Material-Dependent Formation and Degradation of Bone Matrix—Comparison of Two Cryogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Scaffold Manufacturing and Sterilization
2.2. Physical Characterization of Scaffolds
2.2.1. Pore Structure
2.2.2. Porosity and Water Uptake Rate
2.2.3. Permeability
2.2.4. Mineral Content
2.2.5. Matrix Stiffness
2.3. Cell Culture
2.3.1. Cell Lines
2.3.2. Cell Seeding on the Scaffolds
2.3.3. Osteogenic Differentiation of SCP-1 Cells
2.3.4. Osteogenic Maturation of SaOS-2 Cells
2.3.5. Osteoclastic Differentiation of THP-1 Cells
2.4. Functional Testings
2.4.1. Live–Dead-Staining
2.4.2. Mitochondrial Activity (Resazurin Conversion)
2.4.3. Quantification of Total DNA
2.4.4. Dot Blot Analysis
2.4.5. Changes in the Scaffolds’ Stiffness and Mineral Content
2.5. Statistical Analysis
3. Results
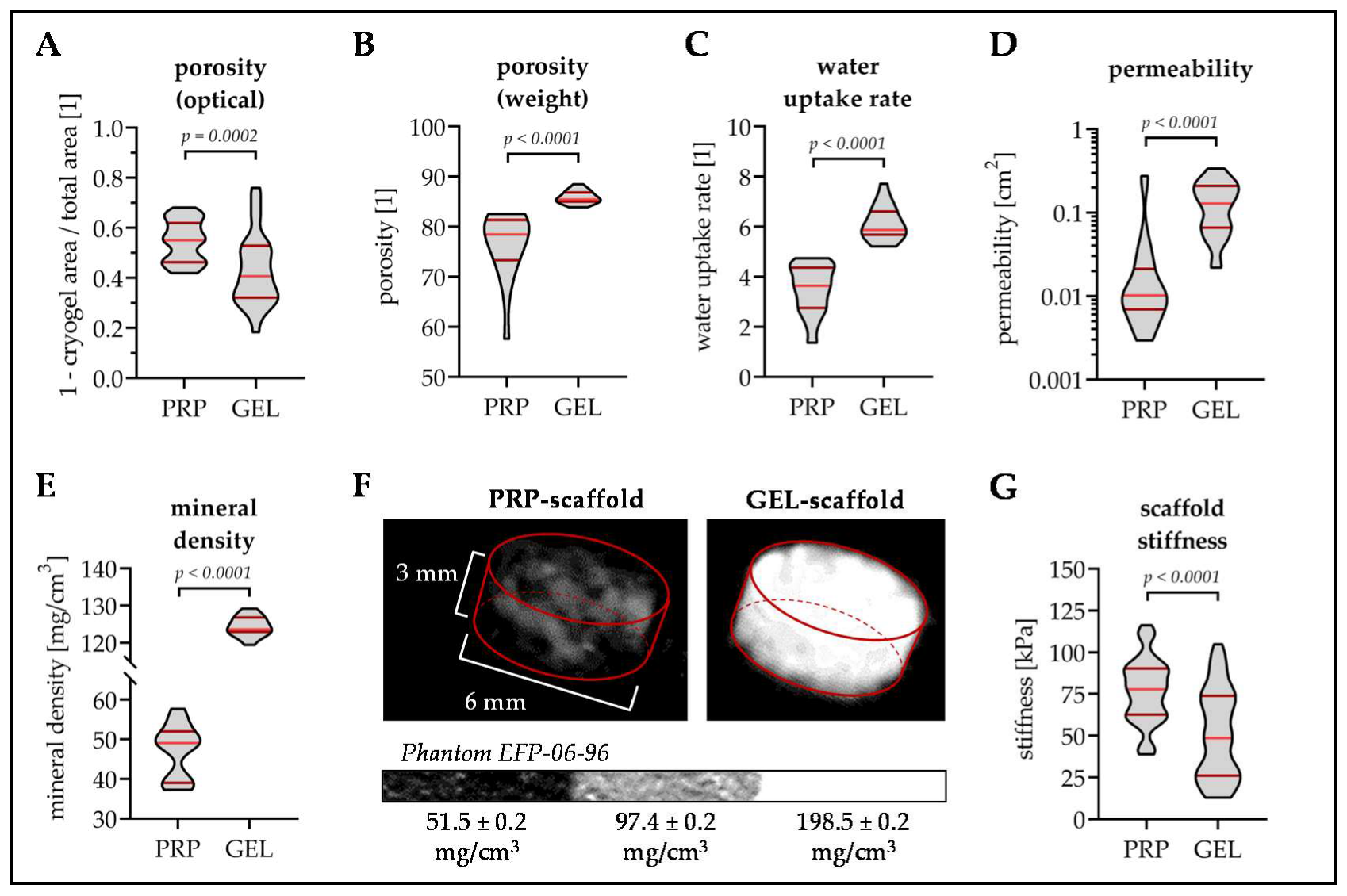
3.1. GEL Scaffolds Have Larger Pores Than PRP Scaffolds
3.2. PRP Scaffolds Have A Higher Porosity and Stiffness Than GEL Scaffolds
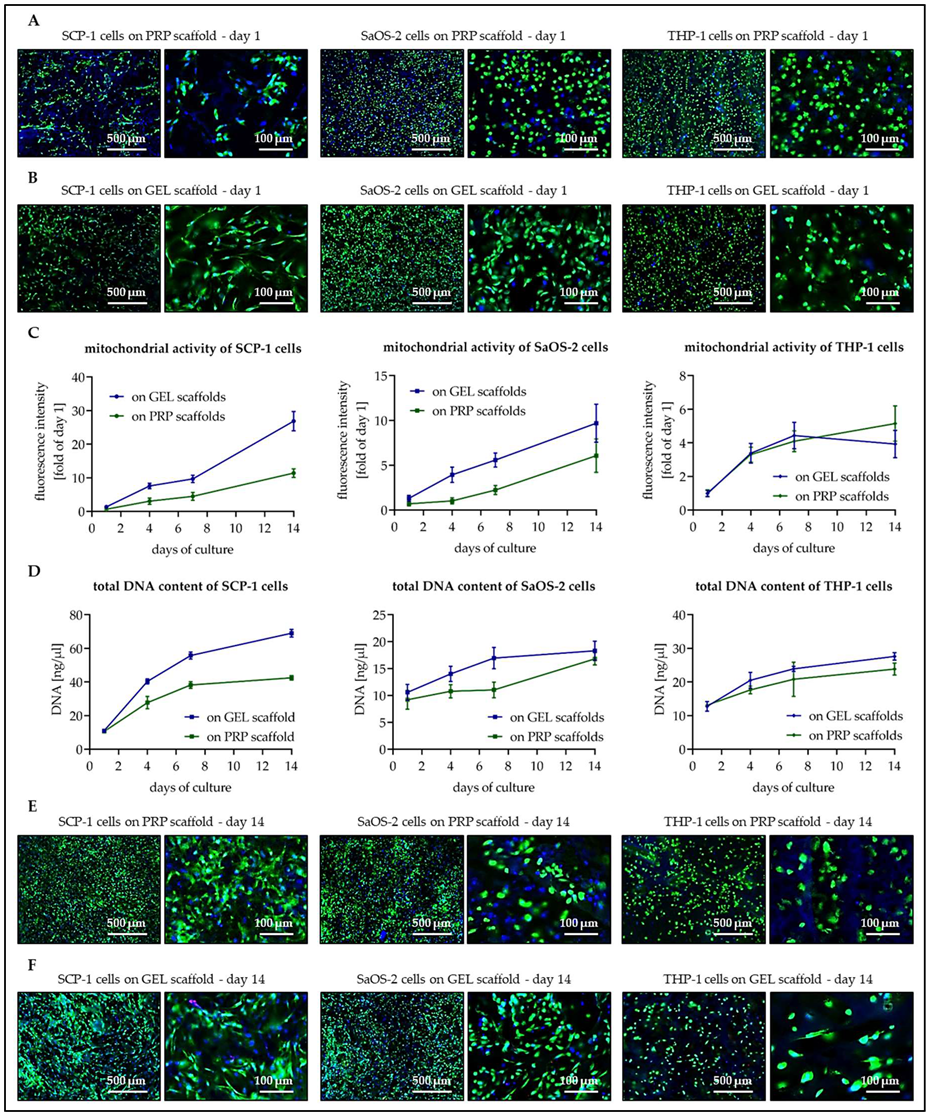
3.3. Osteogenic Cells Show Better Cell Attachment and Faster Proliferation on GEL Than on PRP Scaffolds
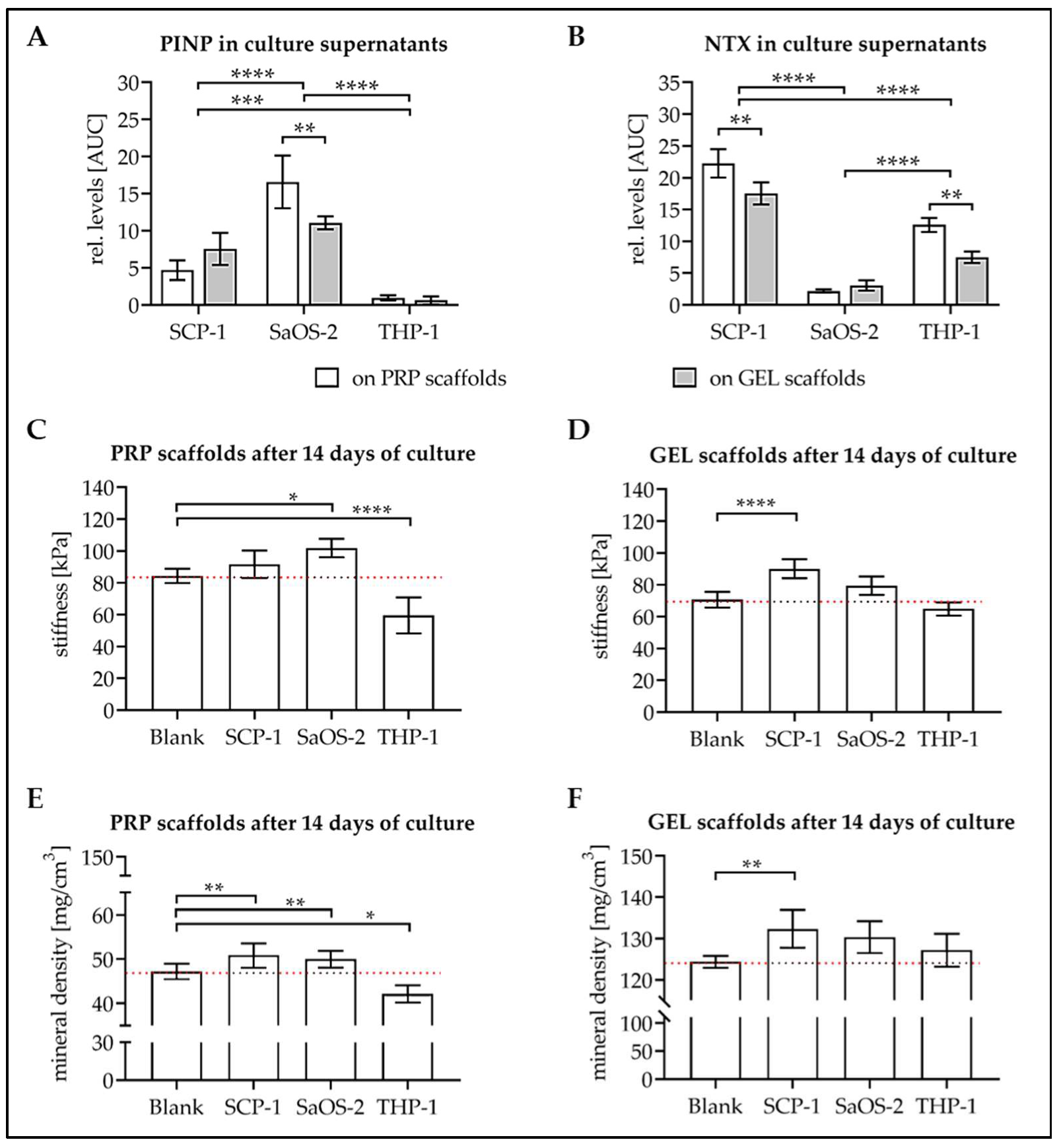
3.4. Levels of Osteoblast and Osteoclast Markers in Culture Supernatant
3.5. Culture with the Cells Alters Stiffness and Mineral Content of the Scaffolds
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abreviations
| AAc | acrylic acid |
| AAm | acrylamide |
| ALP | alkaline phosphatase |
| AMPS | 2-acrylamido-2-methylpropane sulfonic acid sodium salt |
| APS | ammonium persulfate |
| BAAm | N,N-methylene(bis)acrylamide |
| BDDE | 1,4-butanediol diglycidyl ether |
| CTSK | cathepsin K |
| EGDE | ethylene glycol diglycidyl ether |
| GEL | gelatin |
| HA | hydroxyapatite |
| HEMA | 2-hydroxyethyl methacrylate |
| M-CSF | macrophage colony-stimulating factor |
| MMA | methyl-methacrylate |
| NTX | collagen-type I N-telopeptide |
| OPG | Osteoprotegerin |
| PEGDA | poly-ethylene glycol diacrylate |
| PINP | procollagen type I N-terminal propeptide |
| PRP | platelet-rich plasma |
| RANKL | receptor activator of nuclear factor kappa-Β ligand |
| TEMED | N,N,N,N-tetramethyl-ethylenediamine |
| TRAP5b | tartrate-resistant acidic phosphatase 5b |
References
- Smith, J.O.; Aarvold, A.; Tayton, E.R.; Dunlop, D.G.; Oreffo, R.O. Skeletal tissue regeneration: Current approaches, challenges, and novel reconstructive strategies for an aging population. Tissue Eng. B Rev. 2011, 17, 307–320. [Google Scholar] [CrossRef]
- Kao, S.T.; Scott, D.D. A review of bone substitutes. Oral Maxillofac. Surg. Clin. N. Am. 2007, 19, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Granero-Molto, F.; Weis, J.A.; Miga, M.I.; Landis, B.; Myers, T.J.; O’Rear, L.; Longobardi, L.; Jansen, E.D.; Mortlock, D.P.; Spagnoli, A. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells 2009, 27, 1887–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, Y.; Harada, N.; Sato, K.; Abe, S.; Yamanaka, K.; Matushita, T. Stem cell therapy: Is there a future for reconstruction of large bone defects? Injury 2016, 47 (Suppl. 1), S47–S51. [Google Scholar] [CrossRef]
- Brydone, A.S.; Meek, D.; Maclaine, S. Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc. Inst. Mech. Eng. H 2010, 224, 1329–1343. [Google Scholar] [CrossRef] [PubMed]
- Toosi, S.; Naderi-Meshkin, H.; Kalalinia, F.; Peivandi, M.T.; Hossein Khani, H.; Bahrami, A.R.; Heirani-Tabasi, A.; Mirahmadi, M.; Behravan, J. Comparative characteristics of mesenchymal stem cells derived from reamer-irrigator-aspirator, iliac crest bone marrow, and adipose tissue. Cell. Mol. Biol. 2016, 62, 68–74. [Google Scholar]
- Haussling, V.; Deninger, S.; Vidoni, L.; Rinderknecht, H.; Ruoss, M.; Arnscheidt, C.; Athanasopulu, K.; Kemkemer, R.; Nussler, A.K.; Ehnert, S. Impact of Four Protein Additives in Cryogels on Osteogenic Differentiation of Adipose-Derived Mesenchymal Stem Cells. Bioengineeringb 2019, 6, 67. [Google Scholar] [CrossRef] [Green Version]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef]
- Bulnheim, U.; Müller, P.; Neumann, H.G.; Peters, K.; Unger, R.E.; Kirkpatrick, C.J.; Rychly, J. Endothelial cells stimulate osteogenic differentiation of mesenchymal stem cells on calcium phosphate scaffolds. J. Tissue Eng. Regen. Med. 2014, 8, 831–840. [Google Scholar] [CrossRef]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [Green Version]
- Memic, A.; Colombani, T.; Eggermont, L.J.; Rezaeeyazdi, M.; Steingold, J.; Rogers, Z.J.; Navare, K.J.; Mohammed, H.S.; Bencherif, S.A. Latest Advances in Cryogel Technology for Biomedical Applications. Adv. Ther. 2019, 2, 1800114. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Li, X.; Liu, X.; Zhang, N.; Wen, X. Effects of substrate stiffness on adipogenic and osteogenic differentiation of human mesenchymal stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 40, 316–323. [Google Scholar] [CrossRef]
- Dawson, J.I.; Oreffo, R.O. Bridging the regeneration gap: Stem cells, biomaterials and clinical translation in bone tissue engineering. Arch. Biochem. Biophys. 2008, 473, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Jacobs, C.R. Mechanically induced osteogenic lineage commitment of stem cells. Stem. Cell Res. 2013, 4, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludag, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef]
- Hixon, K.R.; Lu, T.; Sell, S.A. A comprehensive review of cryogels and their roles in tissue engineering applications. Acta Biomater. 2017, 62, 29–41. [Google Scholar] [CrossRef]
- Savina, I.N.; Ingavle, G.C.; Cundy, A.B.; Mikhalovsky, S.V. A simple method for the production of large volume 3D macroporous hydrogels for advanced biotechnological, medical and environmental applications. Sci. Rep. 2016, 6, 21154. [Google Scholar] [CrossRef] [Green Version]
- Saylan, Y.; Denizli, A. Supermacroporous Composite Cryogels in Biomedical Applications. Gels 2019, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Hwang, Y.; Kashif, M.; Jeong, D.; Kim, G. Evaluation of Bone Regeneration on Polyhydroxyethyl-polymethyl Methacrylate Membrane in a Rabbit Calvarial Defect Model. Vivo 2016, 30, 587–591. [Google Scholar]
- Kumari, J.; Karande, A.A.; Kumar, A. Combined Effect of Cryogel Matrix and Temperature-Reversible Soluble–Insoluble Polymer for the Development of in Vitro Human Liver Tissue. ACS Appl. Mater. Interfaces 2016, 8, 264–277. [Google Scholar] [CrossRef]
- Backer, A.; Goppert, B.; Sturm, S.; Abaffy, P.; Sollich, T.; Gruhl, F.J. Impact of adjustable cryogel properties on the performance of prostate cancer cells in 3D. SpringerPlus 2016, 5, 902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szczepanska, J.; Poplawski, T.; Synowiec, E.; Pawlowska, E.; Chojnacki, C.J.; Chojnacki, J.; Blasiak, J. 2-hydroxylethyl methacrylate (HEMA), a tooth restoration component, exerts its genotoxic effects in human gingival fibroblasts trough methacrylic acid, an immediate product of its degradation. Mol. Biol. Rep. 2012, 39, 1561–1574. [Google Scholar] [CrossRef] [Green Version]
- Doube, M.; Klosowski, M.M.; Arganda-Carreras, I.; Cordelieres, F.P.; Dougherty, R.P.; Jackson, J.S.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef] [Green Version]
- Ruoss, M.; Haussling, V.; Schugner, F.; Olde Damink, L.H.H.; Lee, S.M.L.; Ge, L.; Ehnert, S.; Nussler, A.K. A Standardized Collagen-Based Scaffold Improves Human Hepatocyte Shipment and Allows Metabolic Studies over 10 Days. Bioengineering 2018, 5, 86. [Google Scholar] [CrossRef] [Green Version]
- Tamjid, E.; Simchi, A.; Dunlop John, W.C.; Fratzl, P.; Bagheri, R.; Vossoughi, M. Tissue growth into three-dimensional composite scaffolds with controlled micro-features and nanotopographical surfaces. J. Biomed. Mater. Res. A 2013, 101, 2796–2807. [Google Scholar] [CrossRef] [PubMed]
- Bocker, W.; Yin, Z.; Drosse, I.; Haasters, F.; Rossmann, O.; Wierer, M.; Popov, C.; Locher, M.; Mutschler, W.; Docheva, D.; et al. Introducing a single-cell-derived human mesenchymal stem cell line expressing hTERT after lentiviral gene transfer. J. Cell. Mol. Med. 2008, 12, 1347–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aspera-Werz, R.H.; Chen, T.; Ehnert, S.; Zhu, S.; Frohlich, T.; Nussler, A.K. Cigarette Smoke Induces the Risk of Metabolic Bone Diseases: Transforming Growth Factor Beta Signaling Impairment via Dysfunctional Primary Cilia Affects Migration, Proliferation, and Differentiation of Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 2915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehnert, S.; Linnemann, C.; Aspera-Werz, R.H.; Bykova, D.; Biermann, S.; Fecht, L.; De Zwart, P.M.; Nussler, A.K.; Stuby, F. Immune Cell Induced Migration of Osteoprogenitor Cells Is Mediated by TGF-beta Dependent Upregulation of NOX4 and Activation of Focal Adhesion Kinase. Int. J. Mol. Sci. 2018, 19, 2239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.H.; Si, Y.; Xu, G.; Chen, X.M.; Xiong, H.; Lai, L.; Zheng, Y.Q.; Zhang, Z.G. High-dose PMA with RANKL and MCSF induces THP1 cell differentiation into human functional osteoclasts in vitro. Mol. Med. Rep. 2017, 16, 8380–8384. [Google Scholar] [CrossRef] [Green Version]
- Jain, K.G.; Mohanty, S.; Ray, A.R.; Malhotra, R.; Airan, B. Culture & differentiation of mesenchymal stem cell into osteoblast on degradable biomedical composite scaffold: In vitro study. Indian J. Med. Res. 2015, 142, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Mayr-Wohlfart, U.; Fiedler, J.; Gunther, K.P.; Puhl, W.; Kessler, S. Proliferation and differentiation rates of a human osteoblast-like cell line (SaOS-2) in contact with different bone substitute materials. J. Biomed. Mater. Res. 2001, 57, 132–139. [Google Scholar] [CrossRef]
- Ayobian-Markazi, N.; Fourootan, T.; Kharazifar, M.J. Comparison of cell viability and morphology of a human osteoblast-like cell line (SaOS-2) seeded on various bone substitute materials: An in vitro study. Dent. Res. J. 2012, 9, 86–92. [Google Scholar] [CrossRef]
- George, J.; Kuboki, Y.; Miyata, T. Differentiation of mesenchymal stem cells into osteoblasts on honeycomb collagen scaffolds. Biotechnol. Bioeng. 2006, 95, 404–411. [Google Scholar] [CrossRef]
- Ofner, C.M., 3rd; Bubnis, W.A. Chemical and swelling evaluations of amino group crosslinking in gelatin and modified gelatin matrices. Pharm. Res. 1996, 13, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Yates, K.; Vogt, C.; Qian, Z.; Frost, M.C.; Zhao, F. Increasing mechanical strength of gelatin hydrogels by divalent metal ion removal. Sci. Rep. 2014, 4, 4706. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef]
- Thein-Han, W.W.; Misra, R.D. Biomimetic chitosan-nanohydroxyapatite composite scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 1182–1197. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Dong, C.; Yang, L.; Lv, Y. 3D Scaffolds with Different Stiffness but the Same Microstructure for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2015, 7, 15790–15802. [Google Scholar] [CrossRef]
- Faia-Torres, A.B.; Guimond-Lischer, S.; Rottmar, M.; Charnley, M.; Goren, T.; Maniura-Weber, K.; Spencer, N.D.; Reis, R.L.; Textor, M.; Neves, N.M. Differential regulation of osteogenic differentiation of stem cells on surface roughness gradients. Biomaterials 2014, 35, 9023–9032. [Google Scholar] [CrossRef] [PubMed]
- Faia-Torres, A.B.; Charnley, M.; Goren, T.; Guimond-Lischer, S.; Rottmar, M.; Maniura-Weber, K.; Spencer, N.D.; Reis, R.L.; Textor, M.; Neves, N.M. Osteogenic differentiation of human mesenchymal stem cells in the absence of osteogenic supplements: A surface-roughness gradient study. Acta Biomater. 2015, 28, 64–75. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, P.S.; Loboa, E.G. Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways. Tissue Eng. B Rev. 2012, 18, 436–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joaquin, D.; Grigola, M.; Kwon, G.; Blasius, C.; Han, Y.; Perlitz, D.; Jiang, J.; Ziegler, Y.; Nardulli, A.; Hsia, K.J. Cell migration and organization in three-dimensional in vitro culture driven by stiffness gradient. Biotechnol. Bioeng. 2016, 113, 2496–2506. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, M.J.; Dennis, C.; Yang, X.B.; Kirkham, J. Tissue non-specific alkaline phosphatase production by human dental pulp stromal cells is enhanced by high density cell culture. Cell Tissue Res. 2015, 361, 529–540. [Google Scholar] [CrossRef] [Green Version]
- Rochet, N.; Leroy, P.; Far, D.F.; Ollier, L.; Loubat, A.; Rossi, B. CAL72: A human osteosarcoma cell line with unique effects on hematopoietic cells. Eur. J. Haematol. 2003, 70, 43–52. [Google Scholar] [CrossRef]
- Sun, M.; Chi, G.; Xu, J.; Tan, Y.; Xu, J.; Lv, S.; Xu, Z.; Xia, Y.; Li, L.; Li, Y. Extracellular matrix stiffness controls osteogenic differentiation of mesenchymal stem cells mediated by integrin alpha5. Stem. Cell Res. 2018, 9, 52. [Google Scholar] [CrossRef]
- Shapira, L.; Halabi, A. Behavior of two osteoblast-like cell lines cultured on machined or rough titanium surfaces. Clin. Oral Implant. Res. 2009, 20, 50–55. [Google Scholar] [CrossRef]
- Glantschnig, H.; Fisher, J.E.; Wesolowski, G.; Rodan, G.A.; Reszka, A.A. M-CSF, TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003, 10, 1165–1177. [Google Scholar] [CrossRef] [Green Version]
- Berube, P.; Yang, Y.; Carnes, D.L.; Stover, R.E.; Boland, E.J.; Ong, J.L. The effect of sputtered calcium phosphate coatings of different crystallinity on osteoblast differentiation. J. Periodontol. 2005, 76, 1697–1709. [Google Scholar] [CrossRef]
- Kostenuik, P.J.; Shalhoub, V. Osteoprotegerin: A physiological and pharmacological inhibitor of bone resorption. Curr. Pharm. Des. 2001, 7, 613–635. [Google Scholar] [CrossRef] [PubMed]
- Pandit, V.; Zuidema, J.M.; Venuto, K.N.; Macione, J.; Dai, G.; Gilbert, R.J.; Kotha, S.P. Evaluation of multifunctional polysaccharide hydrogels with varying stiffness for bone tissue engineering. Tissue Eng. A 2013, 19, 2452–2463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotz, E.M.; Berger, M.B.; Boyan, B.D.; Schwartz, Z. Regulation of mesenchymal stem cell differentiation on microstructured titanium surfaces by semaphorin 3A. Bone 2020, 134, 115260. [Google Scholar] [CrossRef]
- Daculsi, G.; Passuti, N. Effect of the macroporosity for osseous substitution of calcium phosphate ceramics. Biomaterials 1990, 11, 86–87. [Google Scholar]
- Murphy, C.M.; O’Brien, F.J. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adhes. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Kopesky, P.; Tiedemann, K.; Alkekhia, D.; Zechner, C.; Millard, B.; Schoeberl, B.; Komarova, S.V. Autocrine signaling is a key regulatory element during osteoclastogenesis. Biol. Open 2014, 3, 767–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, N.; Udagawa, N.; Suda, T. Vitamin D endocrine system and osteoclasts. BoneKEy Rep. 2014, 3, 495. [Google Scholar] [CrossRef] [Green Version]
- Crotti, T.N.; O’Sullivan, R.P.; Shen, Z.; Flannery, M.R.; Fajardo, R.J.; Ross, F.P.; Goldring, S.R.; McHugh, K.P. Bone matrix regulates osteoclast differentiation and annexin A8 gene expression. J. Cell. Physiol. 2011, 226, 3413–3421. [Google Scholar] [CrossRef]
- Cappariello, A.; Maurizi, A.; Veeriah, V.; Teti, A. The Great Beauty of the osteoclast. Arch. Biochem. Biophys. 2014, 558, 70–78. [Google Scholar] [CrossRef]
- Rucci, N.; Teti, A. The “love-hate” relationship between osteoclasts and bone matrix. Matrix Biol. 2016, 52–54, 176–190. [Google Scholar] [CrossRef] [PubMed]



| Monomers | Cross-linker | Starter |
|---|---|---|
| alginate | G:M ratio | Ca2+, Mg2+, etc. |
| gelatin | Glutaraldehyde | |
| collagen | ||
| fibrinogen | ||
| serum albumin | ||
| hyaluronic acid | ||
| chitosan | ||
| acrylamide (AAm) and its derivatives | N,N-methylene(bis) acrylamide (BAAm) or methyl-methacrylate (MMA) or poly-ethylene glycol diacrylate (PEGDA) or biodegradable cross-linkers | ammonium persulfate (APS) + N,N,N,N-tetramethyl- ethylenediamine (TEMED) |
| acrylic acid (AAc) | ||
| 2-hydroxyethyl methacrylate (HEMA) | ||
| ethylene glycol diglycidyl ether (EGDE) | ||
| 1,4-butanediol diglycidyl ether (BDDE) | ||
| 2-acrylamido-2-methylpropane sulfonic acid sodium salt (AMPS) | ||
| Target Protein | Role | Species | Order # | Company |
|---|---|---|---|---|
| ALP | early osteogenic marker | goat | sc-23430 | Santa Cruz, Heidelberg, GER |
| Cathepsin K | osteoclast marker | mouse | sc-48353 | Santa Cruz, Heidelberg, GER |
| M-CSF | inducer for osteoclastogenesis | rabbit | 500-P44 | Peprotech, Hamburg, GER |
| NTX | collagen type I degradation | rabbit | PAA639hu01 | Cloud-Clone, Aachen, GER |
| OPG | inhibitor for RANKL | rabbit | 500-P149 | Peprotech, Hamburg, GER |
| PINP | collagen type I formation | rabbit | abx131414 | Abbexa, Aachen, GER |
| RANKL | inducer for osteoclastogenesis | mouse | 500-M46 | Peprotech, Hamburg, GER |
| TRAP5b | osteoclast marker | mouse | sc-376875 | Santa Cruz, Heidelberg, GER |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, W.; Häussling, V.; Aspera-Werz, R.H.; Springer, F.; Rinderknecht, H.; Braun, B.; Küper, M.A.; Nussler, A.K.; Ehnert, S. Material-Dependent Formation and Degradation of Bone Matrix—Comparison of Two Cryogels. Bioengineering 2020, 7, 52. https://doi.org/10.3390/bioengineering7020052
Weng W, Häussling V, Aspera-Werz RH, Springer F, Rinderknecht H, Braun B, Küper MA, Nussler AK, Ehnert S. Material-Dependent Formation and Degradation of Bone Matrix—Comparison of Two Cryogels. Bioengineering. 2020; 7(2):52. https://doi.org/10.3390/bioengineering7020052
Chicago/Turabian StyleWeng, Weidong, Victor Häussling, Romina H. Aspera-Werz, Fabian Springer, Helen Rinderknecht, Bianca Braun, Markus A. Küper, Andreas K. Nussler, and Sabrina Ehnert. 2020. "Material-Dependent Formation and Degradation of Bone Matrix—Comparison of Two Cryogels" Bioengineering 7, no. 2: 52. https://doi.org/10.3390/bioengineering7020052
APA StyleWeng, W., Häussling, V., Aspera-Werz, R. H., Springer, F., Rinderknecht, H., Braun, B., Küper, M. A., Nussler, A. K., & Ehnert, S. (2020). Material-Dependent Formation and Degradation of Bone Matrix—Comparison of Two Cryogels. Bioengineering, 7(2), 52. https://doi.org/10.3390/bioengineering7020052






