Influence of Saline Buffers over the Stability of High-Annealed Gold Nanoparticles Formed on Coverslips for Biological and Chemosensing Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
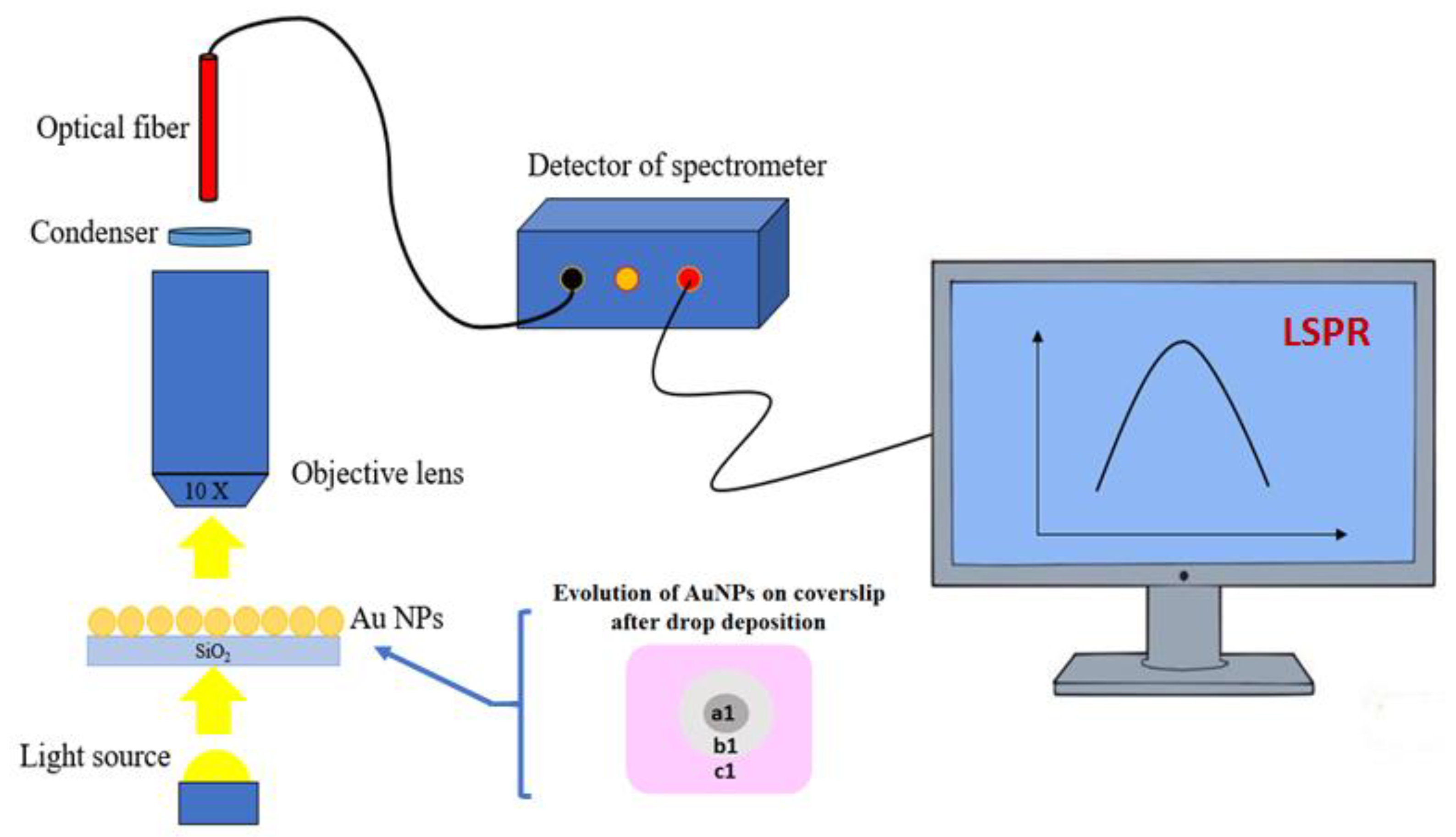
2.2. Instruments
2.3. Data Analysis
2.4. Preparation of Saline Buffers
2.5. Preparation of Annealed Gold Nanostructures on Coverslips
2.6. Effect of Buffers over the Stability of Annealed Gold on Thin Glasses
3. Results and Discussions
3.1. Characterization of Annealed Gold Coated Coverslips—Bare AuNPs
3.2. Influence of Water and Saline Buffers over the Stability of Annealed AuNPs on Coverslips
3.2.1. Study of Water Solvent
3.2.2. Study of SSPE Buffer
3.2.3. Study of PBS Buffer
3.3. Sensing of Chemical BPE Molecules in Aqueous Solution with SEM, AFM and LSPR Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malekzad, H.; Zangabad, P.S.; Mirshekari, H.; Karimi, M.; Hamblin, M.R. Noble metal nanoparticles in biosensors: Recent studies and applications. Nanotechnol. Rev. 2017, 6, 301–329. [Google Scholar] [CrossRef]
- Cobley, C.M.; Chen, J.; Cho, E.C.; Wang, L.V.; Xia, Y. Gold nanostructures: A class of multifunctional materials for biomedical applications. Chem. Soc. Rev. 2011, 40, 44–56. [Google Scholar] [CrossRef]
- You, Z.; Qiu, Q.; Chen, H.; Feng, Y.; Wang, X.; Wang, Y.; Ying, Y. Laser-induced noble metal nanoparticle-graphene composites enabled flexible biosensor for pathogen detection. Biosens. Bioelectron. 2020, 150, 111896. [Google Scholar] [CrossRef] [PubMed]
- Presnova, G.; Presnov, D.; Krupenin, V.; Grigorenko, V.; Trifonov, A.; Andreeva, I.; Ignatenko, O.; Egorov, A.; Rubtsova, M. Biosensor based on a silicon nanowire field-effect transistor functionalized by gold nanoparticles for the highly sensitive determination of prostate specific antigen. Biosens. Bioelectron. 2017, 88, 283–289. [Google Scholar] [CrossRef]
- Qin, L.; Zeng, G.; Lai, C.; Huang, D.; Xu, P.; Zhang, C.; Cheng, M.; Liu, X.; Liu, S.; Li, B.; et al. “Gold rush” in modern science: Fabrication strategies and typical advanced applications of gold nanoparticles in sensing. Coord. Chem. Rev. 2018, 359, 1–31. [Google Scholar] [CrossRef]
- Qu, F.; Huang, W.; You, J. A fluorescent sensor for detecting dopamine and tyrosinase activity by dual-emission carbon dots and gold nanoparticles. Colloids Surf. B Biointerfaces 2018, 162, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ali, M.R.K.; Chen, K.; Fang, N.; El-Sayed, M.A. Gold nanoparticles in biological optical imaging. NanoToday 2019, 24, 120–140. [Google Scholar] [CrossRef]
- Zeng, S.; Yong, K.-T.; Roy, I.; Dinh, X.-Q.; Yu, X.; Luan, F. A Review on functionalized gold nanoparticles for biosensing applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Guliy, O.I.; Kanevskiy, M.V.; Fomin, A.S.; Staroverov, S.A.; Bunin, V.D. Progress in the use of an electro-optical sensor for virus detection. Opt. Commun. 2020, 465, 125605. [Google Scholar] [CrossRef]
- Alharbi, R.; Irannejad, M.; Yavuz, M. A short review on the role of the metal-graphene hybrid nanostructure in promoting the localized surface plasmon resonance sensor performance. Sensors 2019, 19, 862. [Google Scholar] [CrossRef]
- Jatschka, J.; Dathe, A.; Csáki, A.; Fritzsche, W.; Stranik, Q. Propagating and localized surface plasmon resonance sensing—A critical comparison based on measurements and theory. Sens. Bio Sens. Res. 2016, 7, 62–70. [Google Scholar] [CrossRef]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Schmid, G.; Chi, L.F. Metal clusters and colloids. Adv. Mater. 1998, 10, 515–526. [Google Scholar] [CrossRef]
- Stobiecka, M.; Deeb, J.; Hepel, M. Ligand exchange effects in gold nanoparticle assembly induced by oxidative stress biomarkers: Homocysteine and cysteine. Biophys. Chem. 2010, 146, 98–107. [Google Scholar] [CrossRef]
- Stobiecka, M.; Coopersmith, K.; Hepel, M. Resonance elastic light scattering (RELS) spectroscopy of fast non-Langmuirian ligand-exchange in glutathione-induced gold nanoparticle assembly. J. Colloid Interface Sci. 2020, 350, 168–177. [Google Scholar] [CrossRef]
- Stobiecka, M.; Hepel, M. Rapid functionalization of metal nanoparticles by moderator-tunable ligand-exchange process for biosensor designs. Sens. Actuators B Chem. 2020, 149, 373–380. [Google Scholar] [CrossRef]
- Jain, N.; Bhargava, A.; Majumdar, S.; Tarafdar, J.C.; Panwar, J. Extracellular biosynthesis and characterization of silver nanoparticles using Aspergillus flavusNJP08: A mechanism perspective. Nanoscale 2011, 3, 635–641. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Mycocrystallization of gold ions by the fungus Cylindrocladium floridanum. World J. Microbiol. Biotechnol. 2013, 29, 2207–2211. [Google Scholar] [CrossRef]
- Singh, R.; Thakur, P.; Thakur, A.; Kumar, H.; Chawla, P.; V. Rohit, J.; Kaushik, R.; Kumar, N. Colorimetric sensing approaches of surface-modified gold and silver nanoparticles for detection of residual pesticides: A review. Int. J. Environ. Anal. Chem. 2020, 1–17, in press. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.-M.; Eritja, R.; Oliveira-Brett, A.M. Electrochemical and AFM characterization of G-quadruplex electrochemical biosensors and applications. J. Nucleic Acids 2018, 2018, 5307106. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, T.; Pan, Y.; Ciacchi, L.C.; Xu, B.; Wei, G. AFM-based force spectroscopy for bioimaging and biosensing. RSC Adv. 2016, 6, 12893–12912. [Google Scholar] [CrossRef]
- Manzano, M.; Vizzini, P.; Jia, K.; Adam, P.-M.; Ionescu, R.E. Development of localized surface plasmon resonance biosensors for the detection of Brettanomyces bruxellensis in wine. Sens. Actuators B Chem. 2016, 223, 295–300. [Google Scholar] [CrossRef]
- Miranda-Castro, R.; Sánchez-Salcedo, R.; Suárez-Álvarez, B.; Los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J. Thioaromatic DNA monolayers for target-amplification-free electrochemical sensing of environmental pathogenic bacteria. Biosens. Bioelectron 2017, 92, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Batule, B.S.; Seok, Y.; Kim, M.-G. Paper-based nucleic acid testing system for simple and early diagnosis of mosquito-borne RNA viruses from human serum. Biosens. Bioelectron. 2020, 151, 111998. [Google Scholar] [CrossRef]
- Liu, L.; Wei, Y.; Jiao, S.; Zhu, S.; Liu, X. A novel label-free strategy for the ultrasensitive miRNA-182 detection based on MoS2/Ti3C2 nanohybrids. Biosens. Bioelectron. 2019, 137, 45–51. [Google Scholar] [CrossRef]
- Hamid, R.; Zhad, L.Z.; Rodríguez Torres, Y.M.; Lai, R.Y. A reagentless and reusable electrochemical aptamer-based sensor for rapid detection of Cd (II). J. Electroanal. Chem. 2017, 803, 89–94. [Google Scholar]
- Tan, Y.; Qiu, J.; Cui, M.; Wei, X.; Zhao, M.; Qiu, B.; Chen, G. An immobilization free DNAzyme based electrochemical biosensor for lead determination. Analyst 2016, 141, 1121–1126. [Google Scholar] [CrossRef]
- Akhtar, N.; Emran, M.Y.; Shenashen, M.A.; Khalifa, H.; Osaka, T.; Faheem, A.; Homma, T.; Kawarada, H.; El-Safty, S.A. Fabrication of photo-electrochemical biosensors for ultrasensitive screening of mono-bioactive molecules: The effect of geometrical structures and crystal surfaces. J. Mater. Chem. B 2017, 5, 7985–7996. [Google Scholar] [CrossRef]
- Zhou, L.; Poggesi, S.; Casari Bariani, G.; Mittapalli, R.; Adam, P.-M.; Manzano, M.; Ionescu, R.E. Robust SERS platforms based on annealed gold nanostructures formed on ultrafine glass substrates for various (bio)applications. Biosensors 2019, 9, 53. [Google Scholar] [CrossRef]








| [BPE] | Fresh Functionalization | After One Week | After Two Weeks | After Three Weeks | After Four Weeks | After Five Weeks | |
|---|---|---|---|---|---|---|---|
| 10−3 M | λmax (nm) | 548.210 | 549.904 | 550.792 | 553.910 | 552.660 | 552.660 |
| ODmax | 0.13240 | 0.16940 | 0.18220 | 0.17680 | 0.16668 | 0.16760 | |
| 10−5 M | λmax (nm) | 548.566 | 549.814 | 550.260 | 553.110 | 552.660 | 552.660 |
| ODmax | 0.12480 | 0.16760 | 0.18100 | 0.17440 | 0.17420 | 0.17540 | |
| 10−7 M | λmax (nm) | 550.706 | 549.636 | 548.566 | 553.110 | 559.780 | 552.660 |
| ODmax | 0.12300 | 0.14680 | 0.15720 | 0.14320 | 0.13960 | 0.14600 | |
| 10−9 M | λmax (nm) | 551.864 | 551.062 | 550.440 | 553.288 | 559.780 | 559.780 |
| ODmax | 0.10820 | 0.13380 | 0.14560 | 0.12980 | 0.14600 | 0.14680 | |
| 10−11 M | λmax (nm) | 551.864 | 539.290 | 542.412 | 548.654 | 559.870 | 552.660 |
| ODmax | 0.10740 | 0.13020 | 0.14540 | 0.13940 | 0.12640 | 0.13140 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Ionescu, R.E. Influence of Saline Buffers over the Stability of High-Annealed Gold Nanoparticles Formed on Coverslips for Biological and Chemosensing Applications. Bioengineering 2020, 7, 68. https://doi.org/10.3390/bioengineering7030068
Zhou L, Ionescu RE. Influence of Saline Buffers over the Stability of High-Annealed Gold Nanoparticles Formed on Coverslips for Biological and Chemosensing Applications. Bioengineering. 2020; 7(3):68. https://doi.org/10.3390/bioengineering7030068
Chicago/Turabian StyleZhou, Lan, and Rodica Elena Ionescu. 2020. "Influence of Saline Buffers over the Stability of High-Annealed Gold Nanoparticles Formed on Coverslips for Biological and Chemosensing Applications" Bioengineering 7, no. 3: 68. https://doi.org/10.3390/bioengineering7030068
APA StyleZhou, L., & Ionescu, R. E. (2020). Influence of Saline Buffers over the Stability of High-Annealed Gold Nanoparticles Formed on Coverslips for Biological and Chemosensing Applications. Bioengineering, 7(3), 68. https://doi.org/10.3390/bioengineering7030068






