Processing High-Solid and High-Ammonia Rich Manures in a Two-Stage (Liquid-Solid) Low-Temperature Anaerobic Digestion Process: Start-Up and Operating Strategies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock and Inoculum
2.2. Experimental Setup of Two-Stage (Liquid–Solid) Anaerobic Digesters
2.3. Analytical Methods
2.3.1. Biogas Analysis
2.3.2. Physiochemical Analysis
3. Results and Discussion
3.1. Characteristics of the Feedstock and the Inoculum
3.2. Influence of Operational Parameters in the Two-Stage AD Process Treating CM + DM Mixture
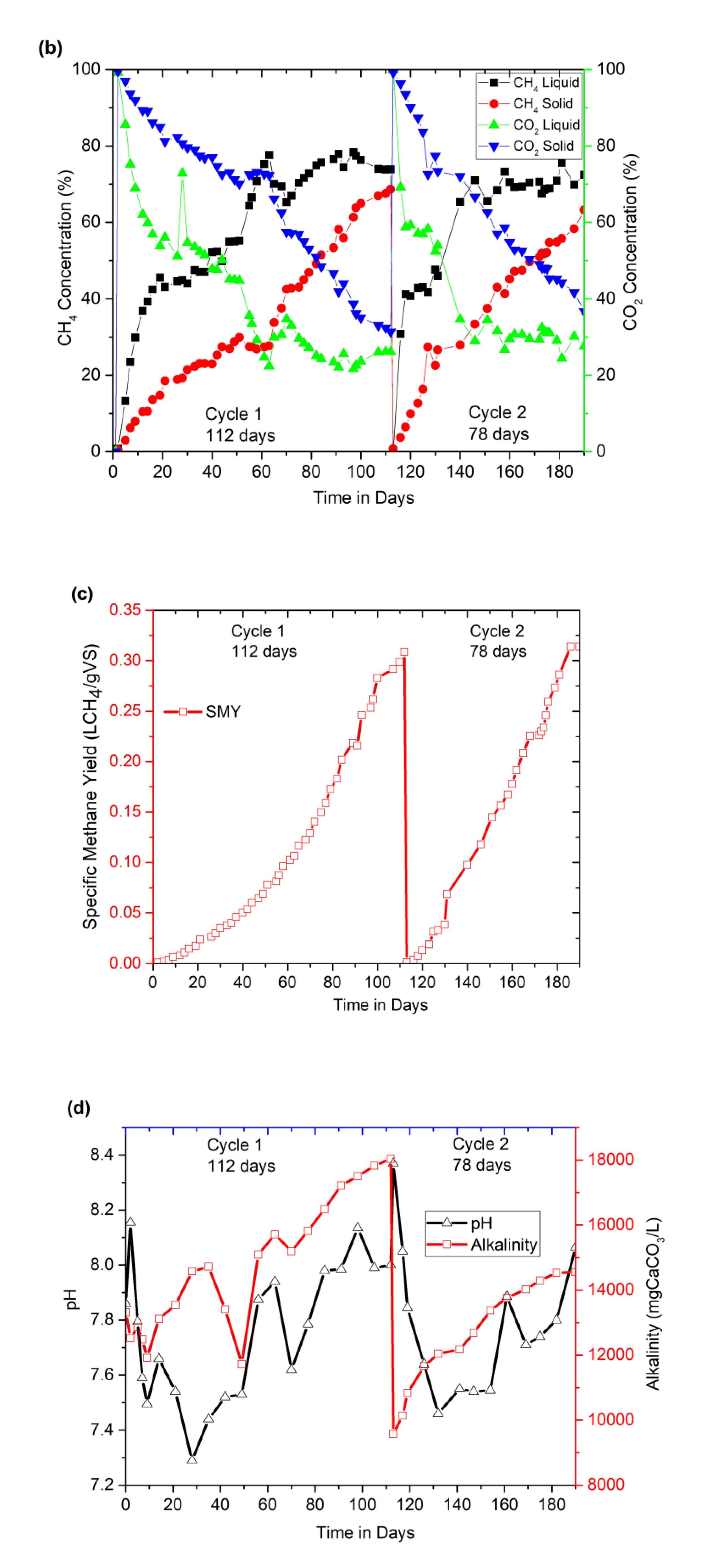
3.2.1. Performance of the Two-Stage AD at Different Cycles and OLRs: Biogas and Methane Production and Digester Buffering Indicators
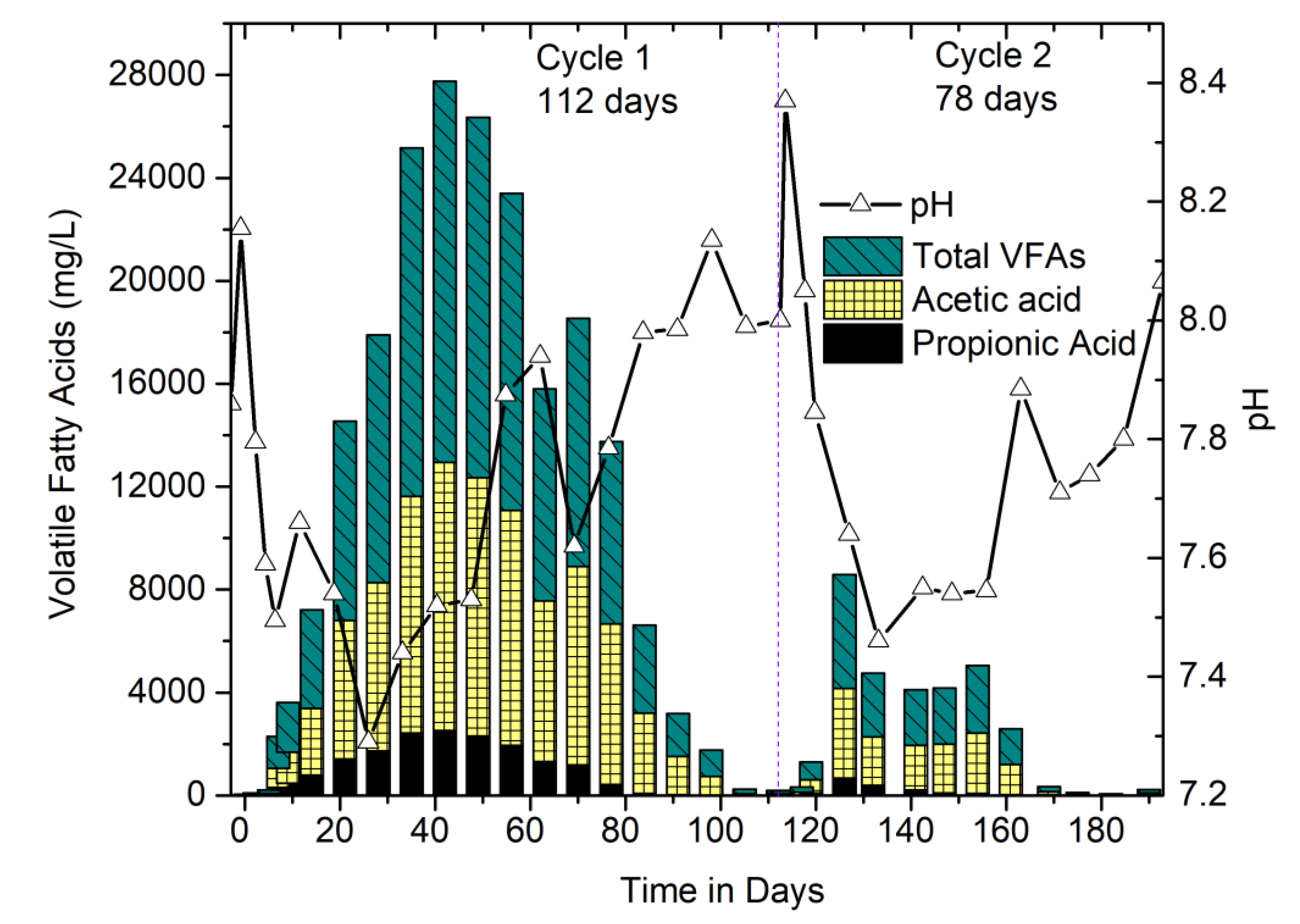
3.2.2. Performance Monitoring of Digesters: Correlation between VFAs, pH, and Methane Concentration
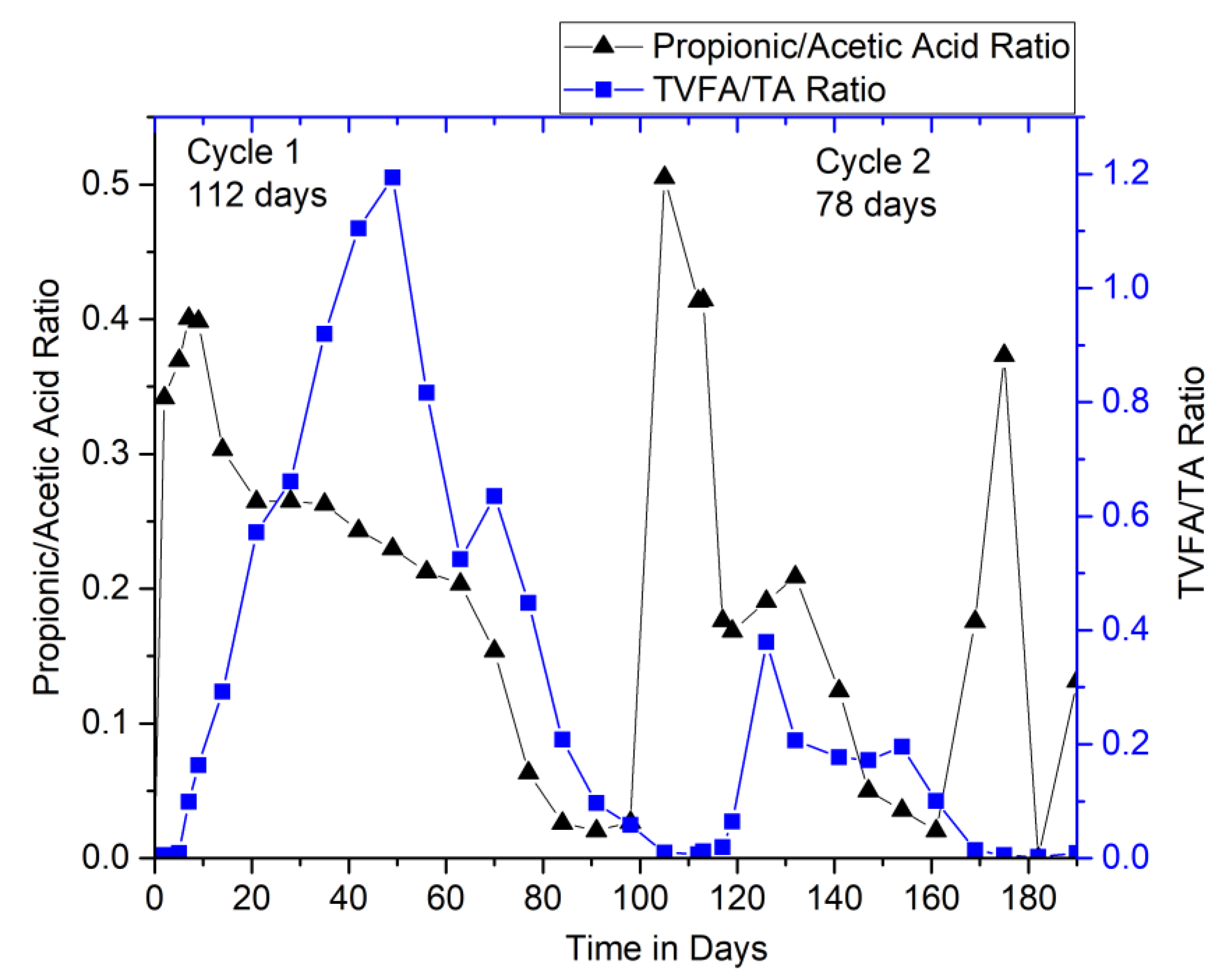
3.2.3. Performance Monitoring of Digesters: Ratio Limits
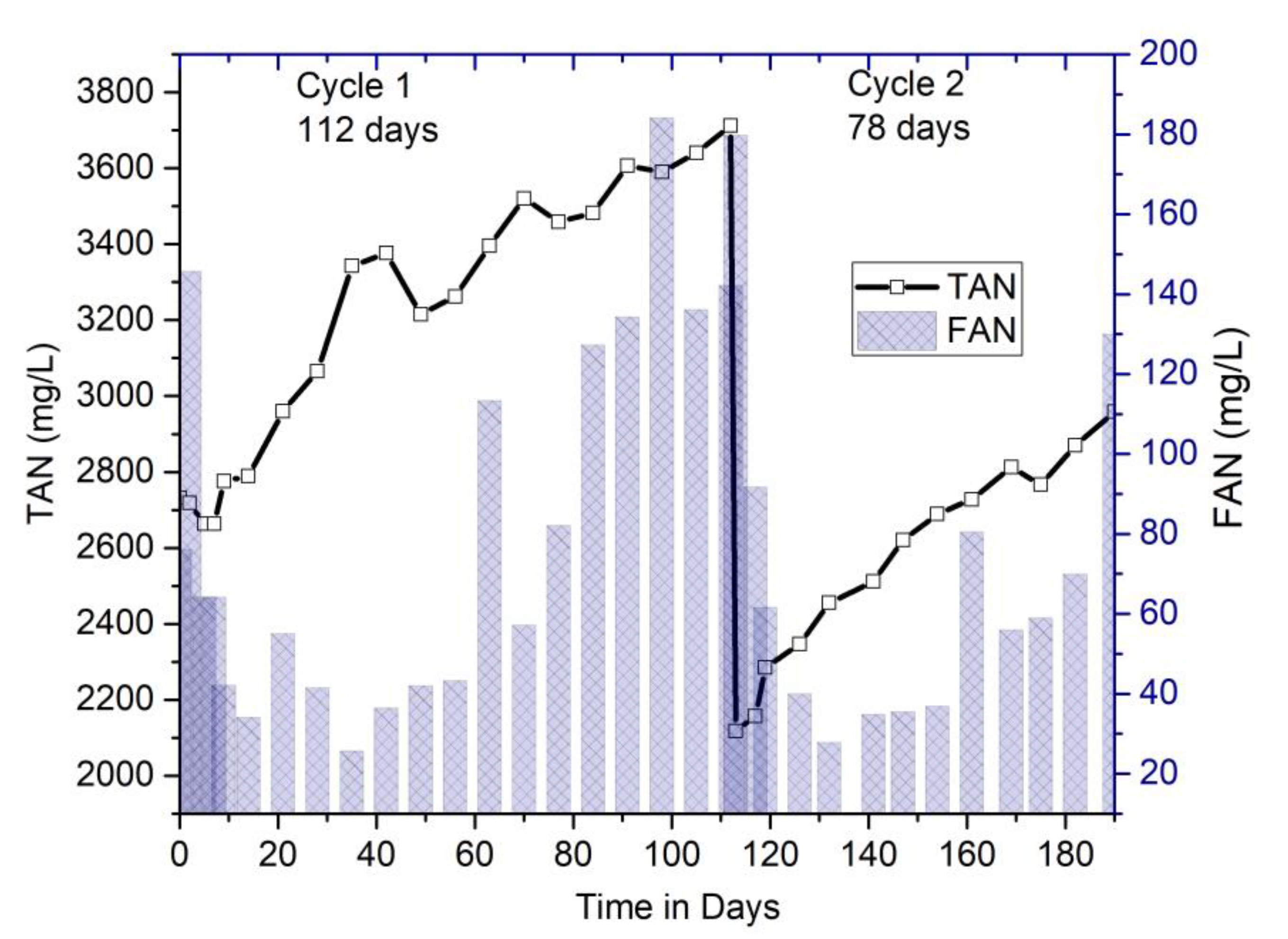
3.2.4. Performance Monitoring of Digesters: Relationship between pH, Ammonia (TAN, FAN), and Temperature
3.3. Comparative Study of Two-Stage (Liquid–Solid) AD of CM and Co-Digestion of CM + DM
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- High Level Expert Forum—How to Feed the World in 2050. Available online: http://www.fao.org/fileadmin/templates/wsfs/docs/Issues_papers/HLEF2050_Global_Agriculture.pdf (accessed on 3 March 2020).
- AVEC (Association of Poultry Processors and Poultry Trade in the EU Countries). Annual Report 2016. Available online: http://www.avec-poultry.eu/wp-content/uploads/2018/04/AVEC-2016-BAT.pdf (accessed on 8 June 2018).
- Fatma, A.; Namba, N.; Kesseva, M.R.; Nishio, N.; Nakashimada, Y. Enhancement of methane production from co-digestion of chicken manure with agricultural wastes. Bioresour. Technol. 2014, 159, 80–87. [Google Scholar]
- MacLeod, M.; Gerber, P.; Mottet, A. Greenhouse Gas Emissions from Pig and Chicken Supply Chains; Food and Agriculture Organization: Rome, Italy, 2012. [Google Scholar]
- Werner, F.; Wang, X.; Gabauer, W.; Ortner, M.; Li, Z. Tackling ammonia inhibition for efficient biogas production from chicken manure: Status and technical trends in Europe and China. Renew. Sustain. Energy Rev. 2018, 97, 186–199. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2001: The Scientific Basis; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Sun, X.; Lu, P.; Jiang, T.; Schuchardt, F.; Li, G. Influence of bulking agents on CH4, N2O, and NH3 emissions during rapid composting of pig manure from the Chinese Ganqinfen system. J. Zhejiang Univ. Sci. B 2014, 15, 353–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, D.; Henihan, A.M.; Bowen, B.; Lynch, D.; McDonnell, K.; Kwapinski, W. Utilisation of poultry litter as an energy feedstock. Biomass Bioenergy 2013, 49, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Kelleher, B.P.; Leahy, J.J.; Henihan, A.M.; O’Dwyer, T.F.; Sutton, D.; Leahy, M.J. Advances in poultry litter disposal technology—A review. Bioresour. Technol. 2002, 83, 27–36. [Google Scholar] [CrossRef]
- Rajinikanth, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar]
- Suleyman, K.Y.S.; Kocak, E. Anaerobic digestion technology in poultry and livestock waste treatment—A literature review. Waste Manag. Res. 2009, 27, 3–18. [Google Scholar]
- Matheri, A.N.; Ndiweni, S.N.; Belaid, M.; Muzenda, E.; Hubert, R. Optimising biogas production from anaerobic co-digestion of chicken manure and organic fraction of municipal solid waste. Renew. Sustain. Energy Rev. 2017, 80, 756–764. [Google Scholar] [CrossRef]
- Singh, K.; Lee, K.; Worley, J.; Risse, L.M.; Das, K.C. Anaerobic digestion of poultry litter: A review. Appl. Eng. Agric. 2010, 26, 677–688. [Google Scholar] [CrossRef]
- Siddique, M.N.I.; Munaim, M.S.A.; Wahid, Z.A. Mesophilic and thermophilic biomethane production by co-digesting pretreated petrochemical wastewater with beef and dairy cattle manure. J. Ind. Eng. Chem. 2014, 20, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Chen, S.; Li, X.; Lar, J.S.; He, Y.; Zhu, B. Anaerobic co-digestion of kitchen waste with cattle manure for biogas production. Energy Fuels 2009, 23, 2225–2228. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Rice, E.W.; Greenberg, A.E.; Franson, M.A.H.A. APHA: Standard Methods for the Examination of Water and Wastewater; Centennial Edition; APHA, AWWA, WEF: Washington, DC, USA, 2005. [Google Scholar]
- Massé, D.I.; Masse, L.; Croteau, F. The effect of temperature fluctuations on psychrophilic anaerobic sequencing batch reactors treating swine manure. Bioresour. Technol. 2003, 89, 57–62. [Google Scholar] [CrossRef]
- Esposito, G.; Frunzo, L.; Giordano, A.; Liotta, F.; Panico, A.; Pirozzi, F. Anaerobic co-digestion of organic wastes. Rev. Environ. Sci. Bio Technol. 2012, 11, 325–341. [Google Scholar] [CrossRef]
- Khanal, S.K. Anaerobic Biotechnology for Bioenergy Production: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Li, H.L.; Guo, X.L.; Cao, F.F.; Wang, Y. Process evolution of dry anaerobic co-digestion of cattle manure with kitchen waste. Chem. Biochem. Eng. Q. 2014, 28, 61–166. [Google Scholar]
- Rajagopal, R.; Ramirez, I.; Steyer, J.P.; Mehrotra, I.; Kumar, P.; Escudie, R.; Torrijos, M. Experimental and modeling investigations of a hybrid upflow anaerobic sludge-filter bed (UASFB) reactor. Water Sci. Technol. 2008, 58, 109–117. [Google Scholar]
- Brown, D.; Li, Y. Solid state anaerobic co-digestion of yard waste and food waste for biogas production. Bioresour. Technol. 2013, 127, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Ehimen, E.A.; Sun, Z.F.; Carrington, C.G.; Birch, E.J.; Eaton-Rye, J.J. Anaerobic digestion of microalgae residues resulting from the biodiesel production process. Appl. Energy 2011, 88, 3454–3463. [Google Scholar] [CrossRef]
- Hansen, K.H.; Angelidaki, I.; Ahring, B.K. Anaerobic digestion of swine manure: Inhibition by ammonia. Water Res. 1998, 32, 5–12. [Google Scholar] [CrossRef]




| Cycle 1 | Cycle 2 | |
|---|---|---|
| Total weight of feedstock treated | 7 kg (CM + DM) | 4.7 kg (CM + DM)+ 4.7 kg (Dry inoculum from cycle 1)) = 9.4 kg |
| Quantity increment (%) per cycle | - | 34% w/w |
| Mix ratio (CM:DM) | 1:1 | 1:1 |
| Volume of liquid inoculum | 25 L | 25 L |
| Solid substrate: liquid inoculum digester volumetric ratio | 1:3.6 | 1:2.6 |
| OLR (gVS/L.d) * | 3.7 | 4.7 |
| CM (C1) | CM + DM (C1) | CM (C2) | CM + DM (C2) | |
|---|---|---|---|---|
| Cycle length (retention time or treatment period) | 70 | 112 | 85 | 78 |
| Quantity of raw manure treated (kg) | 5.4 | 7 | 6.5 | 4.7 |
| Total volume of HSAD (L) | 60 | 40 | 60 | 40 |
| Total amount of solid material treated in HSAD (kg) | 10 | 7 | 10.8 | 9.4 |
| Total volume of liquid digester (L) | 60 | 40 | 60 | 40 |
| Active volume of liquid digester (L) | 25 | |||
| Quantity and frequency of liquid inoculum percolated-recirculated | 5L-thrice a week | |||
| Mode of operation | Batch | |||
| Temperature (°C) | 20 ± 1 | |||
| OLR (gVS/L.d) | 4.3 | 3.7 | 4.6 | 4.7 |
| Parameter | Cycle 1 | Cycle 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| CM | DM | Inoculum | CM + DM | CM | DM | Inoculum | CM + DM | |
| pH | 8.68 | 7.58 | 7.86 | 8.2 | 8.88 | 8.13 | 8.37 | 8.1 |
| CODt (mg/L) | 568,017 | 208,433 | 7121 | 405,534 | 565,885 | 188,341 | 5968 | 402,921 |
| CODs (mg/L) | 114,768 | 44,852 | 4415 | 94,044 | 111,545 | 34,017 | 3915 | 96,944 |
| Alkalinity (asmg/L CaCO3) | 33,282 | 13,932 | 13,313 | 12,649 | 30,486 | 11,126 | 9575 | - |
| TS (%) | 65 | 23.9 | 1.28 | 48 | 73 | 21.58 | 1.02 | 51 |
| VS (%) | 56 | 21.3 | 0.54 | 42 | 65 | 19.23 | 0.40 | 45 |
| TKN (mg/L) | 21,962 | 6749 | 3151 | 13,613 | 23,072 | 5194 | 2359 | 13,472 |
| NH3 (mg/L) | 6070 | 1389 | 2732 | 3470 | 7229 | 1795 | 2117 | - |
| TVFA (mg/L) | 11,588 | 6973 | 24 | 10,582 | 10,914 | 6499 | 116 | - |
| CODt/TKN | 25.8 | 31 | 2 | 30 | 25 | 36 | 3 | 30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahato, P.; Goyette, B.; Rahaman, M.S.; Rajagopal, R. Processing High-Solid and High-Ammonia Rich Manures in a Two-Stage (Liquid-Solid) Low-Temperature Anaerobic Digestion Process: Start-Up and Operating Strategies. Bioengineering 2020, 7, 80. https://doi.org/10.3390/bioengineering7030080
Mahato P, Goyette B, Rahaman MS, Rajagopal R. Processing High-Solid and High-Ammonia Rich Manures in a Two-Stage (Liquid-Solid) Low-Temperature Anaerobic Digestion Process: Start-Up and Operating Strategies. Bioengineering. 2020; 7(3):80. https://doi.org/10.3390/bioengineering7030080
Chicago/Turabian StyleMahato, Prativa, Bernard Goyette, Md. Saifur Rahaman, and Rajinikanth Rajagopal. 2020. "Processing High-Solid and High-Ammonia Rich Manures in a Two-Stage (Liquid-Solid) Low-Temperature Anaerobic Digestion Process: Start-Up and Operating Strategies" Bioengineering 7, no. 3: 80. https://doi.org/10.3390/bioengineering7030080
APA StyleMahato, P., Goyette, B., Rahaman, M. S., & Rajagopal, R. (2020). Processing High-Solid and High-Ammonia Rich Manures in a Two-Stage (Liquid-Solid) Low-Temperature Anaerobic Digestion Process: Start-Up and Operating Strategies. Bioengineering, 7(3), 80. https://doi.org/10.3390/bioengineering7030080






