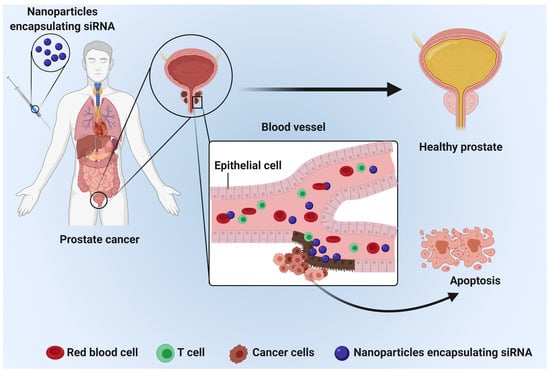
Progress in Delivery of siRNA-Based Therapeutics Employing Nano-Vehicles for Treatment of Prostate Cancer
Abstract
:1. Introduction
2. siRNA Structure and Function: A Brief Overview
3. siRNA Targets Signaling Pathways: Focus on PCa Therapy
4. The Dark Side of siRNA Delivery System: Challenges and Opportunities
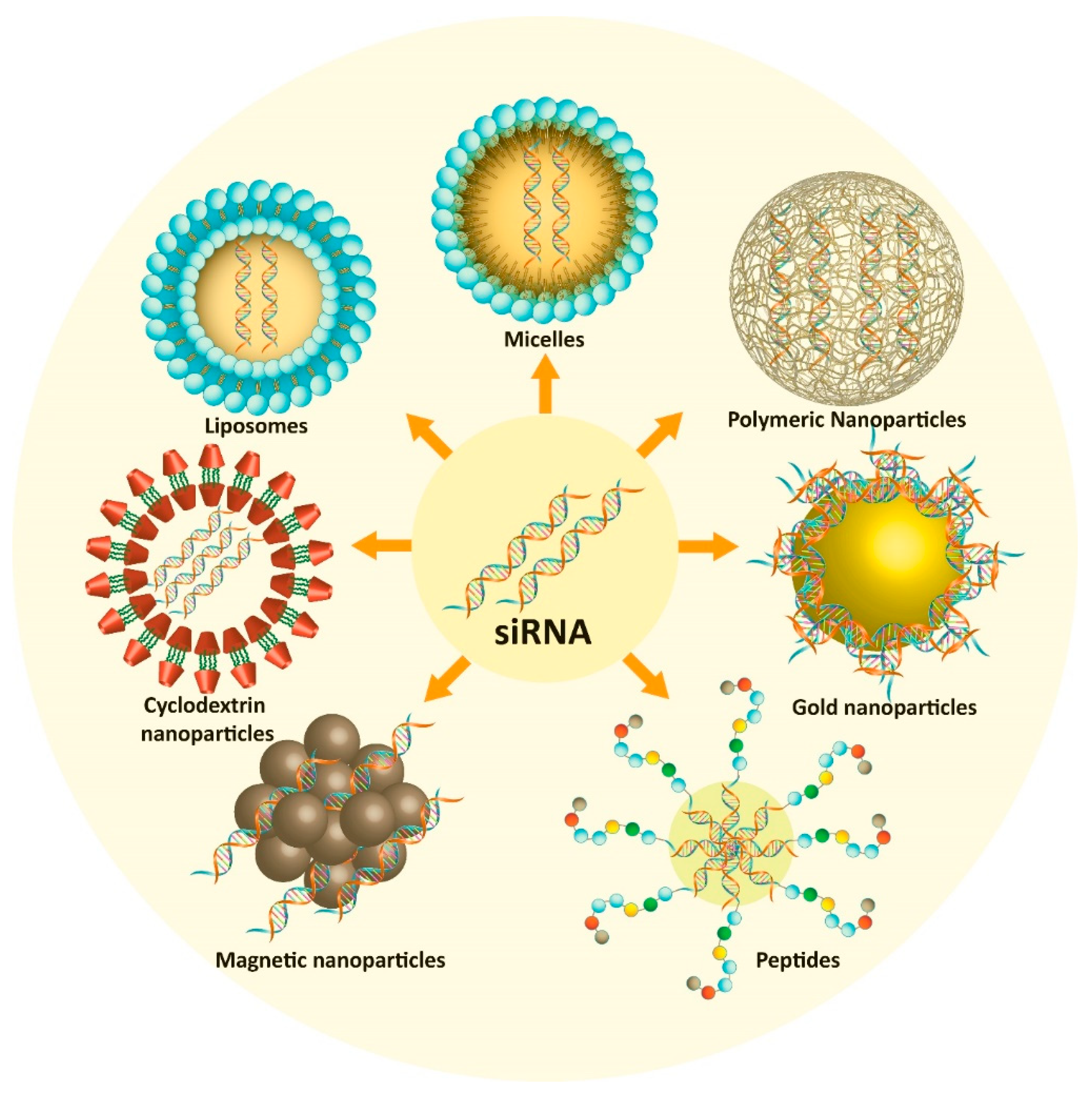
5. Nano-Vehicles
5.1. Polymeric Nanoparticles
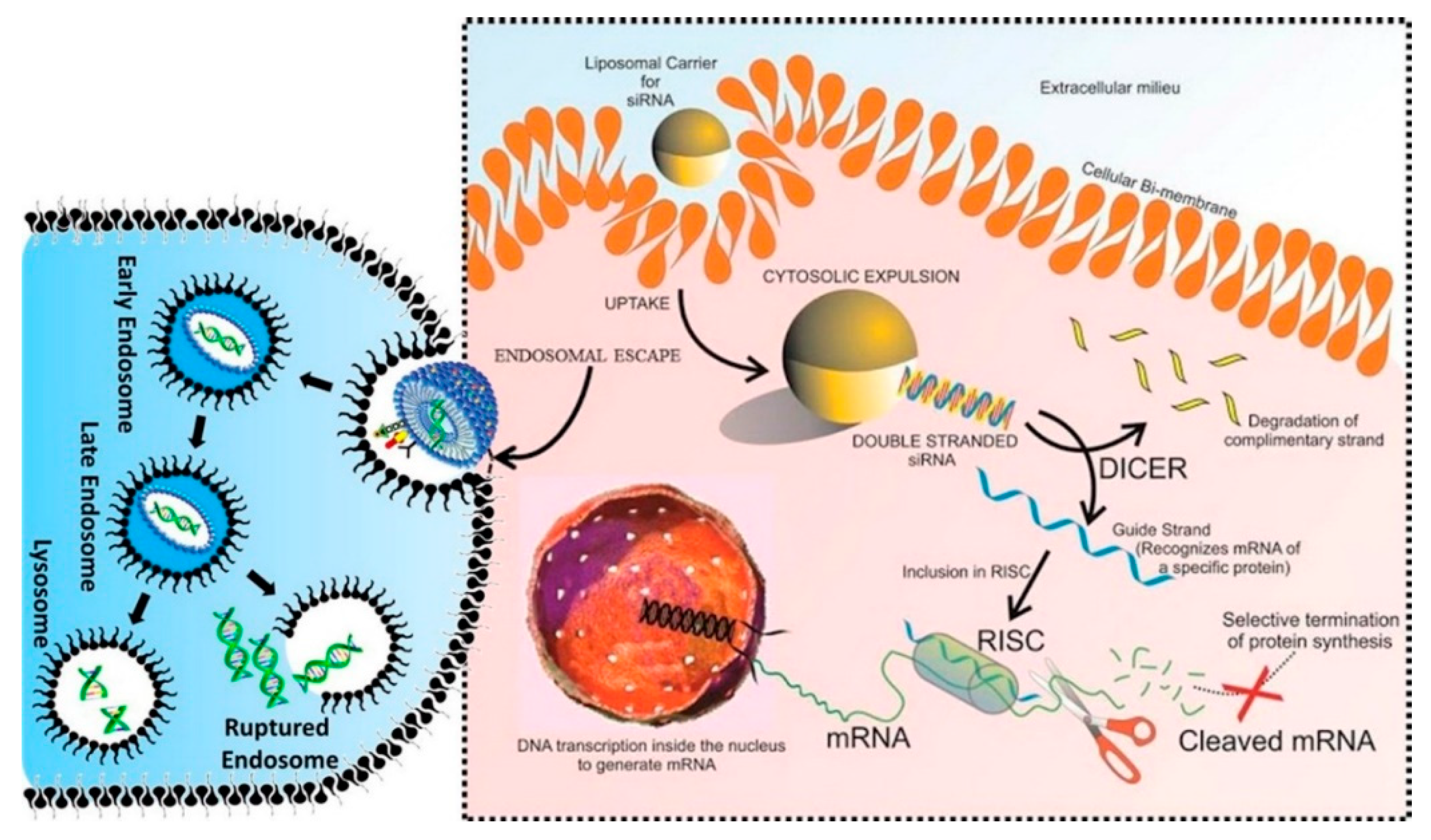
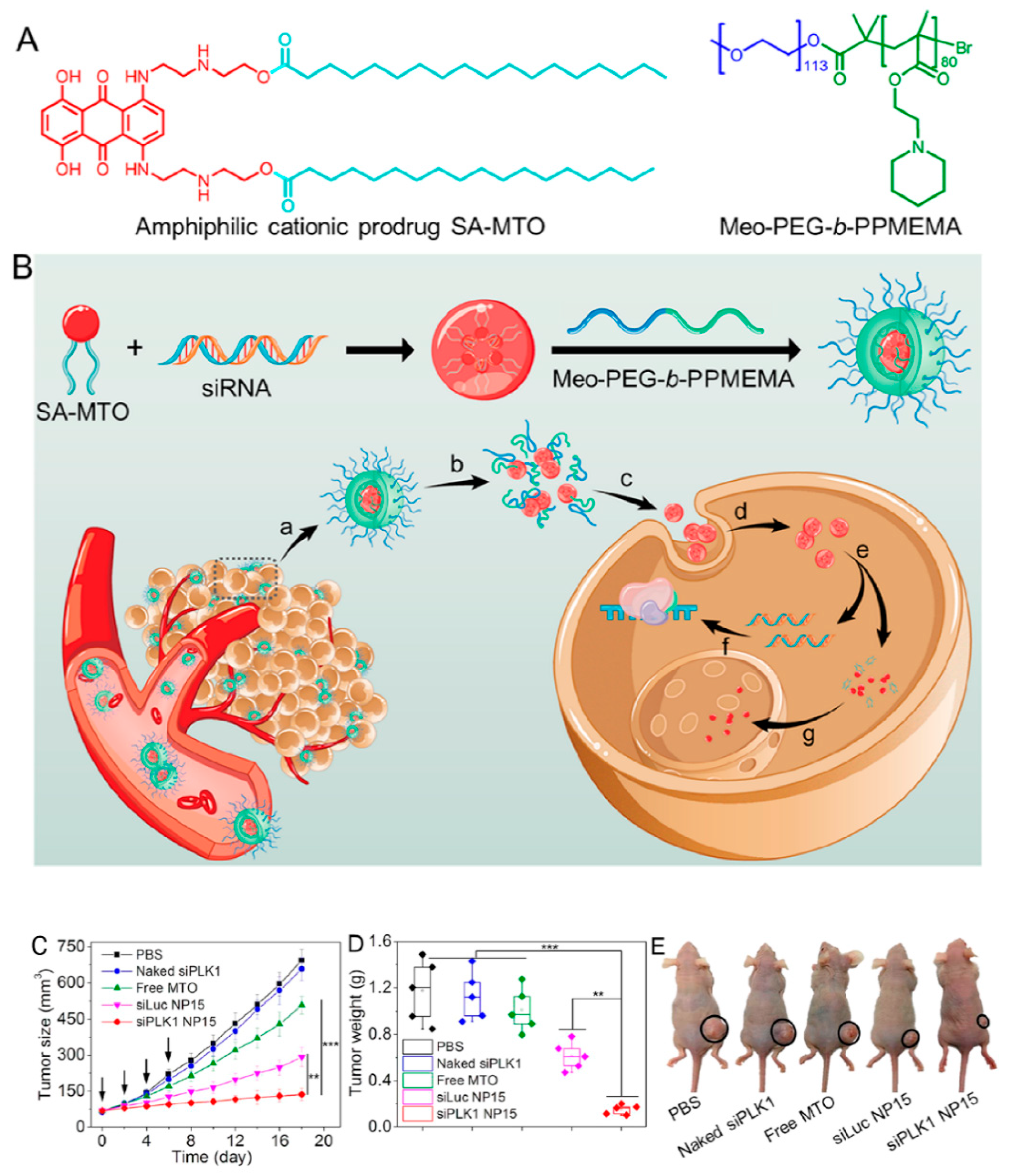
5.2. Lipid Nanostructures
5.3. Peptides
5.4. Cyclodextrin
5.5. Magnetic Nanoparticles
5.6. Gold Nanoparticles
6. Conclusions and Remarks
Funding
Conflicts of Interest
Abbreviations
| PCa | prostate cancer |
| LHRH | luteinizing hormone releasing hormone |
| ARs | androgen receptors |
| mCRPC | metastasis castration-resistant prostate cancer |
| PSA | prostate-specific antigen |
| DRE | digital rectal examination |
| FZD | Frizzled |
| KRT5 | keratin 5 |
| miR | microRNA |
| lncRNAs | long non-coding RNAs |
| siRNA | small interfering RNA |
| RNAi | RNA interference |
| RISC | RNA-induced silencing complex |
| mRNA | messenger RNA |
| PEP | phosphoenopyruvate |
| NF-κB | nuclear factor kappa B |
| Bcl-2 | B-cell lymphoma 1 |
| SATB1 | special AT-rich sequence-binding protein 1 |
| TRIM24 | tripartite motif-containing protein 24 |
| CIP2A | cancerous inhibitor of protein phosphatase 2A |
| PARP1 | poly(ADP-ribose) polymerase-1 |
| EMT | epithelial-to-mesenchymal transition |
| HIF-α | hypoxia-inducible factor-1α |
| ROS | reactive oxygen species |
| JNK | c-Jun N-terminal kinase |
| MAPK | mitogen-activated protein kinase |
| ESM-1 | endothelial cell-specific molecule-1 |
| VEGF | vascular endothelial growth factor |
| SALL4 | Sal-like 4 |
| TLRs | Toll-like receptors |
| RGD | arginine–glycine–aspartic acid |
| PHB1 | prohibitin-1 |
| PSMA | prostate-specific membrane antigen |
| i.v. | intravenous |
| SPIONs | superparamagnetic iron oxide nanoparticles |
| EPR | enhanced permeability and retention |
| ADAM10 | a disintegrin and metalloproteinase 10 |
| GRPR | gastrin-releasing peptide receptor |
| CDCA1 | cell division cycle-associated protein 1 |
| SPR | surface plasmon resonance |
| PLK1 | polo-like kinase 1 |
| Tf | transferrin |
| ZEB1 | zinc finger E-box binding homeobox 1 |
| DANCR | differentiation antagonizing non-protein coding RNA |
| MEG3 | lncRNA maternally expressed gene 3 |
| PCA3 | prostate cancer antigen 3 |
| DRAIC | downregulated RNA in cancer |
| PCAT29 | prostate cancer-associated transcript 29 |
| GAS5 | growth arrest-specific 5 |
| CTBP1-AS | C-terminal binding protein 1 antisense |
| PCGEM | prostate cancer gene expression marker 1 |
| MALAT-1 | metastasis-associated lung adenocarcinoma transcript 1 |
| NEAT1 | nuclear-enriched abundant transcript 1 |
| PCAT5 | prostate cancer-associated transcript 5 |
| SChLAP1 | second chromosome locus associated with prostate 1 |
| HOTAIR | HOX transcript antisense RNA |
| SOCS2-AS1 | cytokine signaling 2 antisense transcript 1 |
| TIMP 2/3 | tissue inhibitor of metalloproteinase |
| EZH2 | enhancer of zeste homolog |
| ZNF217 | zinc finger protein 217 |
| ZEB1 | zinc-finger E-box binding homeobox 1 |
| PRUNE2 | prune homolog 2 |
| NKX3-1 | homeobox protein Nkx 3.1 |
| FOXA1 | forkhead box protein A1 |
| BCL4 | B-cell lymphoma like-2 like protein 4 |
| SMAD3 | mothers against decapentaplegic homolog 3 |
| CTBP1 | C-terminal binding protein 1 antisense |
| HDAC-Sin3A | histone decarboxylase paired amphipathic helix protein Sin3a complex |
| TMEM48 | transmembrane protein 48 |
| CKS2 | cyclin-dependent kinase regulatory subunit 2 |
| hnRNP A1 | heterogeneous nuclear ribonucleoprotein A1 |
| U2AF65 | U2 small nuclear RNA auxiliary factor 2 |
| DAB2IP | disabled homolog 2-interacting protein |
| TMPRSS2 | transmembrane protease, serine 2 |
| ERG | ETS (E-twenty-six)-related gene |
| SWI/SFN complex | switch/sucrose non-fermentable complex |
| TNSF10 | tumor necrosis factor superfamily member 10 |
| MDM2 | mouse double minute 2 homolog |
References
- Rawla, P. Epidemiology of prostate cancer. World J. Oncol. 2019, 10, 63. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Tang, H.; Thayanithy, V.; Subramanian, S.; Oberg, A.L.; Cunningham, J.M.; Cerhan, J.R.; Steer, C.J.; Thibodeau, S.N. Gene networks and microRNAs implicated in aggressive prostate cancer. Cancer Res. 2009, 69, 9490–9497. [Google Scholar] [CrossRef] [Green Version]
- Czerwińska, M.; Bilewicz, A.; Kruszewski, M.; Wegierek-Ciuk, A.; Lankoff, A. Targeted Radionuclide Therapy of Prostate Cancer—From Basic Research to Clinical Perspectives. Molecules 2020, 25, 1743. [Google Scholar] [CrossRef] [Green Version]
- Rice, M.A.; Malhotra, S.V.; Stoyanova, T.I. Second-generation antiandrogens in castration resistant prostate cancer. Front. Oncol. 2019, 9, 801. [Google Scholar] [CrossRef]
- Nuhn, P.; De Bono, J.S.; Fizazi, K.; Freedland, S.J.; Grilli, M.; Kantoff, P.W.; Sonpavde, G.; Sternberg, C.N.; Yegnasubramanian, S.; Antonarakis, E.S. Update on systemic prostate cancer therapies: Management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur. Urol. 2019, 75, 88–99. [Google Scholar] [CrossRef]
- Nguyen-Nielsen, M.; Borre, M. Diagnostic and therapeutic strategies for prostate cancer. In Seminars in Nuclear Medicine; Saunders: Philadelphia, PA, USA, 2016; pp. 484–490. [Google Scholar]
- Cattrini, C.; Castro, E.; Lozano, R.; Zanardi, E.; Rubagotti, A.; Boccardo, F.; Olmos, D. Current Treatment Options for Metastatic Hormone-Sensitive Prostate Cancer. Cancers 2019, 11, 1355. [Google Scholar] [CrossRef] [Green Version]
- Boettcher, A.N.; Usman, A.; Morgans, A.K.; VanderWeele, D.; Sosman, J.; Wu, J. Past, current, and future of immunotherapies for prostate cancer. Front. Oncol. 2019, 9, 884. [Google Scholar] [CrossRef] [Green Version]
- Kurth, I.; Hein, L.; Mäbert, K.; Peitzsch, C.; Koi, L.; Cojoc, M.; Kunz-Schughart, L.; Baumann, M.; Dubrovska, A. Cancer stem cell related markers of radioresistance in head and neck squamous cell carcinoma. Oncotarget 2015, 6, 34494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arechaga-Ocampo, E.; Lopez-Camarillo, C.; Villegas-Sepulveda, N.; Gonzalez-De la Rosa, C.H.; Perez-Añorve, I.X.; Roldan-Perez, R.; Flores-Perez, A.; Peña-Curiel, O.; Angeles-Zaragoza, O.; Rangel Corona, R. Tumor suppressor miR-29c regulates radioresistance in lung cancer cells. Tumor Biol. 2017, 39, 1010428317695010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, K.M.; Dong, S.; Fan, M.; Li, J.J. Nuclear factor-κB p65 inhibits mitogen-activated protein kinase signaling pathway in radioresistant breast cancer cells. Mol. Cancer Res. 2006, 4, 945–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazawa, M.; Hosokawa, Y.; Monzen, S.; Yoshino, H.; Kashiwakura, I. Regulation of DNA damage response and cell cycle in radiation-resistant HL60 myeloid leukemia cells. Oncol. Rep. 2012, 28, 55–61. [Google Scholar]
- Elsasser-Beile, U.; Buhler, P.; Wolf, P. Targeted therapies for prostate cancer against the prostate specific membrane antigen. Curr. Drug Targets 2009, 10, 118–125. [Google Scholar] [CrossRef]
- Egidi, M.G.; Cochetti, G.; Guelfi, G.; Zampini, D.; Diverio, S.; Poli, G.; Mearini, E. Stability assessment of candidate reference genes in urine sediment of prostate cancer patients for miRNA applications. Dis. Markers 2015. [Google Scholar] [CrossRef]
- Slovin, S.F. Targeting castration-resistant prostate cancer with monoclonal antibodies and constructs. Immunotherapy 2013, 5, 1347–1355. [Google Scholar] [CrossRef]
- Barve, A.; Jin, W.; Cheng, K. Prostate cancer relevant antigens and enzymes for targeted drug delivery. J. Control. Release 2014, 187, 118–132. [Google Scholar] [CrossRef] [Green Version]
- Diao, W.; Cai, H.; Chen, L.; Jin, X.; Liao, X.; Jia, Z. Recent advances in prostate-specific membrane antigen-based radiopharmaceuticals. Curr. Top. Med. Chem. 2019, 19, 33–56. [Google Scholar] [CrossRef]
- Mohan, C.D.; Rangappa, S.; Preetham, H.D.; Chandra Nayak, S.; Gupta, V.K.; Basappa, S.; Sethi, G.; Rangappa, K.S. Targeting STAT3 signaling pathway in cancer by agents derived from Mother Nature. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef]
- Kashyap, D.; Tuli, H.S.; Yerer, M.B.; Sharma, A.; Sak, K.; Srivastava, S.; Pandey, A.; Garg, V.K.; Sethi, G.; Bishayee, A. Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.J.F.; Marchi, S.; Petroni, G.; Kroemer, G.; Galluzzi, L.; Pervaiz, S. Noncanonical Cell Fate Regulation by Bcl-2 Proteins. Trends Cell Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hazari, Y.; Bravo-San Pedro, J.M.; Hetz, C.; Galluzzi, L.; Kroemer, G. Autophagy in hepatic adaptation to stress. J. Hepatol. 2020, 72, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; de la Roche, M.; Djamgoz, M.B.A.; Siddik, Z.H. Overview of the oncogenic signaling pathways in colorectal cancer: Mechanistic insights. Semin. Cancer Biol. 2019, 58, 65–79. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Fuentes-Mattei, E.; Fayyaz, S.; Raj, P.; Goblirsch, M.; Poltronieri, P.; Calin, G.A. Interplay between epigenetic abnormalities and deregulated expression of microRNAs in cancer. Semin. Cancer Biol. 2019, 58, 47–55. [Google Scholar] [CrossRef]
- Fayyaz, S.; Javed, Z.; Attar, R.; Farooqi, A.A.; Yaylim, I.; Ahmad, A. MicroRNA regulation of TRAIL mediated signaling in different cancers: Control of micro steering wheels during the journey from bench-top to the bedside. Semin. Cancer Biol. 2019, 58, 56–64. [Google Scholar] [CrossRef]
- Wang, Y.; Singhal, U.; Qiao, Y.; Kasputis, T.; Chung, J.S.; Zhao, H.; Chammaa, F.; Belardo, J.A.; Roth, T.M.; Zhang, H.; et al. Wnt Signaling Drives Prostate Cancer Bone Metastatic Tropism and Invasion. Transl. Oncol. 2020, 13, 100747. [Google Scholar] [CrossRef]
- Du, H.; Wang, X.; Dong, R.; Hu, D.; Xiong, Y. miR-601 inhibits proliferation, migration and invasion of prostate cancer stem cells by targeting KRT5 to inactivate the Wnt signaling pathway. Int. J. Clin. Exp. Pathol. 2019, 12, 4361–4379. [Google Scholar]
- Chen, W.; Yu, Z.; Huang, W.; Yang, Y.; Wang, F.; Huang, H. LncRNA LINC00665 Promotes Prostate Cancer Progression via miR-1224–5p/SND1 Axis. Oncotargets Ther. 2020, 13, 2527–2535. [Google Scholar] [CrossRef] [Green Version]
- Qian, C.; Liao, C.H.; Tan, B.F.; Chen, Y.F.; Dang, B.W.; Chen, J.L.; Liu, C.B. LncRNA PROX1-AS1 promotes proliferation, invasion, and migration in prostate cancer via targeting miR-647. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2938–2944. [Google Scholar] [CrossRef]
- Cao, H.; Gao, R.; Chen, L.; Feng, Y. TRIM66 promotes malignant progression of prostate carcinoma through the JAK/STAT pathway. Febs Open Bio 2020, 10, 515–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, D.; Yu, Y.; Wang, W.; Wu, K.; Liu, D.; Yang, Y.; Zhang, C.; Qi, Y.; Zhao, S. Long noncoding RNA PART1 promotes progression of non-small cell lung cancer cells via JAK-STAT signaling pathway. Cancer Med. 2019, 8, 6064–6081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; He, H.; Jiang, K.; Jiang, P.; He, H.; Feng, S.; Chen, K.; Shao, J.; Deng, G. FAM46C inhibits cell proliferation and cell cycle progression and promotes apoptosis through PTEN/AKT signaling pathway and is associated with chemosensitivity in prostate cancer. Aging 2020, 12, 6352–6369. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Chen, H.; Wang, P.; Yang, N.; Bao, J. Downregulation of lncRNA ZEB1-AS1 Represses Cell Proliferation, Migration, and Invasion Through Mediating PI3K/AKT/mTOR Signaling by miR-342–3p/CUL4B Axis in Prostate Cancer. Cancer Biother. Radiopharm. 2020. [Google Scholar] [CrossRef]
- Gong, H.; Zhou, L.; Khelfat, L.; Qiu, G.; Wang, Y.; Mao, K.; Chen, W. Rho-Associated Protein Kinase (ROCK) Promotes Proliferation and Migration of PC-3 and DU145 Prostate Cancer Cells by Targeting LIM Kinase 1 (LIMK1) and Matrix Metalloproteinase-2 (MMP-2). Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 3090–3099. [Google Scholar] [CrossRef]
- Julka, P.K.; Verma, A.; Gupta, K. Personalized Treatment Approach to Metastatic Castration-Resistant Prostate Cancer with BRCA2 and PTEN Mutations: A Case Report. Case Rep. Oncol. 2020, 13, 55–61. [Google Scholar] [CrossRef]
- Mu, D.; Zhou, G.; Li, J.; Su, B.; Guo, H. Ursolic acid activates the apoptosis of prostate cancer via ROCK/PTEN mediated mitochondrial translocation of cofilin-1. Oncol. Lett. 2018, 15, 3202–3206. [Google Scholar] [CrossRef]
- Lu, X.; Yang, F.; Chen, D.; Zhao, Q.; Chen, D.; Ping, H.; Xing, N. Quercetin reverses docetaxel resistance in prostate cancer via androgen receptor and PI3K/Akt signaling pathways. Int. J. Biol. Sci. 2020, 16, 1121–1134. [Google Scholar] [CrossRef]
- Hajiasgharzadeh, K.; Somi, M.H.; Shanehbandi, D.; Mokhtarzadeh, A.; Baradaran, B. Small interfering RNA–mediated gene suppression as a therapeutic intervention in hepatocellular carcinoma. J. Cell. Physiol. 2019, 234, 3263–3276. [Google Scholar] [CrossRef]
- Roedig, H.; Damiescu, R.; Zeng-Brouwers, J.; Kutija, I.; Trebicka, J.; Wygrecka, M.; Schaefer, L. Danger matrix molecules orchestrate CD14/CD44 signaling in cancer development. Semin. Cancer Biol. 2020, 62, 31–47. [Google Scholar] [CrossRef]
- Lee, J.H.; Mohan, C.D.; Deivasigamani, A.; Jung, Y.Y.; Rangappa, S.; Basappa, S.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Garg, M.; et al. Brusatol suppresses STAT3-driven metastasis by downregulating epithelial-mesenchymal transition in hepatocellular carcinoma. J. Adv. Res. 2020. [Google Scholar] [CrossRef]
- Kirtonia, A.; Pandya, G.; Sethi, G.; Pandey, A.K.; Das, B.C.; Garg, M. A comprehensive review of genetic alterations and molecular targeted therapies for the implementation of personalized medicine in acute myeloid leukemia. J. Mol. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mirza, B.; Croley, C.R.; Ahmad, M.; Pumarol, J.; Das, N.; Sethi, G.; Bishayee, A. Mango (Mangifera indica L.): A magnificent plant with cancer preventive and anticancer therapeutic potential. Crit. Rev. Food Sci. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Mishra, S.R.; Behera, B.P.; Mahapatra, K.K.; Panigrahi, D.P.; Bhol, C.S.; Praharaj, P.P.; Sethi, G.; Patra, S.K.; Bhutia, S.K. Autophagy-modulating phytochemicals in cancer therapeutics: Current evidences and future perspectives. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef]
- Lee, J.H.; Mohan, C.D.; Shanmugam, M.K.; Rangappa, S.; Sethi, G.; Siveen, K.S.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Basappa, S.; et al. Vitexin abrogates invasion and survival of hepatocellular carcinoma cells through targeting STAT3 signaling pathway. Biochimie 2020, 175, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Kansara, S.; Pandey, V.; Lobie, P.E.; Sethi, G.; Garg, M.; Pandey, A.K. Mechanistic Involvement of Long Non-Coding RNAs in Oncotherapeutics Resistance in Triple-Negative Breast Cancer. Cells 2020, 9, 1511. [Google Scholar] [CrossRef]
- Lee, J.H.; Chinnathambi, A.; Alharbi, S.A.; Shair, O.H.M.; Sethi, G.; Ahn, K.S. Farnesol abrogates epithelial to mesenchymal transition process through regulating Akt/mTOR pathway. Pharmacol. Res. 2019, 150, 104504. [Google Scholar] [CrossRef]
- Ko, J.-H.; Yang, M.H.; Baek, S.H.; Nam, D.; Jung, S.H.; Ahn, K.S. Theacrine attenuates epithelial mesenchymal transition in human breast cancer MDA-MB-231 cells. Phytother. Res. 2019, 33, 1934–1942. [Google Scholar] [CrossRef]
- Varghese, E.; Liskova, A.; Kubatka, P.; Samuel, S.M.; Büsselberg, D. Anti-Angiogenic Effects of Phytochemicals on miRNA Regulating Breast Cancer Progression. Biomolecules 2020, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Krishnamachary, B.; Pachecho-Torres, J.; Penet, M.F.; Bhujwalla, Z.M. Theranostic small interfering RNA nanoparticles in cancer precision nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechno. 2019, 2, e1595. [Google Scholar] [CrossRef]
- Kim, H.J.; Yi, Y.; Kim, A.; Miyata, K. Small Delivery Vehicles of siRNA for Enhanced Cancer Targeting. Biomacromolecules 2018, 19, 2377–2390. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yu, W.; Xu, Z.; Hu, J.; Liu, J.; Wang, F. Multifunctional siRNA-Laden Hybrid Nanoplatform for Non-Invasive PA/IR Dual-Modal Imaging-Guided Enhanced Photogenetherapy. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef] [PubMed]
- Dharamdasani, V.; Mandal, A.; Qi, Q.M.; Suzuki, I.; Bentley, M.; Mitragotri, S. Topical delivery of siRNA into skin using ionic liquids. J. Control. Release Off. J. Control. Release Soc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Parashar, D.; Geethadevi, A.; Aure, M.R.; Mishra, J.; George, J.; Chen, C.; Mishra, M.K.; Tahiri, A.; Zhao, W.; Nair, B.; et al. miRNA551b-3p Activates an Oncostatin Signaling Module for the Progression of Triple-Negative Breast Cancer. Cell Rep. 2019, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambeth, L.S.; Smith, C.A. Short hairpin RNA-mediated gene silencing. In siRNA Design; Springer: Berlin, Germany, 2013; pp. 205–232. [Google Scholar]
- Springer, A.D.; Dowdy, S.F. GalNAc-siRNA conjugates: Leading the way for delivery of RNAi therapeutics. Nucleic Acid Ther. 2018, 28, 109–118. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef]
- Jinek, M.; Doudna, J.A. A three-dimensional view of the molecular machinery of RNA interference. Nature 2009, 457, 405–412. [Google Scholar] [CrossRef]
- Fellmann, C.; Lowe, S.W. Stable RNA interference rules for silencing. Nat. Cell Biol. 2014, 16, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Barata, P.; Sood, A.K.; Hong, D.S. RNA-targeted therapeutics in cancer clinical trials: Current status and future directions. Cancer Treat. Rev. 2016, 50, 35–47. [Google Scholar] [CrossRef]
- Wang, W.-T.; Han, C.; Sun, Y.-M.; Chen, T.-Q.; Chen, Y.-Q. Noncoding RNAs in cancer therapy resistance and targeted drug development. J. Hematol. Oncol. 2019, 12, 55. [Google Scholar] [CrossRef]
- Luo, W.; Semenza, G.L. Emerging roles of PKM2 in cell metabolism and cancer progression. Trends Endocrinol. Metab. 2012, 23, 560–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Lu, Z. Regulation and function of pyruvate kinase M2 in cancer. Cancer Lett. 2013, 339, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, N.; Ojo, D.; Yan, J.; Tang, D. PKM2 contributes to cancer metabolism. Cancer Lett. 2015, 356, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, P.; Li, Z. The multifaceted regulation and functions of PKM2 in tumor progression. Biochim. Et Biophys. Acta 2014, 1846, 285–296. [Google Scholar] [CrossRef]
- Ding, G.B.; Meng, X.; Yang, P.; Li, B.; Stauber, R.H.; Li, Z. Integration of Polylactide into Polyethylenimine Facilitates the Safe and Effective Intracellular siRNA Delivery. Polymers 2020, 12, 445. [Google Scholar] [CrossRef] [Green Version]
- Puar, Y.R.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; Sethi, G.; Tergaonkar, V. Evidence for the Involvement of the Master Transcription Factor NF-κB in Cancer Initiation and Progression. Biomedicines 2018, 6, 82. [Google Scholar] [CrossRef] [Green Version]
- Ibaraki, H.; Kanazawa, T.; Owada, M.; Iwaya, K.; Takashima, Y.; Seta, Y. Anti-Metastatic Effects on Melanoma via Intravenous Administration of Anti-NF-kappaB siRNA Complexed with Functional Peptide-Modified Nano-Micelles. Pharmaceutics 2020, 12, 64. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.H.; Chen, R.Y.; Hsieh, H.P.; Tsai, S.J.; Chang, K.C.; Yen, C.J.; Huang, Y.C.; Liu, Y.W.; Lee, J.C.; Lai, Y.C.; et al. A selective Aurora-A 5′-UTR siRNA inhibits tumor growth and metastasis. Cancer Lett. 2020, 472, 97–107. [Google Scholar] [CrossRef]
- Choi, K.Y.; Correa, S.; Min, J.; Li, J.; Roy, S.; Laccetti, K.H.; Dreaden, E.; Kong, S.; Heo, R.; Roh, Y.H.; et al. Binary Targeting of siRNA to Hematologic Cancer Cells In Vivo using Layer-by-Layer Nanoparticles. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Zhupanyn, P.; Ewe, A.; Buch, T.; Malek, A.; Rademacher, P.; Muller, C.; Reinert, A.; Jaimes, Y.; Aigner, A. Extracellular vesicle (ECV)-modified polyethylenimine (PEI) complexes for enhanced siRNA delivery in vitro and in vivo. J. Control. Release Off. J. Control. Release Soc. 2020, 319, 63–76. [Google Scholar] [CrossRef]
- Zuckerman, J.E.; Gritli, I.; Tolcher, A.; Heidel, J.D.; Lim, D.; Morgan, R.; Chmielowski, B.; Ribas, A.; Davis, M.E.; Yen, Y. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc. Natl. Acad. Sci. USA 2014, 111, 11449–11454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tattermusch, A.; Brockdorff, N. A scaffold for X chromosome inactivation. Hum. Genet. 2011, 130, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Y.; Zhang, F.W.; Tong, J.; Liu, F. MiR-191–5p inhibits lung adenocarcinoma by repressing SATB1 to inhibit Wnt pathway. Mol. Genet. Genom. Med. 2020, 8, e1043. [Google Scholar] [CrossRef] [PubMed]
- Glatzel-Plucinska, N.; Piotrowska, A.; Dziegiel, P.; Podhorska-Okolow, M. The Role of SATB1 in Tumour Progression and Metastasis. Int. J. Mol. Sci. 2019, 20, 4156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Yang, C.S.; Ma, Z.X.; Chen, J.C.; Zheng, J.N.; Sun, X.Q.; Wang, J.Q. Inhibition of prostate cancer DU145 cell growth with small interfering RNA targeting the SATB1 gene. Exp. Ther. Med. 2018, 15, 3028–3033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takei, Y. siRNA-Based Drug Targeting Human Bcl-xL Against Cancers. Methods Mol. Biol. (Clifton N.J.) 2019, 1974, 31–40. [Google Scholar] [CrossRef]
- Huang, L.; Li, X.; Ye, H.; Liu, Y.; Liang, X.; Yang, C.; Hua, L.; Yan, Z.; Zhang, X. Long non-coding RNA NCK1-AS1 promotes the tumorigenesis of glioma through sponging microRNA-138–2-3p and activating the TRIM24/Wnt/beta-catenin axis. J. Exp. Clin. Cancer Res. 2020, 39, 63. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.P.; Cai, L.C.; Wang, X.Y.; Cheng, S.Y.; Zhang, D.M.; Jian, W.G.; Wang, T.D.; Yang, J.K.; Yang, K.B.; Zhang, C. BMP8A promotes survival and drug resistance via Nrf2/TRIM24 signaling pathway in clear cell renal cell carcinoma. Cancer Sci. 2020. [Google Scholar] [CrossRef] [Green Version]
- Offermann, A.; Roth, D.; Hupe, M.C.; Hohensteiner, S.; Becker, F.; Joerg, V.; Carlsson, J.; Kuempers, C.; Ribbat-Idel, J.; Tharun, L.; et al. TRIM24 as an independent prognostic biomarker for prostate cancer. Urol. Oncol. 2019, 37, 576.e1–576.e10. [Google Scholar] [CrossRef]
- Shi, S.J.; Wang, L.J.; Han, D.H.; Wu, J.H.; Jiao, D.; Zhang, K.L.; Chen, J.W.; Li, Y.; Yang, F.; Zhang, J.L.; et al. Therapeutic effects of human monoclonal PSMA antibody-mediated TRIM24 siRNA delivery in PSMA-positive castration-resistant prostate cancer. Theranostics 2019, 9, 1247–1263. [Google Scholar] [CrossRef]
- Chen, W.; Liang, J.L.; Zhou, K.; Zeng, Q.L.; Ye, J.W.; Huang, M.J. Effect of CIP2A and its mechanism of action in the malignant biological behavior of colorectal cancer. Cell Commun. Signal. CCS 2020, 18, 67. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Fang, L.; Zang, Y.; Ren, J.; Xu, Z. CIP2A Promotes Proliferation, Invasion and Chemoresistance to Cisplatin in Renal Cell Carcinoma. J. Cancer 2018, 9, 4029–4038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaarala, M.H.; Vaisanen, M.R.; Ristimaki, A. CIP2A expression is increased in prostate cancer. J. Exp. Clin. Cancer Res. 2010, 29, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallai, R.; Bhaskar, A.; Barnett-Bernodat, N.; Gallo-Ebert, C.; Pusey, M.; Nickels, J.T., Jr.; Rice, L.M. Leucine-rich repeat-containing protein 59 mediates nuclear import of cancerous inhibitor of PP2A in prostate cancer cells. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2015, 36, 6383–6390. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.F.; Cheng, P.; Sun, C.; Wang, H.; Wang, W. Inhibitory effects of polyphyllins I and VII on human cisplatin-resistant NSCLC via p53 upregulation and CIP2A/AKT/mTOR signaling axis inhibition. Chin. J. Nat. Med. 2019, 17, 768–777. [Google Scholar] [CrossRef]
- Liu, P.; Xiang, Y.; Liu, X.; Zhang, T.; Yang, R.; Chen, S.; Xu, L.; Yu, Q.; Zhao, H.; Zhang, L.; et al. Cucurbitacin B Induces the Lysosomal Degradation of EGFR and Suppresses the CIP2A/PP2A/Akt Signaling Axis in Gefitinib-Resistant Non-Small Cell Lung Cancer. Molecules 2019, 24, 647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razi Soofiyani, S.; Mohammad Hoseini, A.; Mohammadi, A.; Khaze Shahgoli, V.; Baradaran, B.; Hejazi, M.S. siRNA-Mediated Silencing of CIP2A Enhances Docetaxel Activity Against PC-3 Prostate Cancer Cells. Adv. Pharm. Bull. 2017, 7, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Ray Chaudhuri, A.; Nussenzweig, A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017, 18, 610–621. [Google Scholar] [CrossRef]
- Schiewer, M.J.; Knudsen, K.E. Transcriptional roles of PARP1 in cancer. Mol. Cancer Res. 2014, 12, 1069–1080. [Google Scholar] [CrossRef] [Green Version]
- Bankier, C.; Cheong, Y.; Mahalingam, S.; Edirisinghe, M.; Ren, G.; Cloutman-Green, E.; Ciric, L. A comparison of methods to assess the antimicrobial activity of nanoparticle combinations on bacterial cells. PLoS ONE 2018, 13, e0192093. [Google Scholar] [CrossRef] [Green Version]
- Smolle, M.A.; Bauernhofer, T.; Pummer, K.; Calin, G.A.; Pichler, M. Current insights into long non-coding RNAs (LncRNAs) in prostate cancer. Int. J. Mol. Sci. 2017, 18, 473. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Purkerson, J.M.; Freeman, R.S.; Schwaderer, A.L.; Schwartz, G.J. Acidosis induces antimicrobial peptide expression and resistance to uropathogenic E. coli infection in kidney collecting duct cells via HIF-1alpha. Am. J. Physiol. Ren. Physiol. 2020, 318, F468–F474. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, S.Y.; Zhao, J.F.; Han, X.G.; Wang, H.B.; Sun, M.L. HIF-1alpha or HOTTIP/CTCF Promotes Head and Neck Squamous Cell Carcinoma Progression and Drug Resistance by Targeting HOXA9. Mol. Ther. Nucleic Acids 2020, 20, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Jin, F.; Xiao, D.; Wang, H.; Huang, C.; Wang, X.; Gao, S.; Liu, J.; Yang, S.; Hao, J. IL-37/ STAT3/ HIF-1alpha negative feedback signaling drives gemcitabine resistance in pancreatic cancer. Theranostics 2020, 10, 4088–4100. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zou, X.; Zhang, D.; Liu, S.; Duan, Z.; Liu, L. Self-enforcing HMGB1/NF-kappaB/HIF-1alpha Feedback Loop Promotes Cisplatin Resistance in Hepatocellular Carcinoma Cells. J. Cancer 2020, 11, 3893–3902. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Li, Y.; Zeng, J.; Wang, B.; Ji, K.; Tang, Y.; Sun, Q. Knockdown of HIF-1alpha by siRNA-expressing plasmid delivered by attenuated Salmonella enhances the antitumor effects of cisplatin on prostate cancer. Sci. Rep. 2017, 7, 7546. [Google Scholar] [CrossRef] [Green Version]
- Davis, R.J. Signal transduction by the JNK group of MAP kinases. Cell 2000, 103, 239–252. [Google Scholar] [CrossRef] [Green Version]
- Kralova, J.; Sheely, J.I.; Liss, A.S.; Bose, H.R., Jr. ERK and JNK activation is essential for oncogenic transformation by v-Rel. Oncogene 2010, 29, 6267–6279. [Google Scholar] [CrossRef] [Green Version]
- Parra, E.; Ferreira, J. Modulation of the response of prostate cancer cell lines to cisplatin treatment using small interfering RNA. Oncol. Rep. 2013, 30, 1936–1942. [Google Scholar] [CrossRef]
- Radwan, D.R.; Matloob, A.; Mikhail, S.; Saad, L.; Guirguis, D. Metal Organic Framework-Graphene Nano-Composites for High Adsorption Removal of DBT as hazard material in Liquid Fuel. J. Hazard. Mater. 2019, 373, 447–458. [Google Scholar]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Rennel, E.; Mellberg, S.; Dimberg, A.; Petersson, L.; Botling, J.; Ameur, A.; Westholm, J.O.; Komorowski, J.; Lassalle, P.; Cross, M.J.; et al. Endocan is a VEGF-A and PI3K regulated gene with increased expression in human renal cancer. Exp. Cell Res. 2007, 313, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Belperio, J.A.; Keane, M.P.; Arenberg, D.A.; Addison, C.L.; Ehlert, J.E.; Burdick, M.D.; Strieter, R.M. CXC chemokines in angiogenesis. J. Leukoc. Biol. 2000, 68, 1–8. [Google Scholar] [PubMed]
- Rebollo, J.; Geliebter, J.; Reyes, N. ESM-1 siRNA Knockdown Decreased Migration and Expression of CXCL3 in Prostate Cancer Cells. Int. J. Biomed. Sci. IJBS 2017, 13, 35–42. [Google Scholar] [PubMed]
- Sun, J.; Zhao, Z.; Zhang, W.; Tang, Q.; Yang, F.; Hu, X.; Liu, C.; Song, B.; Zhang, B.; Wang, H. Spalt-Like Protein 4 (SALL4) Promotes Angiogenesis by Activating Vascular Endothelial Growth Factor A (VEGFA) Signaling. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e920851. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.M.; Shi, X.L.; Xing, K.L.; Zhou, H.X.; Lu, L.L.; Wu, W.Z. miR-296–5p suppresses stem cell potency of hepatocellular carcinoma cells via regulating Brg1/Sall4 axis. Cell. Signal. 2020, 72, 109650. [Google Scholar] [CrossRef]
- Liu, K.F.; Shan, Y.X. Effects of siRNA-mediated silencing of Sal-like 4 expression on proliferation and apoptosis of prostate cancer C4–2 cells. Genet. Mol. Res. GMR 2016, 15. [Google Scholar] [CrossRef]
- Urbinati, G.; de Waziers, I.; Slamic, M.; Foussigniere, T.; Ali, H.M.; Desmaele, D.; Couvreur, P.; Massaad-Massade, L. Knocking Down TMPRSS2-ERG Fusion Oncogene by siRNA Could be an Alternative Treatment to Flutamide. Mol. Ther. Nucleic Acids 2016, 5, e301. [Google Scholar] [CrossRef]
- Liu, C.Y.; Xu, P.C.; Chen, D.G.; Fan, X.H.; Li, M.Q.; Yang, X.; Xu, Y.P. Effect of siRNA targeting HER2/neu on the proliferation and viability of prostate cancer PC-3M cells. Genet. Mol. Res. GMR 2015, 14, 17145–17153. [Google Scholar] [CrossRef]
- Li, B.K.; Guo, K.; Li, C.Y.; Li, H.L.; Zhao, P.P.; Chen, K.; Liu, C.X. Influence of suppression of CapG gene expression by siRNA on the growth and metastasis of human prostate cancer cells. Genet. Mol. Res. GMR 2015, 14, 15769–15778. [Google Scholar] [CrossRef]
- Urbinati, G.; Ali, H.M.; Rousseau, Q.; Chapuis, H.; Desmaele, D.; Couvreur, P.; Massaad-Massade, L. Antineoplastic Effects of siRNA against TMPRSS2-ERG Junction Oncogene in Prostate Cancer. PLoS ONE 2015, 10, e0125277. [Google Scholar] [CrossRef] [PubMed]
- Chile, S.A.; Ray, K.B.; Shaikh, S.; Rajagopal, V.; Rao, H.S.; Ramana, V.; Kumar, A.S. Evaluation of target mRNA cleavage by aurorakinase B specific siRNA in prostate and hepatic cancer cells and its therapeutic potential in mouse models of liver cancer. Indian J. Exp. Biol. 2014, 52, 943–951. [Google Scholar] [PubMed]
- Dan, C.; Zhu, H.C.; Liu, X.H.; Yao, Q.S. [RelB-siRNA enhanced the radiosensitivity of murine prostate cancer cell line RM-1]. Zhonghua Yi Xue Za Zhi 2013, 93, 3355–3359. [Google Scholar]
- Jiang, T.; Zhou, C.; Gu, J.; Liu, Y.; Zhao, L.; Li, W.; Wang, G.; Li, Y.; Cai, L. Enhanced therapeutic effect of cisplatin on the prostate cancer in tumor-bearing mice by transfecting the attenuated Salmonella carrying a plasmid co-expressing p53 gene and mdm2 siRNA. Cancer Lett. 2013, 337, 133–142. [Google Scholar] [CrossRef]
- Pan, B.; Zheng, S.; Liu, C.; Xu, Y. Suppression of IGHG1 gene expression by siRNA leads to growth inhibition and apoptosis induction in human prostate cancer cell. Mol. Biol. Rep. 2013, 40, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Deezagi, A.; Ansari-Majd, S.; Vaseli-Hagh, N. Induced apoptosis in human prostate cancer cells by blocking of vascular endothelial growth factor by siRNA. Clin. Transl. Oncol. 2012, 14, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Shao, Y.; Liang, Z.; Shao, C.; Wang, B.; Guo, B.; Li, N.; Zhao, X.; Li, Y. Effects of a human plasma membrane-associated sialidase siRNA on prostate cancer invasion. Biochem. Biophys. Res. Commun. 2011, 416, 270–276. [Google Scholar] [CrossRef]
- Wu, W.; Kong, Z.; Duan, X.; Zhu, H.; Li, S.; Zeng, S.; Liang, Y.; Iliakis, G.; Gui, Z.; Yang, D. Inhibition of PARP1 by small interfering RNA enhances docetaxel activity against human prostate cancer PC3 cells. Biochem. Biophys. Res. Commun. 2013, 442, 127–132. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, J.; Yan, C.; Hou, J.; Pu, J.; Zhang, G.; Fu, Z.; Wang, X. Effect of small interfering RNA targeting hypoxia-inducible factor-1α on radiosensitivity of PC3 cell line. Urology 2012, 79, e717–e724. [Google Scholar] [CrossRef]
- Izumi, K.; Fang, L.Y.; Mizokami, A.; Namiki, M.; Li, L.; Lin, W.J.; Chang, C. Targeting the androgen receptor with siRNA promotes prostate cancer metastasis through enhanced macrophage recruitment via CCL2/CCR2-induced STAT3 activation. Embo Mol. Med. 2013, 5, 1383–1401. [Google Scholar] [CrossRef]
- Parra, E.; Gutierrez, L.; Ferreira, J. Increased expression of p21Waf1/Cip1 and JNK with costimulation of prostate cancer cell activation by an siRNA Egr-1 inhibitor. Oncol. Rep. 2013, 30, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Lin, P.; Liu, X.; Li, D.; Liu, Z.J.; Zou, H.F.; Jiang, Y.; Zhao, X.F.; Feng, J.L.; Yu, X.G. [Inhibitory effect of siRNA targeting ADAM17 on the proliferation of prostate cancer PC-3 cells]. Zhonghua Nan Ke Xue = Natl. J. Androl. 2012, 18, 687–691. [Google Scholar]
- Du, Y.F.; Liang, L.; Shi, Y.; Long, Q.Z.; Zeng, J.; Wang, X.Y.; He, D.L. Multi-target siRNA based on DNMT3A/B homologous conserved region influences cell cycle and apoptosis of human prostate cancer cell line TSU-PR1. Genet. Mol. Biol. 2012, 35, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Bee, A.; Brewer, D.; Beesley, C.; Dodson, A.; Forootan, S.; Dickinson, T.; Gerard, P.; Lane, B.; Yao, S.; Cooper, C.S.; et al. siRNA knockdown of ribosomal protein gene RPL19 abrogates the aggressive phenotype of human prostate cancer. PLoS ONE 2011, 6, e22672. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Sun, Y.; Li, L.; Niu, Y.; Lin, W.; Lin, C.; Antonarakis, E.S.; Luo, J.; Yeh, S.; Chang, C. Preclinical Study using Malat1 Small Interfering RNA or Androgen Receptor Splicing Variant 7 Degradation Enhancer ASC-J9((R)) to Suppress Enzalutamide-resistant Prostate Cancer Progression. Eur. Urol. 2017, 72, 835–844. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Yang, W.; Wang, Z.; Hu, Z.; Zeng, X.; Yang, C.; Wang, Y.; Zhang, Y.; Li, F.; Liu, Z.; et al. Knockdown of glucose-regulated protein 78/binding immunoglobulin heavy chain protein expression by asymmetric small interfering RNA induces apoptosis in prostate cancer cells and attenuates migratory capability. Mol. Med. Rep. 2015, 11, 249–256. [Google Scholar] [CrossRef]
- Kim, S.S.; Cho, H.J.; Kang, J.Y.; Kang, H.K.; Yoo, T.K. Inhibition of androgen receptor expression with small interfering RNA enhances cancer cell apoptosis by suppressing survival factors in androgen insensitive, late stage LNCaP cells. Sci. World J. 2013, 2013, 519397. [Google Scholar] [CrossRef]
- Parra, E. Inhibition of JNK-1 by small interfering RNA induces apoptotic signaling in PC-3 prostate cancer cells. Int. J. Mol. Med. 2012, 30, 923–930. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Guo, Z.Q.; Ji, S.Q.; Zhang, M.; Jiang, N.; Li, X.S.; Zhou, L.Q. Small interfering RNA targeting HMGN5 induces apoptosis via modulation of a mitochondrial pathway and Bcl-2 family proteins in prostate cancer cells. Asian J. Androl. 2012, 14, 487–492. [Google Scholar] [CrossRef] [Green Version]
- Weizhong, Z.; Shuohui, G.; Hanjiao, Q.; Yuhong, M.; Xiaohua, Y.; Jian, C.; Lisen, L. Inhibition of cytohesin-1 by siRNA leads to reduced IGFR signaling in prostate cancer. Braz. J. Med. Biol. Res. 2011, 44, 642–646. [Google Scholar] [CrossRef] [Green Version]
- Parra, E.; Ferreira, J.; Saenz, L. Inhibition of Egr-1 by siRNA in prostate carcinoma cell lines is associated with decreased expression of AP-1 and NF-kappaB. Int. J. Mol. Med. 2011, 28, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Glackin, C.A. Nanoparticle delivery of TWIST small interfering RNA and anticancer drugs: A therapeutic approach for combating cancer. In The Enzymes; Elsevier: Amsterdam, The Netherlands, 2018; Volume 44, pp. 83–101. [Google Scholar]
- Forsbach, A.; Nemorin, J.-G.; Montino, C.; Müller, C.; Samulowitz, U.; Vicari, A.P.; Jurk, M.; Mutwiri, G.K.; Krieg, A.M.; Lipford, G.B. Identification of RNA sequence motifs stimulating sequence-specific TLR8-dependent immune responses. J. Immunol. 2008, 180, 3729–3738. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.; Judge, A.; MacLachlan, I. siRNA and innate immunity. Oligonucleotides 2009, 19, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Behlke, M.A. Chemical modification of siRNAs for in vivo use. Oligonucleotides 2008, 18, 305–320. [Google Scholar] [CrossRef] [Green Version]
- Lima, W.F.; Wu, H.; Nichols, J.G.; Sun, H.; Murray, H.M.; Crooke, S.T. Binding and cleavage specificities of human Argonaute2. J. Biol. Chem. 2009, 284, 26017–26028. [Google Scholar] [CrossRef] [Green Version]
- Haringsma, H.J.; Li, J.J.; Soriano, F.; Kenski, D.M.; Flanagan, W.M.; Willingham, A.T. mRNA knockdown by single strand RNA is improved by chemical modifications. Nucleic Acids Res. 2012, 40, 4125–4136. [Google Scholar] [CrossRef] [Green Version]
- Finlay, J.; Roberts, C.M.; Dong, J.; Zink, J.I.; Tamanoi, F.; Glackin, C.A. Mesoporous silica nanoparticle delivery of chemically modified siRNA against TWIST1 leads to reduced tumor burden. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1657–1666. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Saw, P.E.; Tao, W.; Li, Y.; Ji, X.; Yu, M.; Mahmoudi, M.; Rasmussen, J.; Ayyash, D.; Zhou, Y.; et al. Tumor Microenvironment-Responsive Multistaged Nanoplatform for Systemic RNAi and Cancer Therapy. Nano Lett. 2017, 17, 4427–4435. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Y.; Xie, Y.; Chen, J.; Dou, Y. Apoptosis of A549 cells by small interfering RNA targeting survivin delivery using poly-beta-amino ester/guanidinylated O-carboxymethyl chitosan nanoparticles. Asian J. Pharm. Sci. 2020, 15, 121–128. [Google Scholar] [CrossRef]
- Foged, C. siRNA delivery with lipid-based systems: Promises and pitfalls. Curr. Top. Med. Chem. 2012, 12, 97–107. [Google Scholar] [CrossRef]
- Gooding, M.; Browne, L.P.; Quinteiro, F.M.; Selwood, D.L. siRNA delivery: From lipids to cell-penetrating peptides and their mimics. Chem. Biol. Drug Des. 2012, 80, 787–809. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Morris, V.B.; Labhasetwar, V. Effectiveness of Small Interfering RNA Delivery via Arginine-Rich Polyethylenimine-Based Polyplex in Metastatic and Doxorubicin-Resistant Breast Cancer Cells. J. Pharmacol. Exp. Ther. 2019, 370, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Khan, A. Immunoliposomes-mediated small interfering RNA therapy: A novel approach for the treatment of cancer. Int. J. Health Sci. 2019, 13, 1–9. [Google Scholar]
- Zhao, Y.; Lee, R.J.; Liu, L.; Dong, S.; Zhang, J.; Zhang, Y.; Yao, Y.; Lu, J.; Meng, Q.; Xie, J.; et al. Multifunctional drug carrier based on PEI derivatives loaded with small interfering RNA for therapy of liver cancer. Int. J. Pharm. 2019, 564, 214–224. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Zeng, Q.; Zeng, H.; Liu, X.; Wu, P.; Xie, H.; He, L.; Long, Z.; Lu, X.; et al. Delivery of RIPK4 small interfering RNA for bladder cancer therapy using natural halloysite nanotubes. Sci. Adv. 2019, 5, eaaw6499. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Zheng, Z.; Xue, X.; Zheng, L.; Qin, J.; Li, H.; Zhou, Y.; Fang, G. Targeted eradication of gastric cancer stem cells by CD44 targeting USP22 small interfering RNA-loaded nanoliposomes. Future Oncol. (Lond. Engl.) 2019, 15, 281–295. [Google Scholar] [CrossRef]
- Jia, N.; Wu, H.; Duan, J.; Wei, C.; Wang, K.; Zhang, Y.; Mao, X. Polyethyleneimine-coated Iron Oxide Nanoparticles as a Vehicle for the Delivery of Small Interfering RNA to Macrophages In Vitro and In Vivo. J. Vis. Exp. Jove 2019. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.B.; Zhang, K.; Tam, Y.Y.; Quick, J.; Tam, Y.K.; Lin, P.J.; Chen, S.; Liu, Y.; Nair, J.K.; Zlatev, I.; et al. A Glu-urea-Lys Ligand-conjugated Lipid Nanoparticle/siRNA System Inhibits Androgen Receptor Expression In Vivo. Mol. Ther. Nucleic Acids 2016, 5, e348. [Google Scholar] [CrossRef]
- Ni, X.; Zhang, Y.; Zennami, K.; Castanares, M.; Mukherjee, A.; Raval, R.R.; Zhou, H.; DeWeese, T.L.; Lupold, S.E. Systemic Administration and Targeted Radiosensitization via Chemically Synthetic Aptamer-siRNA Chimeras in Human Tumor Xenografts. Mol. Cancer Ther. 2015, 14, 2797–2804. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Lin, P.J.; Beraldi, E.; Zhang, F.; Kawai, Y.; Leong, J.; Katsumi, H.; Fazli, L.; Fraser, R.; Cullis, P.R.; et al. siRNA Lipid Nanoparticle Potently Silences Clusterin and Delays Progression When Combined with Androgen Receptor Cotargeting in Enzalutamide-Resistant Prostate Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 4845–4855. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Makita, N.; Cao, D.; Mihara, K.; Kadomatsu, K.; Takei, Y. Atelocollagen-mediated intravenous siRNA delivery specific to tumor tissues orthotopically xenografted in prostates of nude mice and its anticancer effects. Nucleic Acid 2015, 25, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.Y.; Wu, I.H.; Chuang, P.H.; Petros, J.A.; Wu, H.C.; Zeng, H.J.; Huang, W.C.; Chung, L.W.; Hsieh, C.L. Targeting L1 cell adhesion molecule expression using liposome-encapsulated siRNA suppresses prostate cancer bone metastasis and growth. Oncotarget 2014, 5, 9911–9929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhang, M.; Tan, K.; Guo, Y.; Tong, H.; Fan, X.; Fang, K.; Li, R. Preparation of nanobubbles carrying androgen receptor siRNA and their inhibitory effects on androgen-independent prostate cancer when combined with ultrasonic irradiation. PLoS ONE 2014, 9, e96586. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.C.; McCarthy, J.; Torres-Fuentes, C.; Cryan, J.F.; Ogier, J.; Darcy, R.; Watson, R.W.; O’Driscoll, C.M. Cyclodextrin mediated delivery of NF-kappaB and SRF siRNA reduces the invasion potential of prostate cancer cells in vitro. Gene Ther. 2015, 22, 802–810. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.; Zhou, J.; Chen, C.; Qu, F.; Rossi, J.J.; Rocchi, P.; Peng, L. Promoting siRNA delivery via enhanced cellular uptake using an arginine-decorated amphiphilic dendrimer. Nanoscale 2015, 7, 3867–3875. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.; Rocchi, P.; Qu, F.; Iovanna, J.L.; Peng, L. Arginine-terminated generation 4 PAMAM dendrimer as an effective nanovector for functional siRNA delivery in vitro and in vivo. Bioconjugate Chem. 2014, 25, 521–532. [Google Scholar] [CrossRef]
- Zeng, S.; Xiong, M.P. Trilayer micelles for combination delivery of rapamycin and siRNA targeting Y-box binding protein-1 (siYB-1). Biomaterials 2013, 34, 6882–6892. [Google Scholar] [CrossRef] [Green Version]
- Gomes-da-Silva, L.C.; Ramalho, J.S.; Pedroso de Lima, M.C.; Simoes, S.; Moreira, J.N. Impact of anti-PLK1 siRNA-containing F3-targeted liposomes on the viability of both cancer and endothelial cells. Eur. J. Pharm. Biopharm. 2013, 85, 356–364. [Google Scholar] [CrossRef] [Green Version]
- Khandare, J.; Calderon, M.; Dagia, N.M.; Haag, R. Multifunctional dendritic polymers in nanomedicine: Opportunities and challenges. Chem. Soc. Rev. 2012, 41, 2824–2848. [Google Scholar] [CrossRef] [Green Version]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2012, 64, 102–115. [Google Scholar] [CrossRef]
- Lee, C.C.; MacKay, J.A.; Fréchet, J.M.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-y.; Makvandi, P.; Zare, E.N.; Tay, F.R.; Niu, L.-N. Advances in Antimicrobial Organic and Inorganic Nanocompounds in Biomedicine. Adv. Funct. Mater. 2020. [Google Scholar] [CrossRef]
- Reebye, V.; Sætrom, P.; Mintz, P.J.; Huang, K.W.; Swiderski, P.; Peng, L.; Liu, C.; Liu, X.; Lindkær-Jensen, S.; Zacharoulis, D. Novel RNA oligonucleotide improves liver function and inhibits liver carcinogenesis in vivo. Hepatology 2014, 59, 216–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Liu, C.; Catapano, C.V.; Peng, L.; Zhou, J.; Rocchi, P. Structurally flexible triethanolamine-core poly (amidoamine) dendrimers as effective nanovectors to deliver RNAi-based therapeutics. Biotechnol. Adv. 2014, 32, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Dufes, C.; Uchegbu, I.F.; Schätzlein, A.G. Dendrimers in gene delivery. Adv. Drug Deliv. Rev. 2005, 57, 2177–2202. [Google Scholar] [CrossRef] [Green Version]
- Sapra, R.; Verma, R.P.; Maurya, G.P.; Dhawan, S.; Babu, J.; Haridas, V. Designer peptide and protein dendrimers: A cross-sectional analysis. Chem. Rev. 2019, 119, 11391–11441. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, Y.; Zhu, D.; Shi, K.; Ma, C.; Zhang, W.; Rocchi, P.; Jiang, L.; Liu, X. Self-assembly of amphiphilic phospholipid peptide dendrimer-based nanovectors for effective delivery of siRNA therapeutics in prostate cancer therapy. J. Control. Release 2020, 322, 416–425. [Google Scholar] [CrossRef]
- Chen, C.-W.; Yeh, M.-K.; Shiau, C.-Y.; Chiang, C.-H.; Lu, D.-W. Efficient downregulation of VEGF in retinal pigment epithelial cells by integrin ligand-labeled liposome-mediated siRNA delivery. Int. J. Nanomed. 2013, 8, 2613. [Google Scholar]
- Hrkach, J.; Von Hoff, D.; Ali, M.M.; Andrianova, E.; Auer, J.; Campbell, T.; De Witt, D.; Figa, M.; Figueiredo, M.; Horhota, A. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med. 2012, 4, 128ra139. [Google Scholar] [CrossRef]
- Zhang, X.; He, Z.; Xiang, L.; Li, L.; Zhang, H.; Lin, F.; Cao, H. Codelivery of GRP78 siRNA and docetaxel via RGD-PEG-DSPE/DOPA/CaP nanoparticles for the treatment of castration-resistant prostate cancer. Drug Des. Dev. Ther. 2019, 13, 1357. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Zou, Y.; Wang, Y.; Huang, X.; Huang, G.; Sumer, B.D.; Boothman, D.A.; Gao, J. Overcoming endosomal barrier by amphotericin B-loaded dual pH-responsive PDMA-b-PDPA micelleplexes for siRNA delivery. ACS Nano 2011, 5, 9246–9255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, K.; Wang, Y.; Huang, X.; Luby-Phelps, K.; Sumer, B.D.; Gao, J. Tunable, ultrasensitive pH-responsive nanoparticles targeting specific endocytic organelles in living cells. Angew. Chem. (Int. Ed. Engl.) 2011, 50, 6109–6114. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Xu, X.D.; Chen, W.H.; Zhang, X.Z. Multi-functional envelope-type nanoparticles assembled from amphiphilic peptidic prodrug with improved anti-tumor activity. ACS Appl. Mater. Interfaces 2014, 6, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Wang, H.Y.; Li, C.; Han, K.; Zhang, X.Z.; Zhuo, R.X. Construction of surfactant-like tetra-tail amphiphilic peptide with RGD ligand for encapsulation of porphyrin for photodynamic therapy. Biomaterials 2011, 32, 1678–1684. [Google Scholar] [CrossRef] [PubMed]
- Barbier-Torres, L.; Lu, S.C. Prohibitin 1 in liver injury and cancer. Exp. Biol. Med. (Maywood N.J.) 2020, 245, 385–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theiss, A.L.; Sitaraman, S.V. The role and therapeutic potential of prohibitin in disease. Biochim. Et Biophys. Acta 2011, 1813, 1137–1143. [Google Scholar] [CrossRef] [Green Version]
- Thuaud, F.; Ribeiro, N.; Nebigil, C.G.; Desaubry, L. Prohibitin ligands in cell death and survival: Mode of action and therapeutic potential. Chem. Biol. 2013, 20, 316–331. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Murphy, L.C.; Nyomba, B.L.; Murphy, L.J. Prohibitin: A potential target for new therapeutics. Trends Mol. Med. 2005, 11, 192–197. [Google Scholar] [CrossRef]
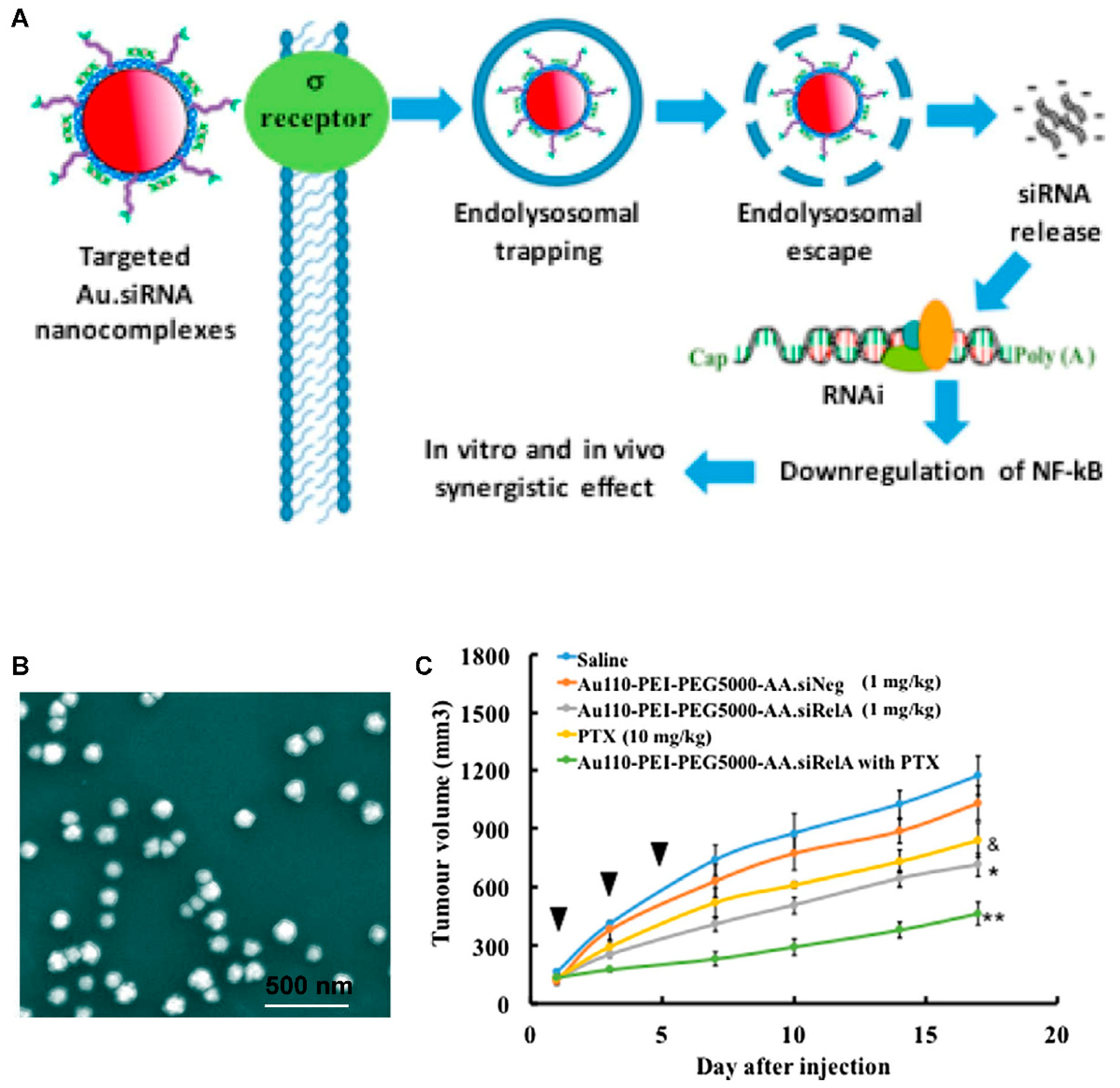
- Xu, X.; Wu, J.; Liu, Y.; Saw, P.E.; Tao, W.; Yu, M.; Zope, H.; Si, M.; Victorious, A.; Rasmussen, J.; et al. Multifunctional Envelope-Type siRNA Delivery Nanoparticle Platform for Prostate Cancer Therapy. ACS Nano 2017, 11, 2618–2627. [Google Scholar] [CrossRef] [Green Version]
- Trimaille, T.; Verrier, B. Micelle-Based Adjuvants for Subunit Vaccine Delivery. Vaccines 2015, 3, 803–813. [Google Scholar] [CrossRef]
- Singh, Y.; Tomar, S.; Khan, S.; Meher, J.G.; Pawar, V.K.; Raval, K.; Sharma, K.; Singh, P.K.; Chaurasia, M.; Reddy, B.S. Bridging small interfering RNA with giant therapeutic outcomes using nanometric liposomes. J. Control. Release 2015, 220, 368–387. [Google Scholar] [CrossRef] [PubMed]
- Croy, S.R.; Kwon, G.S. Polymeric micelles for drug delivery. Curr. Pharm. Des. 2006, 12, 4669–4684. [Google Scholar] [CrossRef]
- Luo, Z.; Shi, S.; Jin, L.; Xu, L.; Yu, J.; Chen, H.; Li, X. Cationic micelle based vaccine induced potent humoral immune response through enhancing antigen uptake and formation of germinal center. Colloids Surf. Biointerfaces 2015, 135, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhong, L.; Jiang, Z.; Pan, H.; Zhang, Y.; Zhu, G.; Bai, L.; Tong, R.; Shi, J.; Duan, X. Cationic micelle-based siRNA delivery for efficient colon cancer gene therapy. Nanoscale Res. Lett. 2019, 14, 193. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Xi, Y.; Chen, M.; Niu, W.; Wang, M.; Ma, P.X.; Lei, B. A highly antibacterial polymeric hybrid micelle with efficiently targeted anticancer siRNA delivery and anti-infection in vitro/in vivo. Nanoscale 2018, 10, 17304–17317. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Hua, L.; Fan, H.; He, Y.; Xu, W.; Zhang, L.; Yang, J.; Deng, F.; Zeng, F. Interplay of PKD3 with SREBP1 Promotes Cell Growth via Upregulating Lipogenesis in Prostate Cancer Cells. J. Cancer 2019, 10, 6395–6404. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wu, Z.; Ding, W.; Xiao, C.; Zhang, Y.; Gao, S.; Gao, Y.; Cai, W. SREBP1 siRNA enhance the docetaxel effect based on a bone-cancer dual-targeting biomimetic nanosystem against bone metastatic castration-resistant prostate cancer. Theranostics 2020, 10, 1619. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Zhang, K.; Tam, Y.Y.; Tam, Y.K.; Belliveau, N.M.; Sung, V.Y.; Lin, P.J.; LeBlanc, E.; Ciufolini, M.A.; Rennie, P.S.; et al. Lipid nanoparticle siRNA systems for silencing the androgen receptor in human prostate cancer in vivo. Int. J. Cancer 2012, 131, E781–E790. [Google Scholar] [CrossRef] [PubMed]
- Hossen, S.; Hossain, M.K.; Basher, M.; Mia, M.; Rahman, M.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Xiang, B.; Dong, D.-W.; Shi, N.-Q.; Gao, W.; Yang, Z.-Z.; Cui, Y.; Cao, D.-Y.; Qi, X.-R. PSA-responsive and PSMA-mediated multifunctional liposomes for targeted therapy of prostate cancer. Biomaterials 2013, 34, 6976–6991. [Google Scholar] [CrossRef]
- Saw, P.E.; Yao, H.; Lin, C.; Tao, W.; Farokhzad, O.C.; Xu, X. Stimuli-Responsive Polymer–Prodrug Hybrid Nanoplatform for Multistage siRNA Delivery and Combination Cancer Therapy. Nano Lett. 2019, 19, 5967–5974. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Lee, W.S.; Yang, J.M.; Shin, S. Basic peptide system for efficient delivery of foreign genes. Biochim. Biophys. Acta (Bba)-Mol. Cell Res. 2003, 1640, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Jamaledin, R.; Di Natale, C.; Onesto, V.; Taraghdari, Z.B.; Zare, E.N.; Makvandi, P.; Vecchione, R.; Netti, P.A. Progress in Microneedle-Mediated Protein Delivery. J. Clin. Med. 2020, 9, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veiman, K.-L.; Mäger, I.; Ezzat, K.; Margus, H.; Lehto, T.; Langel, K.; Kurrikoff, K.; Arukuusk, P.; Suhorutsšenko, J.; Padari, K. PepFect14 peptide vector for efficient gene delivery in cell cultures. Mol. Pharm. 2013, 10, 199–210. [Google Scholar] [CrossRef]
- Cai, X.; Zhu, H.; Zhang, Y.; Gu, Z. Highly efficient and safe delivery of VEGF siRNA by bioreducible fluorinated peptide dendrimers for cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 9402–9415. [Google Scholar] [CrossRef]
- Chen, W.; Li, H.; Liu, Z.; Yuan, W. Lipopolyplex for therapeutic gene delivery and its application for the treatment of Parkinson’s disease. Front. Aging Neurosci. 2016, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Song, Y.; Eldi, P.; Guo, X.; Hayball, J.D.; Garg, S.; Albrecht, H. Targeting prostate cancer cells with hybrid elastin-like polypeptide/liposome nanoparticles. Int. J. Nanomed. 2018, 13, 293. [Google Scholar] [CrossRef] [Green Version]
- Kudsiova, L.; Welser, K.; Campbell, F.; Mohammadi, A.; Dawson, N.; Cui, L.; Hailes, H.C.; Lawrence, M.J.; Tabor, A.B. Delivery of siRNA using ternary complexes containing branched cationic peptides: The role of peptide sequence, branching and targeting. Mol. Biosyst. 2016, 12, 934–951. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, P.; Jhaveri, A.; Pattni, B.; Biswas, S.; Torchilin, V. Transferrin and octaarginine modified dual-functional liposomes with improved cancer cell targeting and enhanced intracellular delivery for the treatment of ovarian cancer. Drug Deliv. 2018, 25, 517–532. [Google Scholar] [CrossRef]
- Begum, A.A.; Toth, I.; Moyle, P.M. Gastrin-releasing peptide receptor-targeted hybrid peptide/phospholipid pDNA/siRNA delivery systems. Nanomedicine 2019, 14, 1153–1171. [Google Scholar] [CrossRef]
- Wigge, P.A.; Kilmartin, J.V. The Ndc80p complex from Saccharomyces cerevisiae contains conserved centromere components and has a function in chromosome segregation. J. Cell Biol. 2001, 152, 349–360. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, J.G.; Howell, B.J.; Canman, J.C.; Hickey, J.M.; Fang, G.; Salmon, E. Nuf2 and Hec1 are required for retention of the checkpoint proteins Mad1 and Mad2 to kinetochores. Curr. Biol. 2003, 13, 2103–2109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obara, W.; Sato, F.; Takeda, K.; Kato, R.; Kato, Y.; Kanehira, M.; Takata, R.; Mimata, H.; Sugai, T.; Nakamura, Y.; et al. Phase I clinical trial of cell division associated 1 (CDCA1) peptide vaccination for castration resistant prostate cancer. Cancer Sci. 2017, 108, 1452–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomita, Y.; Yuno, A.; Tsukamoto, H.; Senju, S.; Yoshimura, S.; Osawa, R.; Kuroda, Y.; Hirayama, M.; Irie, A.; Hamada, A.; et al. Identification of CDCA1-derived long peptides bearing both CD4+ and CD8+ T-cell epitopes: CDCA1-specific CD4+ T-cell immunity in cancer patients. Int. J. Cancer 2014, 134, 352–366. [Google Scholar] [CrossRef]
- Kaneko, N.; Miura, K.; Gu, Z.; Karasawa, H.; Ohnuma, S.; Sasaki, H.; Tsukamoto, N.; Yokoyama, S.; Yamamura, A.; Nagase, H.; et al. siRNA-mediated knockdown against CDCA1 and KNTC2, both frequently overexpressed in colorectal and gastric cancers, suppresses cell proliferation and induces apoptosis. Biochem. Biophys Res. Commun. 2009, 390, 1235–1240. [Google Scholar] [CrossRef]
- Ohnuma, S.; Miura, K.; Horii, A.; Fujibuchi, W.; Kaneko, N.; Gotoh, O.; Nagasaki, H.; Mizoi, T.; Tsukamoto, N.; Kobayashi, T.; et al. Cancer-associated splicing variants of the CDCA1 and MSMB genes expressed in cancer cell lines and surgically resected gastric cancer tissues. Surgery 2009, 145, 57–68. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, C.; Song, B.; Sun, J.; Fu, X.; Yang, F.; Wang, H.; Yan, B. pH low insertion peptide mediated cell division cycle-associated protein 1-siRNA transportation for prostatic cancer therapy targeted to the tumor microenvironment. Biochem. Biophys. Res. Commun. 2018, 503, 1761–1767. [Google Scholar] [CrossRef]
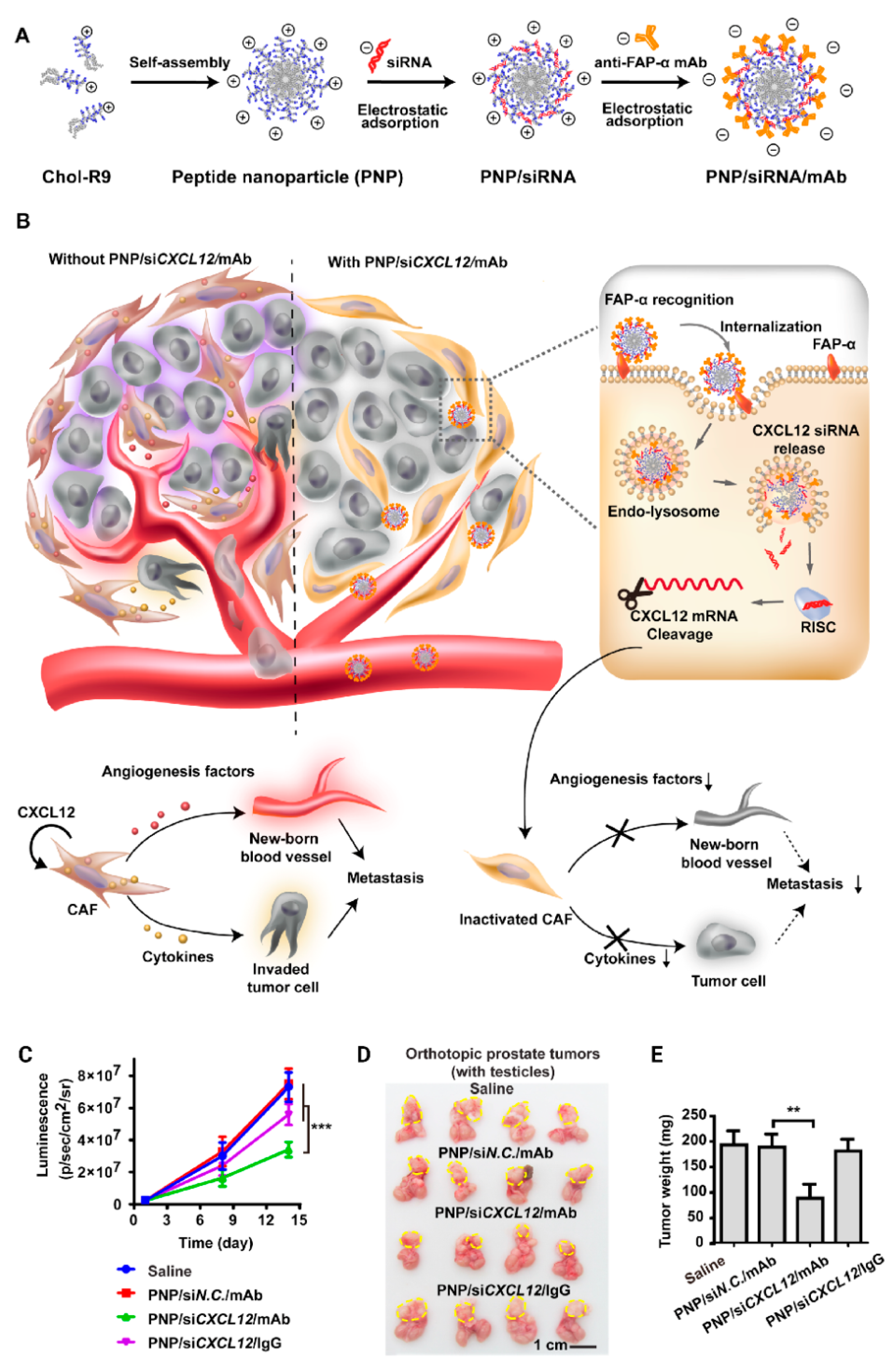
- Lang, J.; Zhao, X.; Qi, Y.; Zhang, Y.; Han, X.; Ding, Y.; Guan, J.; Ji, T.; Zhao, Y.; Nie, G. Reshaping Prostate Tumor Microenvironment To Suppress Metastasis via Cancer-Associated Fibroblast Inactivation with Peptide-Assembly-Based Nanosystem. ACS Nano 2019, 13, 12357–12371. [Google Scholar] [CrossRef]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations II: Solubilization, binding constant, and complexation efficiency. Drug Discov. Today 2016, 21, 363–368. [Google Scholar] [CrossRef]
- Meredith, M.E.; Salameh, T.S.; Banks, W.A. Intranasal Delivery of Proteins and Peptides in the Treatment of Neurodegenerative Diseases. AAPS J. 2015, 17, 780–787. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.E. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: From concept to clinic. Mol. Pharm. 2009, 6, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Lacal, P.M. Neuropilin-1 as Therapeutic Target for Malignant Melanoma. Front. Oncol. 2015, 5, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tse, B.W.C.; Volpert, M.; Ratther, E.; Stylianou, N.; Nouri, M.; McGowan, K.; Lehman, M.L.; McPherson, S.J.; Roshan-Moniri, M.; Butler, M.S.; et al. Neuropilin-1 is upregulated in the adaptive response of prostate tumors to androgen-targeted therapies and is prognostic of metastatic progression and patient mortality. Oncogene 2017, 36, 3417–3427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Huo, S.; Zhang, X.; Liu, J.; Tan, A.; Li, S.; Jin, S.; Xue, X.; Zhao, Y.; Ji, T.; et al. Neuropilin-1-targeted gold nanoparticles enhance therapeutic efficacy of platinum(IV) drug for prostate cancer treatment. ACS Nano 2014, 8, 4205–4220. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Guo, L.; Sun, X.; Shang, M.; Meng, D.; Zhou, X.; Liu, X.; Zhao, Y.; Li, J. UTMD inhibit EMT of breast cancer through the ROS/miR-200c/ZEB1 axis. Sci. Rep. 2020, 10, 6657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Q.; Zhai, X.; Yuan, Y.; Ji, Q.; Zhang, P. lncRNA ZEB1-AS1 inhibits high glucose-induced EMT and fibrogenesis by regulating the miR-216a-5p/BMP7 axis in diabetic nephropathy. Braz. J. Med. Biol. Res. 2020, 53, e9288. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Wang, X.; Li, W.; Yu, G.; Zhu, Z.; Zhang, W. ZEB1 activated-VPS9D1-AS1 promotes the tumorigenesis and progression of prostate cancer by sponging miR-4739 to upregulate MEF2D. Biomed. Pharmacother. 2020, 122, 109557. [Google Scholar] [CrossRef]
- Dai, Y.; Wu, Z.; Lang, C.; Zhang, X.; He, S.; Yang, Q.; Guo, W.; Lai, Y.; Du, H.; Peng, X.; et al. Copy number gain of ZEB1 mediates a double-negative feedback loop with miR-33a-5p that regulates EMT and bone metastasis of prostate cancer dependent on TGF-beta signaling. Theranostics 2019, 9, 6063–6079. [Google Scholar] [CrossRef]
- Evans, J.C.; Malhotra, M.; Sweeney, K.; Darcy, R.; Nelson, C.C.; Hollier, B.G.; O’Driscoll, C.M. Folate-targeted amphiphilic cyclodextrin nanoparticles incorporating a fusogenic peptide deliver therapeutic siRNA and inhibit the invasive capacity of 3D prostate cancer tumours. Int. J. Pharm. 2017, 532, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, M.; Gooding, M.; Evans, J.C.; O’Driscoll, D.; Darcy, R.; O’Driscoll, C.M. Cyclodextrin-siRNA conjugates as versatile gene silencing agents. Eur. J. Pharm. Sci. 2018, 114, 30–37. [Google Scholar] [CrossRef]
- Veiseh, O.; Kievit, F.M.; Mok, H.; Ayesh, J.; Clark, C.; Fang, C.; Leung, M.; Arami, H.; Park, J.O.; Zhang, M. Cell transcytosing poly-arginine coated magnetic nanovector for safe and effective siRNA delivery. Biomaterials 2011, 32, 5717–5725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.W.; Bae, K.H.; Kim, C.; Park, T.G. Clustered magnetite nanocrystals cross-linked with PEI for efficient siRNA delivery. Biomacromolecules 2011, 12, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Gao, Z.; Huang, W.; Jin, M.; Wang, Q. Nanocarrier-mediated co-delivery of chemotherapeutic drugs and gene agents for cancer treatment. Acta Pharm. Sin. B 2015, 5, 169–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makvandi, P.; Wang, C.-y.; Zare, E.N.; Borzacchiello, A.; Niu, L.-n.; Tay, F.R. Metal-based nanomaterials in biomedical applications: Antimicrobial activity and cytotoxicity aspects. Adv. Funct. Mater. 2020, in press. [Google Scholar] [CrossRef]
- Makvandi, P.; Zare, E.N.; Ashtari, B.; Moeini, A.; Tay, F.R.; Niu, L.-N. Polymeric and inorganic nanoscopical antimicrobial fillers in dentistry. Acta Biomater. 2020, 101, 69–101. [Google Scholar] [CrossRef]
- Nedyalkova, M.; Donkova, B.; Romanova, J.; Tzvetkov, G.; Madurga, S.; Simeonov, V. Iron oxide nanoparticles–in vivo/in vitro biomedical applications and in silico studies. Adv. Colloid Interface Sci. 2017, 249, 192–212. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Qiao, H.; Su, Z.; Chen, M.; Ping, Q.; Sun, M. PEGylated carboxymethyl chitosan/calcium phosphate hybrid anionic nanoparticles mediated hTERT siRNA delivery for anticancer therapy. Biomaterials 2014, 35, 7978–7991. [Google Scholar] [CrossRef]
- Etemad-Moghadam, S.; Alaeddini, M. Upregulation of ADAM10 in oral squamous cell carcinoma and its correlation with EGFR, neoangiogenesis and clinicopathologic factors. J. Cranio-Maxillo-Facial Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Facial Surg. 2019, 47, 1583–1588. [Google Scholar] [CrossRef]
- Sun, S.Q.; Ren, L.J.; Liu, J.; Wang, P.; Shan, S.M. Sevoflurane inhibits migration and invasion of colorectal cancer cells by regulating microRNA-34a/ADAM10 axis. Neoplasma 2019, 66, 887–895. [Google Scholar] [CrossRef]
- Panday, R.; Abdalla, A.M.E.; Yu, M.; Li, X.; Ouyang, C.; Yang, G. Functionally modified magnetic nanoparticles for effective siRNA delivery to prostate cancer cells in vitro. J. Biomater. Appl. 2020, 34, 952–964. [Google Scholar] [CrossRef]
- Ng, V.W.; Berti, R.; Lesage, F.; Kakkar, A. Gold: A versatile tool for in vivo imaging. J. Mater. Chem. B 2013, 1, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Hirst, D.; O’sullivan, J. Gold nanoparticles as novel agents for cancer therapy. Br. J. Radiol. 2012, 85, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Jamaledin, R.; Naserzadeh, P.; Afjeh-Dana, E.; Ashtari, B.; Hosseinzadeh, M.; Vecchione, R.; Wu, A.; Tay, F.R.; Borzacchiello, A. Metal-Based Nanostructures/PLGA Nanocomposites: Antimicrobial Activity, Cytotoxicity, and Their Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 3279–3300. [Google Scholar] [CrossRef]
- Zare, E.N.; Makvandi, P.; Ashtari, B.; Rossi, F.; Motahari, A.; Perale, G. Progress in Conductive Polyaniline-Based Nanocomposites for Biomedical Applications: A Review. J. Med. Chem. 2019, 63, 1–22. [Google Scholar] [PubMed]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M. Gold nanoparticles and gold nanoparticle-conjugates for delivery of therapeutic molecules. Progress and challenges. J. Mater. Chem. B 2014, 2, 4204–4220. [Google Scholar] [CrossRef] [PubMed]
- Nassir, A.M.; Ibrahim, I.A.A.; Md, S.; Waris, M.; Tanuja; Ain, M.R.; Ahmad, I.; Shahzad, N. Surface functionalized folate targeted oleuropein nano-liposomes for prostate tumor targeting: Invitro and invivo activity. Life Sci. 2019, 220, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Rahme, K.; Guo, J.; Holmes, J.D. Bioconjugated gold nanoparticles enhance siRNA delivery in prostate cancer cells. In RNA Interference and Cancer Therapy; Springer: Berlin, Germany, 2019; pp. 291–301. [Google Scholar]
- Luan, X.; Rahme, K.; Cong, Z.; Wang, L.; Zou, Y.; He, Y.; Yang, H.; Holmes, J.D.; O’Driscoll, C.M.; Guo, J. Anisamide-targeted PEGylated gold nanoparticles designed to target prostate cancer mediate: Enhanced systemic exposure of siRNA, tumour growth suppression and a synergistic therapeutic response in combination with paclitaxel in mice. Eur. J. Pharm. Biopharm. 2019, 137, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Barr, F.A.; Silljé, H.H.; Nigg, E.A. Polo-like kinases and the orchestration of cell division. Nat. Rev. Mol. Cell Biol. 2004, 5, 429–441. [Google Scholar] [CrossRef]
- Kasahara, K.; Goto, H.; Izawa, I.; Kiyono, T.; Watanabe, N.; Elowe, S.; Nigg, E.A.; Inagaki, M. PI 3-kinase-dependent phosphorylation of Plk1–Ser99 promotes association with 14–3-3γ and is required for metaphase–anaphase transition. Nat. Commun. 2013, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Zhang, Y.; Li, K.; Wang, M.; Li, J.; Qi, Z.; Wu, J.; Wang, Z.; Ling, L.; Liu, H.; et al. miR-593–5p inhibit cell proliferation by targeting PLK1 in non small cell lung cancer cells. Pathol. Res. Pract. 2020, 216, 152786. [Google Scholar] [CrossRef]
- Li, S.S.; Zhu, H.J.; Li, J.Y.; Tian, L.M.; Lv, D.M. MiRNA-875–3p alleviates the progression of colorectal cancer via negatively regulating PLK1 level. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Affatato, R.; Carrassa, L.; Chila, R.; Lupi, M.; Restelli, V.; Damia, G. Identification of PLK1 as a New Therapeutic Target in Mucinous Ovarian Carcinoma. Cancers 2020, 12, 672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, K.H.; Kim, Y.-H.; Wang, Z.; Kim, J.; Kim, J.S.; Kim, S.H.; Kim, K.; Kwon, I.C.; Kiesewetter, D.O.; Chen, X. Engineered Zn (II)-dipicolylamine-gold nanorod provides effective prostate cancer treatment by combining siRNA delivery and photothermal therapy. Theranostics 2017, 7, 4240. [Google Scholar] [CrossRef] [PubMed]
- De Vico, G.; Martano, M.; Maiolino, P.; Carella, F.; Leonardi, L. Expression of transferrin receptor-1 (TFR-1) in canine osteosarcomas. Vet. Med. Sci. 2020. [Google Scholar] [CrossRef]
- Moon, S.J.; Kim, J.H.; Kong, S.H.; Shin, C.S. Protein Expression of Cyclin B1, Transferrin Receptor, and Fibronectin Is Correlated with the Prognosis of Adrenal Cortical Carcinoma. Endocrinol. Metab. (Seoul Korea) 2020, 35, 132–141. [Google Scholar] [CrossRef]
- Nakase, I.; Gallis, B.; Takatani-Nakase, T.; Oh, S.; Lacoste, E.; Singh, N.P.; Goodlett, D.R.; Tanaka, S.; Futaki, S.; Lai, H.; et al. Transferrin receptor-dependent cytotoxicity of artemisinin-transferrin conjugates on prostate cancer cells and induction of apoptosis. Cancer Lett. 2009, 274, 290–298. [Google Scholar] [CrossRef]
- Guo, J.; O’Driscoll, C.M.; Holmes, J.D.; Rahme, K. Bioconjugated gold nanoparticles enhance cellular uptake: A proof of concept study for siRNA delivery in prostate cancer cells. Int. J. Pharm. 2016, 509, 16–27. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, H.; Guo, L.; Wang, Y.; Song, J.; Zhao, X.; Li, C.; Hao, L.; Wang, D.; Tang, J. Ultrasound-mediated nanobubble destruction (UMND) facilitates the delivery of A10–3.2 aptamer targeted and siRNA-loaded cationic nanobubbles for therapy of prostate cancer. Drug Deliv. 2018, 25, 226–240. [Google Scholar] [CrossRef]
- Afkham, A.; Aghebati-Maleki, L.; Siahmansouri, H.; Sadreddini, S.; Ahmadi, M.; Dolati, S.; Afkham, N.M.; Akbarzadeh, P.; Jadidi-Niaragh, F.; Younesi, V. Chitosan (CMD)-mediated co-delivery of SN38 and Snail-specific siRNA as a useful anticancer approach against prostate cancer. Pharmacol. Rep. 2018, 70, 418–425. [Google Scholar] [CrossRef]
- Lee, J.H.; Ku, S.H.; Kim, M.J.; Lee, S.J.; Kim, H.C.; Kim, K.; Kim, S.H.; Kwon, I.C. Rolling circle transcription-based polymeric siRNA nanoparticles for tumor-targeted delivery. J. Control. Release 2017, 263, 29–38. [Google Scholar] [CrossRef]
- Pang, S.T.; Lin, F.W.; Chuang, C.K.; Yang, H.W. Co-Delivery of Docetaxel and p44/42 MAPK siRNA Using PSMA Antibody-Conjugated BSA-PEI Layer-by-Layer Nanoparticles for Prostate Cancer Target Therapy. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Yu, X.; Liu, H.; Wu, D.; She, J.X. Co-targeting EGFR and survivin with a bivalent aptamer-dual siRNA chimera effectively suppresses prostate cancer. Sci. Rep. 2016, 6, 30346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, Y.J.; Yoon, Y.I.; Yoon, T.J.; Lee, H.J. Ultrasound-Guided Delivery of siRNA and a Chemotherapeutic Drug by Using Microbubble Complexes: In Vitro and In Vivo Evaluations in a Prostate Cancer Model. Korean J. Radiol. 2016, 17, 497–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diao, Y.; Liu, J.; Ma, Y.; Su, M.; Zhang, H.; Hao, X. A specific aptamer-cell penetrating peptides complex delivered siRNA efficiently and suppressed prostate tumor growth in vivo. Cancer Biol. Ther. 2016, 17, 498–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgerald, K.A.; Rahme, K.; Guo, J.; Holmes, J.D.; O’Driscoll, C.M. Anisamide-targeted gold nanoparticles for siRNA delivery in prostate cancer–synthesis, physicochemical characterisation and in vitro evaluation. J. Mater. Chem. B 2016, 4, 2242–2252. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Malhotra, M.; Gooding, M.; Sallas, F.; Evans, J.C.; Darcy, R.; O’Driscoll, C.M. A novel, anisamide-targeted cyclodextrin nanoformulation for siRNA delivery to prostate cancer cells expressing the sigma-1 receptor. Int. J. Pharm. 2016, 499, 131–145. [Google Scholar] [CrossRef]
- Saito-Tarashima, N.; Kira, H.; Wada, T.; Miki, K.; Ide, S.; Yamazaki, N.; Matsuda, A.; Minakawa, N. Groove modification of siRNA duplexes to elucidate siRNA-protein interactions using 7-bromo-7-deazaadenosine and 3-bromo-3-deazaadenosine as chemical probes. Org. Biomol. Chem. 2016, 14, 11096–11105. [Google Scholar] [CrossRef]
- Yang, J.; Yu, L.; Zhang, L.; Long, X.; Ji, Y.; Tang, X. Synthesis and Evaluation of Caged siRNA with Terminal Single Vitamin E Modification. Curr. Protoc. Nucleic Acid Chem. 2016, 67, 11–22. [Google Scholar] [CrossRef]
- Ambardekar, V.V.; Han, H.Y.; Varney, M.L.; Vinogradov, S.V.; Singh, R.K.; Vetro, J.A. The modification of siRNA with 3’ cholesterol to increase nuclease protection and suppression of native mRNA by select siRNA polyplexes. Biomaterials 2011, 32, 1404–1411. [Google Scholar] [CrossRef] [Green Version]











| Cell Line | Target Gene | Major Outcomes | Refs |
|---|---|---|---|
| PCa cell line PC-3 (androgen-insensitive cells) | MDM2 | Enhancing cytotoxicity of cisplatin against cancer cells, and induction of caspase-3 and -9 | [116] |
| Human prostate cancer cell lines (PC3, LNCaP) | IGHG1 | Stimulation of apoptosis and inhibition of proliferation | [117] |
| DU-145 (human prostate cancer cell line) | VEGF | Suppressing proliferation and angiogenesis | [118] |
| PC-3M, LNcap and DU145 prostate cancer cell lines | Neu3 | Suppressing migration and metastasis of cancer cells via down-regulation of MMP-2 and MMP-9 | [119] |
| PC3 cells | PARP1 | Enhancing sensitivity of cancer cells into docetaxel chemotherapy via downregulation of PARP1 and subsequent inhibition of EGF/Akt/FOXO1 | [120] |
| PC3 cells | HIF-1α | Downregulation of HIF-1α is corelated with induction of apoptosis and cell-cycle arrest at synthesis (S) and gap 2 (G2)/mitosis (M) phase | [121] |
| LNCaP cells and LAPC4 cells (androgen-sensitive human PCa cell lines), and C4-2 cells (androgen-independent human PCa cell line) | Androgen receptor (AR) | Suppressing metastasis of cancer cells | [122] |
| Human prostate carcinoma cell lines LNCaP and PC-3 | EGR-1 | Enhancing p21 activity and stimulation of apoptosis | [123] |
| PC3 cells | ADAM17 | Interfering with proliferation and DNA synthesis, and stimulation of cell cycle arrest at S phase | [124] |
| Human prostate cancer cell LNCaP and its sublines (C4, C42, C4-2B), ARCaP cell lines IA-8, IF-11, and PC-3, DU-145, TSU-PR1 | DNMT3 | Induction of cell-cycle arrest and apoptosis | [125] |
| Human prostate cell lines PNT2 (benign) and PC-3Mparental (highly malignant) | RPL19 | Impairing proliferation and stimulation of apoptosis | [126] |
| EnzR-PCa cell lines | MALAT1 | Sensitizing cancer cells to androgen therapy | [127] |
| PC-3 and DU145 human prostate cancer cells | GRP78 | Stimulation of apoptosis and suppressing metastasis | [128] |
| LNCaP cells | AR | Stimulation of apoptosis and sensitizing cancer cells to androgen therapy | [129] |
| PC3 cells | JNK-1 | Stimulation of apoptosis, DNA fragmentation, and reducing viability of cancer cells | [130] |
| RWPE-1, DU145, PC-3, and LNCaP cell lines | HMGN5 | Triggering mitochondrial-mediated apoptosis via impairing mitochondrial membrane integrity | [131] |
| Human prostate cancer PC-3 cell lines, which express prostate-specific antigens (PSAs), IGF-1R, and IRS1 (10–12) | Cytohesin-1 | Downregulation of cytohesin-1 is associated with inhibition of IGFR signaling and desirable prognosis | [132] |
| PC-3 and LNCaP prostate carcinoma cell lines | EGR-1 | Triggering apoptosis and inhibition of growth via downregulation of EGR-1, and suppressing its downstream targets NF-κB and AP-1 | [133] |
| Vehicle | Target Gene | In Vitro/In Vivo | Animal Model | Cell Line | Zeta Potential (mV) | Size (nm) | Entrapment Efficiency (EE) (%) | Results | References |
|---|---|---|---|---|---|---|---|---|---|
| Lipid nanoparticle | Androgen receptor (AR) | In vitro In vivo | Mice bearing LNCaP tumors | LNCaP and PC-3 human PCa cell lines | - | Up to 84.5 | - | Downregulation of androgen receptor and interfering with proliferation | [151] |
| Peptide dendrimer | HSP27 | In vitro In vivo | 5.0-week-old male BALB/c nude mice bearing PC3 cells | PC3 cells | +18.5 to +22.3 | 50–70 | - | High cellular uptake, effective gene silencing, and reducing proliferation and viability of cancer cells | [170] |
| Polymeric nanoparticles | GRP78 | In vitro In vivo | PC-3 prostate cancer-bearing mice | PC3 cells | −23.8 to −24.2 | 36.4–39.7 | 82.4 | Co-delivery of siRNA-GRP78 and docetaxel, and suppressing invasion and proliferation of cancer cells | [173] |
| Multifunctional polymeric nanoparticles | PHB1 | In vitro In vivo | LNCaP tumor-bearing male athymic nude mice | Luc-HeLa and PCa cell lines (LNCaP, PC3, DU145, 22RV1) | +14 | 56.6 | 90.6 | Downregulation of PHB1, endosomal penetration, and inhibition of proliferation and invasion of PCa cells | [182] |
| Micelle | SREBP1 | In vitro In vivo | Mouse model | PC-3 and C4-2B cells | +20.3 to +26.9 | 100 | - | Co-delivery of siRNA-SREBP1 and docetaxel, deep tumor penetration, protection of siRNA, and suppressing cancer malignancy | [190] |
| Peptide | EGFP | In vitro | - | PC3 cells | +25.4 | 131.5 | - | Targeted delivery, high cellular uptake, excellent biocompatibility, and reducing malignancy of cancer cells | [203] |
| Peptide | CDCA1 | In vitro In vivo | NOD/SCID mice | Human PCa cell line DU145, PC3, LNCap, and the human prostate epithelial RWPE-1 cells | - | - | - | Downregulation of CDCA1, inhibition of mitosis, and induction of apoptotic cell death | [210] |
| Cyclodextrin conjugate | PLK1 | In vitro | - | U87 and DU145 cells | - | - | - | Downregulation of PLK1, and reducing viability and proliferation of cancer cells | [223] |
| Magnetic nanoparticles | ADAM10 | In vitro | - | PC3 cells | −17.9 | 219.5 | - | Downregulation of ADAM10 and induction of apoptosis in cancer cells | [233] |
| Gold nanoparticles | RelA | In vitro | - | LNCaP cells | +46 to +53 | 113–118 | - | High internalization, endo-lysosomal escape, and reducing proliferation and viability of cancer cells | [240] |
| Multifunctional gold nanorod | PLK1 | In vitro In vivo | PC-3 xenograft tumor | 143B cells | +24.5 to +66.2 | 48.6–51.13 | - | Providing combinational photothermal therapy and gene silencing | [247] |
| Gold nanoparticle | RelA | In vitro | - | PC3 cells | +46 | 118 | - | Downregulation of RelA, and suppressing viability and proliferation of cancer cells | [251] |
| Nanobubble | FoxM1 | In vitro In vivo | Mice bearing PC3 cells | LNCaP cells | +24.07 | 479.83 | - | Improved transfection efficiency, stimulation of apoptosis and cell-cycle arrest, and reducing tumor growth (in vivo) | [252] |
| Chitosan nanoparticles | Snail | In vitro | - | PC-3 human metastatic prostate cancer cell line | +1.8 | 169 | - | Inhibition of metastasis of cancer cells via upregulation of epithelial markers E-cadherin and Claudin-1 | [253] |
| Cyclodextrin nanoparticles | ZEB1 NRP-1 | In vitro | - | PC3 and LNCaP cells | −9.07 to +46.42 | Less than 200 | - | Downregulation of ZEB1 and NRP-1, inhibition of metastasis, and suppressing angiogenesis | [222] |
| Polymeric nanoparticle | VEGF | In vitro In vivo | PC-3 xenograft tumors | PC3 cells | +1.8 | 240 | - | High cellular uptake through endocytosis, targeted delivery, and downregulation of VEGF | [254] |
| Layer-by-layer nanoparticle | P44/42 MAPK | In vitro In vivo | Mouse model | CWR22R cells | +30.5 | 170–179 | 56.7 | Co-delivery of docetaxel and siRNA-MAPK, leading to suppressing invasion and malignancy of cancer cells | [255] |
| Aptamer chimera | EGFR Survivin | In vitro In vivo | Mouse model of PCa | Cell lines including PC3, BXPC3 and T-24 | - | - | - | Selective targeting of PSMA-overexpressing PCa cells, downregulation of EGFR and survivin, and stimulation of apoptosis | [256] |
| Microbubble | Survivin | In vitro In vivo | Xenograft mouse tumor model | Human PCa cell lines PC-3 and LNCaP | - | - | - | Co-delivery of siRNA-survivin and doxorubicin, and suppressing growth and viability of cancer cells (both in vitro and in vivo experiments) | [257] |
| Peptide | Survivin | In vitro In vivo | LNCaP xenografts in nude mice | PC3 cells | - | - | - | Reducing the viability of cancer cells, and induction of apoptosis | [258] |
| Gold nanoparticle | RelA | In vitro | - | PC3 cells | +27.6 | 62.8 | - | Targeting sigma receptor using anisamide-modified gold nanoparticles, silencing RelA gene, and diminishing viability and survival of cancer cells | [259] |
| Cyclodextrin | PLK1 | In vitro | - | DU145 and PC3 cells | +10.28 to +27.8 | Less than 300 nm | - | Selective targeting PCa cells by binding into sigma receptors, downregulation of PLK1 gene, and improving prognosis | [260] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashrafizadeh, M.; Hushmandi, K.; Rahmani Moghadam, E.; Zarrin, V.; Hosseinzadeh Kashani, S.; Bokaie, S.; Najafi, M.; Tavakol, S.; Mohammadinejad, R.; Nabavi, N.; et al. Progress in Delivery of siRNA-Based Therapeutics Employing Nano-Vehicles for Treatment of Prostate Cancer. Bioengineering 2020, 7, 91. https://doi.org/10.3390/bioengineering7030091
Ashrafizadeh M, Hushmandi K, Rahmani Moghadam E, Zarrin V, Hosseinzadeh Kashani S, Bokaie S, Najafi M, Tavakol S, Mohammadinejad R, Nabavi N, et al. Progress in Delivery of siRNA-Based Therapeutics Employing Nano-Vehicles for Treatment of Prostate Cancer. Bioengineering. 2020; 7(3):91. https://doi.org/10.3390/bioengineering7030091
Chicago/Turabian StyleAshrafizadeh, Milad, Kiavash Hushmandi, Ebrahim Rahmani Moghadam, Vahideh Zarrin, Sharareh Hosseinzadeh Kashani, Saied Bokaie, Masoud Najafi, Shima Tavakol, Reza Mohammadinejad, Noushin Nabavi, and et al. 2020. "Progress in Delivery of siRNA-Based Therapeutics Employing Nano-Vehicles for Treatment of Prostate Cancer" Bioengineering 7, no. 3: 91. https://doi.org/10.3390/bioengineering7030091
APA StyleAshrafizadeh, M., Hushmandi, K., Rahmani Moghadam, E., Zarrin, V., Hosseinzadeh Kashani, S., Bokaie, S., Najafi, M., Tavakol, S., Mohammadinejad, R., Nabavi, N., Hsieh, C.-L., Zarepour, A., Zare, E. N., Zarrabi, A., & Makvandi, P. (2020). Progress in Delivery of siRNA-Based Therapeutics Employing Nano-Vehicles for Treatment of Prostate Cancer. Bioengineering, 7(3), 91. https://doi.org/10.3390/bioengineering7030091