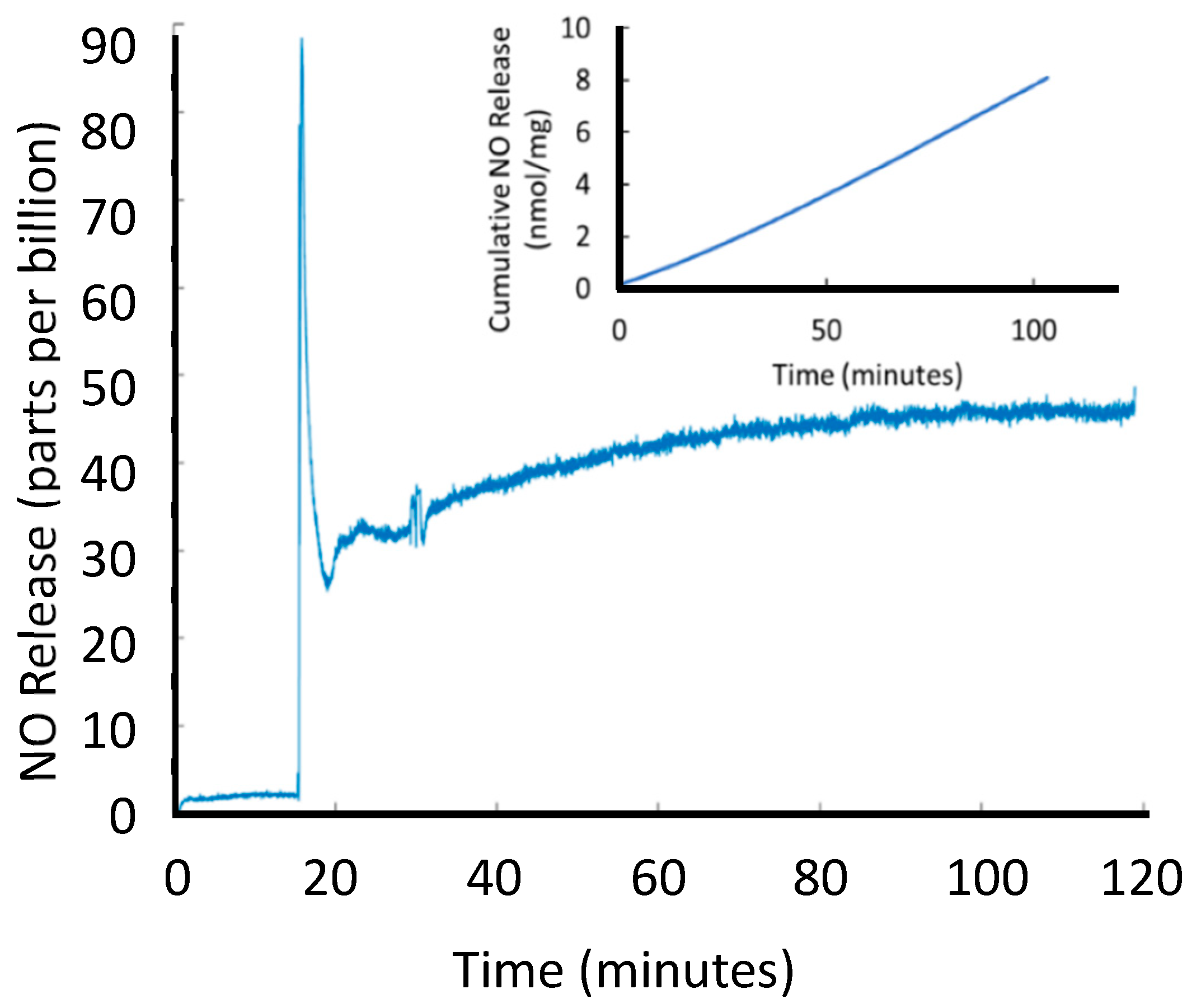
3.2.2. Ion-Mediated NO Release
3.3. Material Characterization
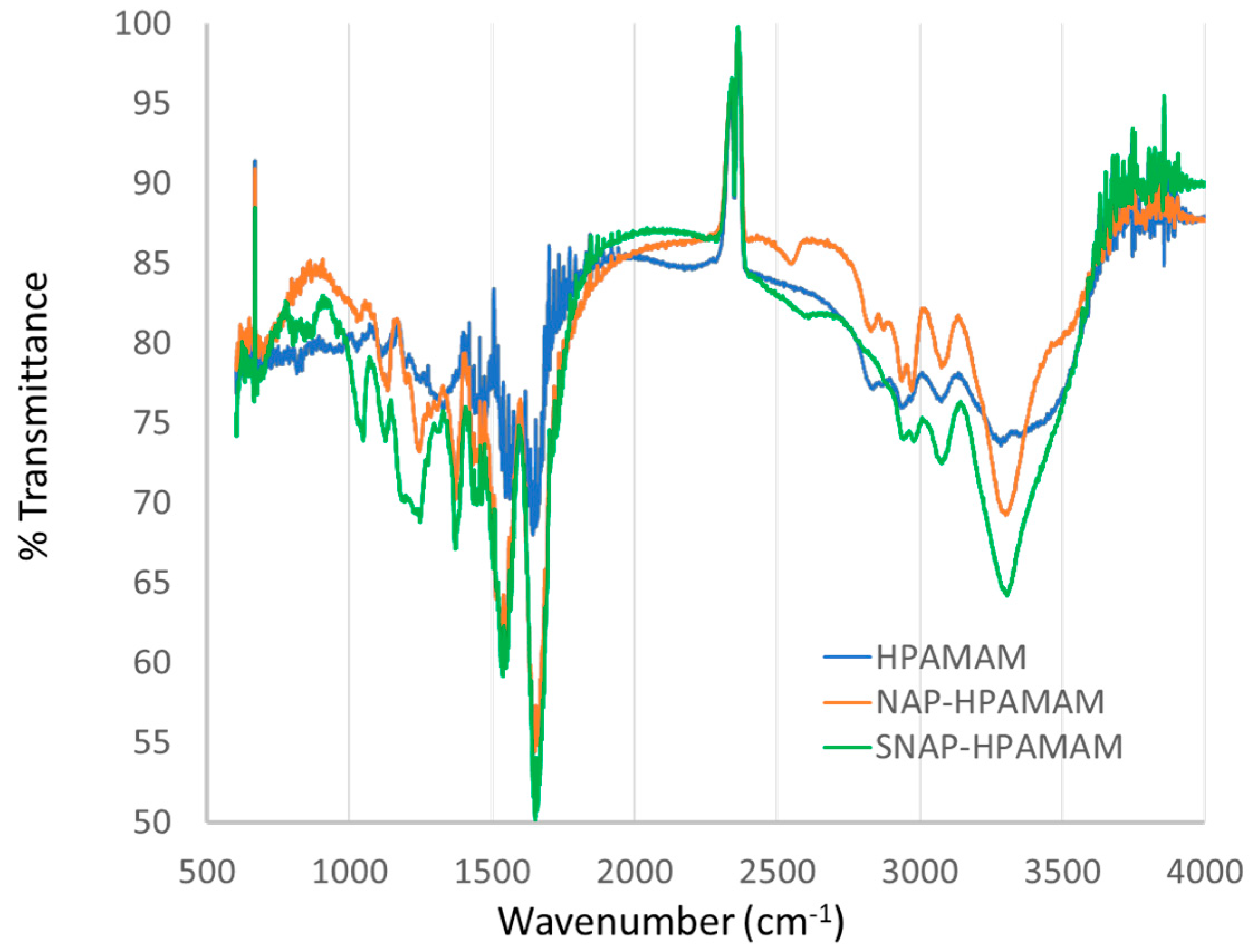
3.3.1. FTIR
3.3.2. NMR
3.3.3. Quantification of Thiols and Primary Amines
3.3.4. Determination of NO Loading
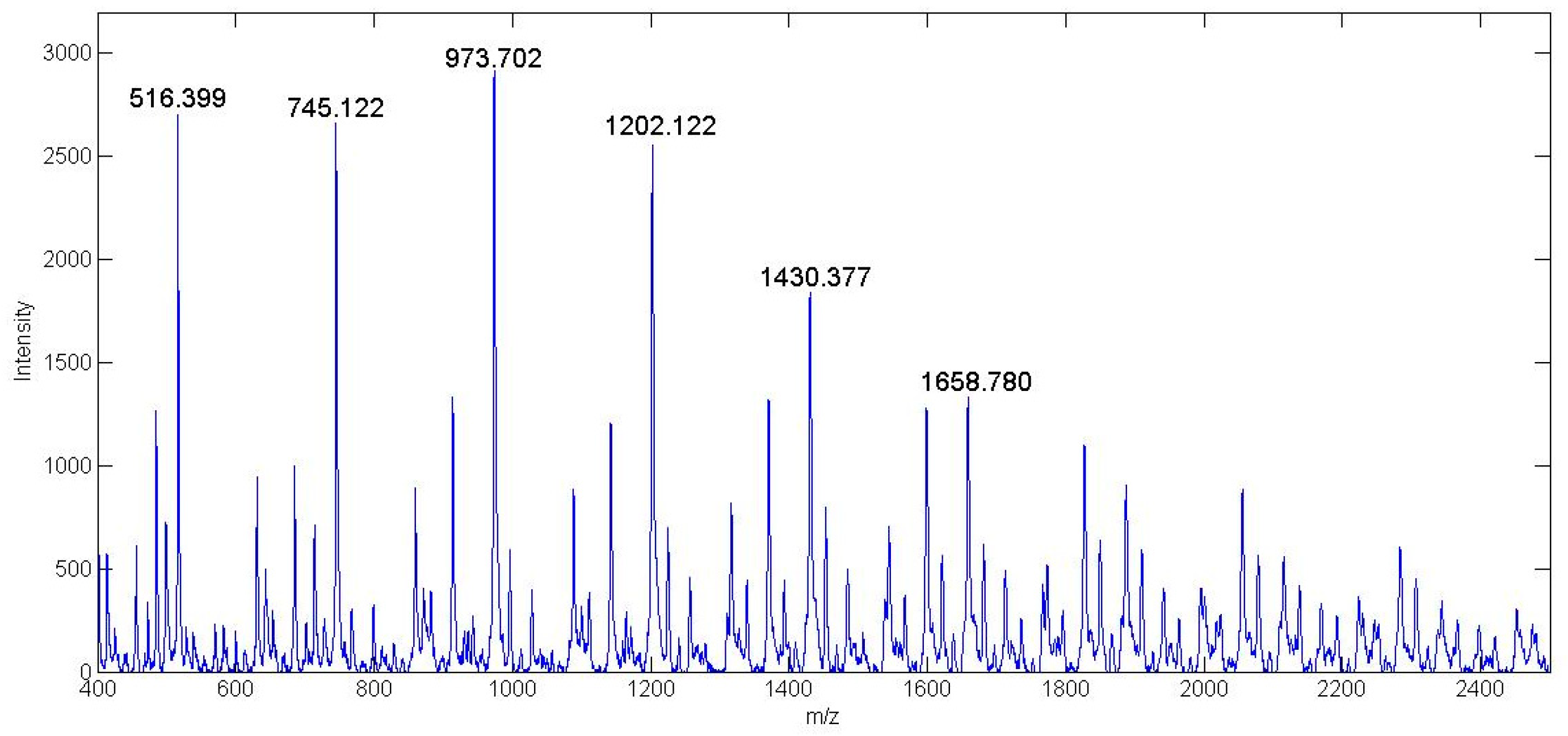
3.3.5. MALDI-TOF
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chaux, A.; Min Ruanet, X.; Fishbein, M.C.; Ouyang, Y.; Kaul, S.; Pass, J.A.; Matloff, J.M. Perivascular delivery of a nitric oxide donor inhibits neointimal hyperplasia in vein grafts implanted in the arterial circulation. J. Thorac. Cardiovasc. Surg. 1998, 115, 604–614. [Google Scholar] [CrossRef]
- Gifford, R. Mediation ofin vivo glucose sensor inflammatory response via nitric oxide release. J. Biomed. Mater. Res. Part A 2005, 75, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.C. Perspectives series: Host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Investig. 1997, 99, 2818. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.H. The chemistry of S-nitrosothiols. Acc. Chem. Res. 1999, 32, 869–876. [Google Scholar] [CrossRef]
- Keefer, L.K. ONOates (1-substituted diazen-1-ium-1, 2-diolates) as nitric oxide donors: Convenient nitric oxide dosage forms. Methods Enzym. 1996, 268, 281–293. [Google Scholar]
- Wang, P.G. Nitric oxide donors: Chemical activities and biological applications. Chem. Rev. 2002, 102, 1091–1134. [Google Scholar] [CrossRef]
- Buhleier, E. Ligand Structure and Complexation, XIII: 2,2’-Bipyridine as a Building Block for New Aza Crown Ethers and Cryptands. Chemsche Ber. 1978, 111, 200–204. [Google Scholar] [CrossRef]
- Newkome, G.R. Micelles. Part 1. Cascade molecules: A new approach to micelles. A [27]-arborol. J. Org. Chem. 1985, 50, 2003–2004. [Google Scholar] [CrossRef]
- Tomalia, D.A. A new class of polymers: Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Gillies, E.R.; Frechet, J.M. Design, synthesis, and biological evaluation of a robust, biodegradable dendrimer. Drug Discov. Today 2005, 10, 35–43. [Google Scholar] [CrossRef]
- Majoros, I.J. PAMAM dendrimer-based multifunctional conjugate for cancer therapy: Synthesis, characterization, and functionality. Biomacromolecules 2006, 7, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-O. Development of biomaterials for gene therapy. Mol. Ther. 2000, 2, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Kukowska-Latallo, J.F. Efficient transfer of genetic material into mammalian cells using Starburst polyamidoamine dendrimers. Proc. Natl. Acad. Sci. USA 1996, 93, 4897–4902. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Izzo, L. Dendrimer biocompatibility and toxicity. Adv. Drug Del. Rev. 2005, 57, 2215–2237. [Google Scholar] [CrossRef] [PubMed]
- Nyitrai, G. Sodium selective ion channel formation in living cell membranes by polyamidoamine dendrimer. Biochim. Biophys. Acta (Bba) Biomembr. 2013, 1828, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.A. Reduced ischemia/reperfusion injury via glutathione-initiated nitric oxide-releasing dendrimers. Nitric Oxide 2010, 22, 30–36. [Google Scholar] [CrossRef]
- Stasko, N.A. S-Nitrosothiol-Modified Dendrimers as Nitric Oxide Delivery Vehicles. Biomacromolecules 2008, 9, 834–841. [Google Scholar] [CrossRef]
- Lu, Y. Nitric oxide-releasing amphiphilic poly (amidoamine) (PAMAM) dendrimers as antibacterial agents. Biomacromolecules 2013, 14, 3589–3598. [Google Scholar] [CrossRef]
- Worley, B.V. Active release of nitric oxide-releasing dendrimers from electrospun polyurethane fibers. Acs Biomater. Sci. Eng. 2016, 2, 426–437. [Google Scholar] [CrossRef]
- Frechet, J.M. Self-condensing vinyl polymerization: An approach to dendritic materials. Science 1995, 269, 1080–1083. [Google Scholar] [CrossRef]
- Kou, Y.; Wan, A. Synthesis of novel N-diazeniumdiolates based on hyperbranched polyethers. Bioorg. Med. Chem. Lett. 2008, 18, 2337–2341. [Google Scholar] [CrossRef] [PubMed]
- Keefer, L.K. Chemistry of the Diazeniumdiolates I. Structural and Spectral Characteristics of the [N (O) NO]−Functional Group. Nitric Oxide 2001, 5, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Klaykruayat, B. Chemical modification of chitosan with cationic hyperbranched dendritic polyamidoamine and its antimicrobial activity on cotton fabric. Carbohydr. Polym. 2010, 80, 197–207. [Google Scholar] [CrossRef]
- Moynihan, H.A.; Roberts, S.M. Preparation of some novel S-nitroso compounds as potential slow-release agents of nitric oxide in vivo. J. Chem. Soc. Perkin Trans. 1 1994, 7, 797–805. [Google Scholar] [CrossRef]
- Yang, B.K. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Free Radic. Res. 2003, 37, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y. Fabrication of nitric oxide-releasing porous polyurethane membranes-coated needle-type implantable glucose biosensors. Chem. Mater. 2011, 23, 4227–4233. [Google Scholar] [CrossRef][Green Version]
- Sun, B. Nitric oxide-releasing dendrimers as antibacterial agents. Biomacromolecules 2012, 13, 3343–3354. [Google Scholar] [CrossRef]
- Yates, C.; Hayes, W. Synthesis and applications of hyperbranched polymers. Eur. Polym. J. 2004, 40, 1257–1281. [Google Scholar] [CrossRef]
- Mikhelson, K. Ion-selective electrodes in PVC matrix. Sens. Actuators B Chem. 1994, 18, 31–37. [Google Scholar] [CrossRef]
- Gierke, G.E. S-Nitroso-N-acetyl-D-penicillamine covalently linked to polydimethylsiloxane (SNAP–PDMS) for use as a controlled photoinitiated nitric oxide release polymer. Sci. Technol. Adv. Mat. 2016, 12, 055007. [Google Scholar] [CrossRef]
- Hopkins, S.P.; Frost, M.C. Synthesis and Characterization of Controlled Nitric Oxide Release from S- Nitroso-N-Acetyl-D-Penicillamine Covalently Linked to Polyvinyl Chloride (SNAP-PVC). Bioengineering 2018, 5, 72. [Google Scholar] [CrossRef] [PubMed]
- Frost, M.C.; Meyerhoff, M.E. Synthesis, characterization, and controlled nitric oxide release from S-nitrosothiol-derivatized fumed silica polymer filler particles. J. Biomed. Mater. Res. Part A 2005, 72A, 409–419. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Frost, M.C. CellNO trap: Novel device for quantitative, real-time, direct measurement of nitric oxide from cultured RAW 267.4 macrophages. Redox Biol. 2016, 8, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Lindström, A.; Hakkarainen, M. Environmentally friendly plasticizers for poly(vinyl chloride)—Improved mechanical properties and compatibility by using branched poly(butylene adipate) as a polymeric plasticize. J. Appl. Polym. Sci. 2006, 100, 2180–2188. [Google Scholar] [CrossRef]
- Liu, J. Design of 3-(4-carboxybenzoyl)-2-quinolinecarboxaldehyde as a reagent for ultrasensitive determination of primary amines by capillary electrophoresis using laser fluorescence detection. Anal. Chem. 1991, 63, 408–412. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hopkins, S.P.; Frost, M.C. S-Nitroso-N-Acetyl-D-Penicillamine Modified Hyperbranched Polyamidoamine for High-Capacity Nitric Oxide Storage and Release. Bioengineering 2020, 7, 9. https://doi.org/10.3390/bioengineering7010009
Hopkins SP, Frost MC. S-Nitroso-N-Acetyl-D-Penicillamine Modified Hyperbranched Polyamidoamine for High-Capacity Nitric Oxide Storage and Release. Bioengineering. 2020; 7(1):9. https://doi.org/10.3390/bioengineering7010009
Chicago/Turabian StyleHopkins, Sean P., and Megan C. Frost. 2020. "S-Nitroso-N-Acetyl-D-Penicillamine Modified Hyperbranched Polyamidoamine for High-Capacity Nitric Oxide Storage and Release" Bioengineering 7, no. 1: 9. https://doi.org/10.3390/bioengineering7010009
APA StyleHopkins, S. P., & Frost, M. C. (2020). S-Nitroso-N-Acetyl-D-Penicillamine Modified Hyperbranched Polyamidoamine for High-Capacity Nitric Oxide Storage and Release. Bioengineering, 7(1), 9. https://doi.org/10.3390/bioengineering7010009



