Characterization of Functional Human Skeletal Myotubes and Neuromuscular Junction Derived—From the Same Induced Pluripotent Stem Cell Source
Abstract
1. Introduction
2. Materials and Methods
2.1. Culturing and Expansion of hiPSC
2.2. Differentiation of Myogenic Progenitorss and Functional Myofibers from hiPSCs
2.3. Immunocytochemistry
2.4. Electrophysiology
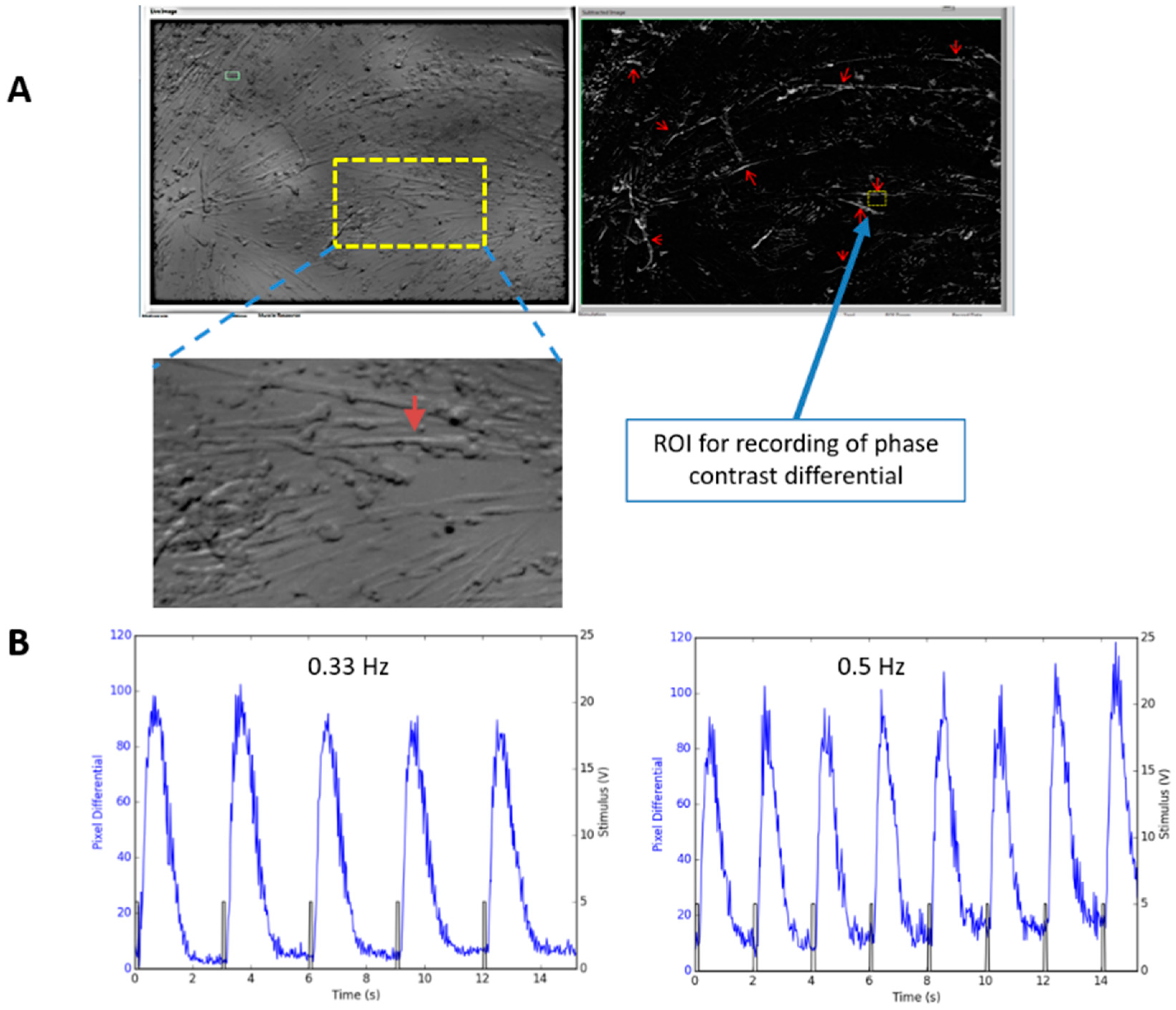
2.5. Phase Contrast Microscopy and Videography
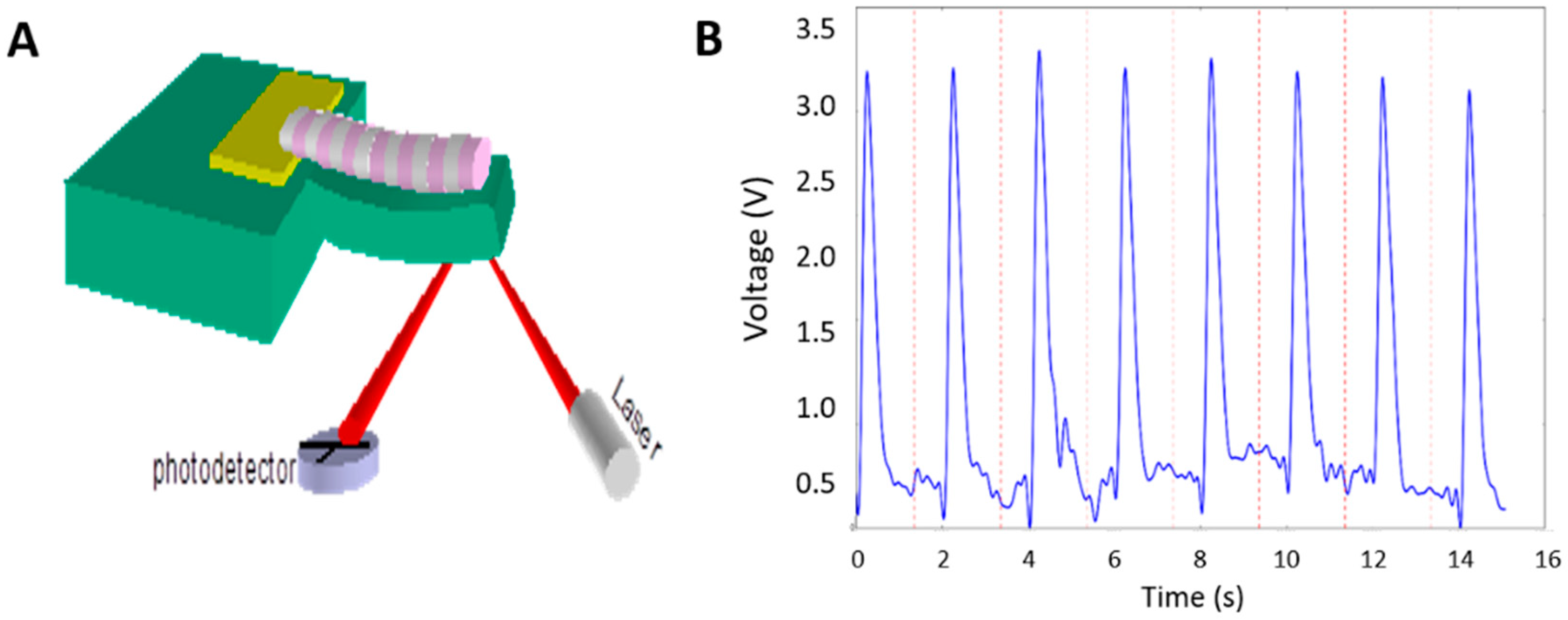
2.6. Cantilever Fabrication and Force Measurement of Myofiber Contraction
2.7. Testing the Formation of Functional NMJs
3. Results
3.1. Myofiber Differentiation Observed by Phase Microscopy
3.2. Characterization of iPSC-Derived Myogenic Progenitors by Immunocytochemical Analysis
3.3. Fiber Type Characterization of iPSC-Derived Myotubes
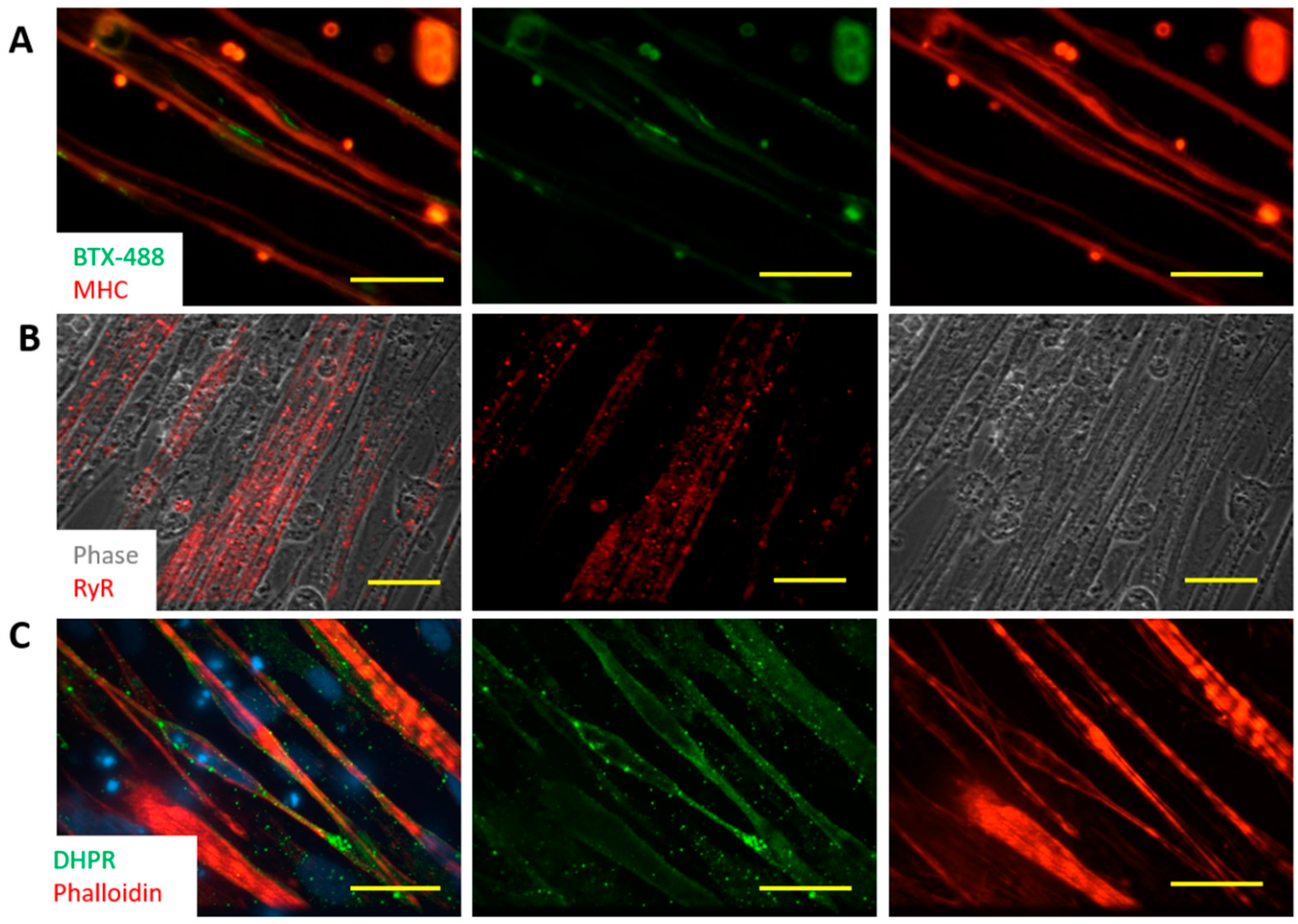
3.4. Characterization of iPSC Myotubes by Immunocytochemistry
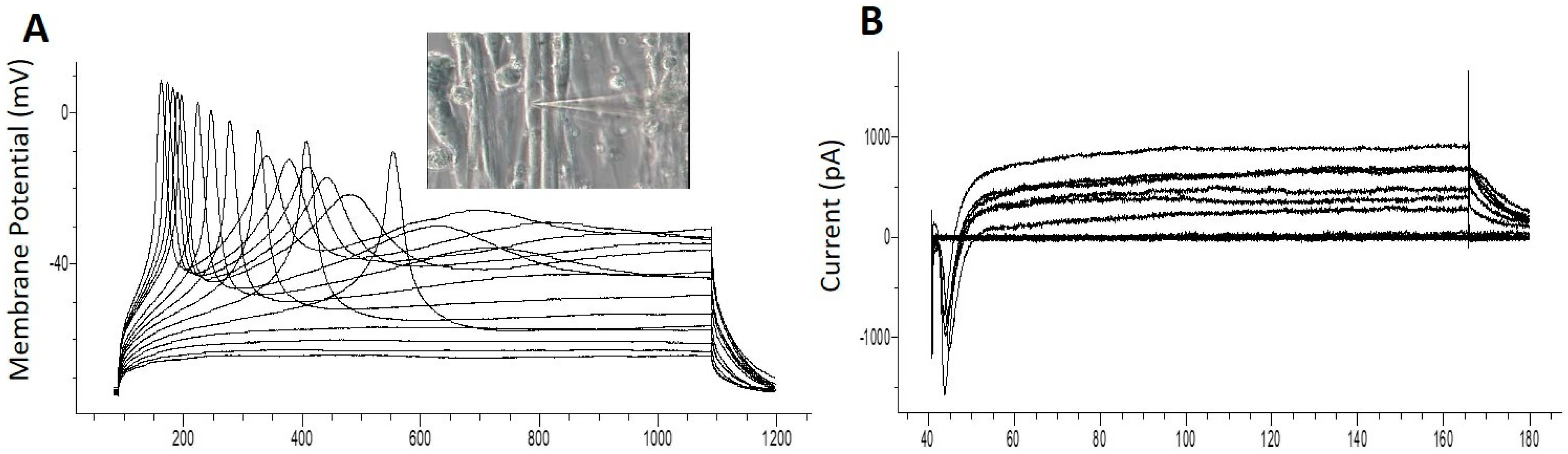
3.5. Patch Clamp Electrophysiology from iPSC Myofibers
3.6. Electrical Stimulation Induced Contraction of Human Myotubes in Culture
3.7. Contraction Profiles of iPSC Myotubes Characterized on Cantilevers
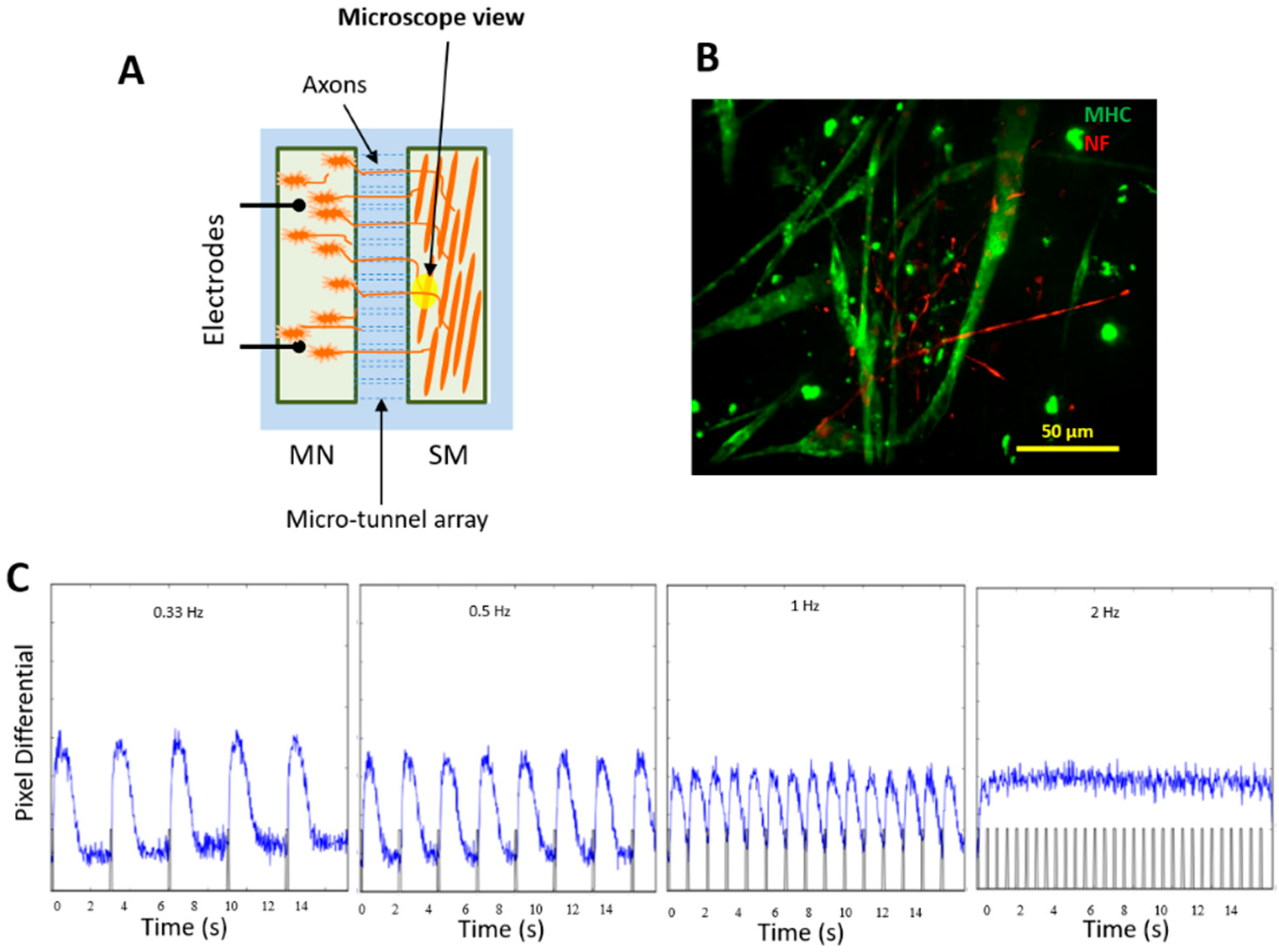
3.8. Innervation of iPSC-SKM by iPSC-MNs from the Same iPSC Line and Formation of Donor-Specific Functional NMJs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Argilés, J.M.; Campos, N.; Lopez-Pedrosa, J.M.; Rueda, R.; Rodriguez-Mañas, L. Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Ortuño-Costela, M.D.C.; García-López, M.; Cerrada, V.; Gallardo, M.E. iPSCs: A powerful tool for skeletal muscle tissue engineering. J. Cell. Mol. Med. 2019, 23, 3784–3794. [Google Scholar] [CrossRef] [PubMed]
- Khodabukus, A.; Prabhu, N.; Wang, J.; Bursac, N. In Vitro Tissue-Engineered Skeletal Muscle Models for Studying Muscle Physiology and Disease. Adv. Healthc. Mater. 2018, 7, e1701498. [Google Scholar] [CrossRef] [PubMed]
- Jodat, Y.A.; Kang, M.G.; Kiaee, K.; Kim, G.J.; Martinez, A.F.H.; Rosenkranz, A.; Bae, H.; Shin, S.R. Human-Derived Organ-on-a-Chip for Personalized Drug Development. Curr. Pharm. Des. 2018, 24, 5471–5486. [Google Scholar] [CrossRef] [PubMed]
- Montarras, D.; Morgan, J.; Collins, C.; Relaix, F.; Zaffran, S.; Cumano, A.; Partridge, T.; Buckingham, M. Direct Isolation of Satellite Cells for Skeletal Muscle Regeneration. Science 2005, 309, 2064–2067. [Google Scholar] [CrossRef]
- Tanaka, A.; Woltjen, K.; Miyake, K.; Hotta, A.; Ikeya, M.; Yamamoto, T.; Nishino, T.; Shoji, E.; Sehara-Fujisawa, A.; Manabe, Y.; et al. Efficient and Reproducible Myogenic Differentiation from Human iPS Cells: Prospects for Modeling Miyoshi Myopathy In Vitro. PLoS ONE 2013, 8, e61540. [Google Scholar] [CrossRef]
- Abujarour, R.; Bennett, M.; Valamehr, B.; Lee, T.T.; Robinson, M.; Robbins, D.; Le, T.; Lai, K.; Flynn, P. Myogenic Differentiation of Muscular Dystrophy-Specific Induced Pluripotent Stem Cells for Use in Drug Discovery. Stem Cells Transl. Med. 2014, 3, 149–160. [Google Scholar] [CrossRef]
- Hosoyama, T.; McGivern, J.V.; Van Dyke, J.M.; Ebert, A.D.; Suzuki, M. Derivation of Myogenic Progenitors Directly from Human Pluripotent Stem Cells Using a Sphere-Based Culture. Stem Cells Transl. Med. 2014, 3, 564–574. [Google Scholar] [CrossRef]
- Chal, J.; Oginuma, M.; Al Tanoury, Z.; Gobert, B.; Sumara, O.; Hick, A.; Bousson, F.; Zidouni, Y.; Mursch, C.; Moncuquet, P.; et al. Differentiation of pluripotent stem cells to muscle fiber to model Duchenne muscular dystrophy. Nat. Biotech. 2015, 33, 962–969. [Google Scholar] [CrossRef]
- Maffioletti, S.M.; Gerli, M.F.M.; Ragazzi, M.; Dastidar, S.; Benedetti, S.; Loperfido, M.; VandenDriessche, T.; Chuah, M.K.; Tedesco, F.S. Efficient derivation and inducible differentiation of expandable skeletal myogenic cells from human ES and patient-specific iPS cells. Nat. Protoc. 2015, 10, 941–958. [Google Scholar] [CrossRef]
- Chal, J.; Al Tanoury, Z.; Hestin, M.; Gobert, B.; Aivio, S.; Hick, A.; Cherrier, T.; Nesmith, A.P.; Parker, K.K.; Pourquie, O. Generation of human muscle fibers and satellite-like cells from human pluripotent stem cells in vitro. Nat. Protoc. 2016, 11, 1833–1850. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, J.; Pagani, F.; De Santis, R.; Limatola, C.; Bozzoni, I.; Di Angelantonio, S.; Rosa, A. Differentiation of control and ALS mutant human iPSCs into functional skeletal muscle cells, a tool for the study of neuromuscolar diseases. Stem Cell Res. 2016, 17, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Demestre, M.; Orth, M.; Föhr, K.J.; Achberger, K.; Ludolph, A.C.; Liebau, S.; Boeckers, T.M. Formation and characterisation of neuromuscular junctions between hiPSC derived motoneurons and myotubes. Stem Cell Res. 2015, 15, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Qian, Y.; Khodabukus, A.; Ribar, T.; Bursac, N. Engineering human pluripotent stem cells into a functional skeletal muscle tissue. Nat. Commun. 2018, 9, 126. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Yoshida, M.; Li, L.-T.; Ikenaka, A.; Oshima, S.; Nakagawa, K.; Sakurai, H.; Matsui, E.; Nakahata, T.; Saito, M.K. iPSC-derived functional human neuromuscular junctions model the pathophysiology of neuromuscular diseases. JCI Insight 2019, 4, e124299. [Google Scholar] [CrossRef]
- Swartz, E.W.; Shintani, G.; Wan, J.; Maffei, J.S.; Wang, S.H.; Miller, B.L.; Havton, L.A.; Coppola, G. Establishment of a Human Induced Pluripotent Stem Cell-Derived Neuromuscular Co-Culture Under Optogenetic Control. BioRxiv 2020, 4, 036400. [Google Scholar] [CrossRef]
- Guo, X.; Smith, V.; Jackson, M.; Tran, M.; Thomas, M.; Patel, A.; Lorusso, E.; Nimbalkar, S.; Cai, Y.; McAleer, C.W.; et al. A human-based functional NMJ system for personalized ALS modeling and drug testing. Adv. Ther. 2020, in press. [Google Scholar] [CrossRef]
- Santhanam, N.; Kumanchik, L.; Guo, X.; Sommerhage, F.; Cai, Y.; Jackson, M.; Martin, C.; Saad, G.; McAleer, C.W.; Wang, Y.; et al. Stem cell derived phenotypic human neuromuscular junction model for dose response evaluation of therapeutics. Biomaterials 2018, 166, 64–78. [Google Scholar] [CrossRef]
- Das, M.; Molnar, P.; Devaraj, H.; Poeta, M.; Hickman, J.J. Electrophysiological and morphological characterization of rat embryonic motoneurons in a defined system. Biotechnol. Prog. 2003, 19, 1756–1761. [Google Scholar] [CrossRef]
- Gao, B.-X.; Ziskind-Conhaim, L. Development of Ionic Currents Underlying Changes in Action Potential Waveforms in Rat Spinal Motoneurons. J. Neurophysiol. 1998, 80, 3047–3061. [Google Scholar] [CrossRef]
- Wilson, K.; Das, M.; Wahl, K.J.; Colton, R.J.; Hickman, J. Measurement of contractile stress generated by cultured rat muscle on silicon cantilevers for toxin detection and muscle performance enhancement. PLoS ONE 2010, 5, e11042. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.S.T.; Long, C.J.; McAleer, C.; Bobbitt, N.; Srinivasan, B.; Hickman, J.J. Utilization of microscale silicon cantilevers to assess cellular contractile function in vitro. J. Vis. Exp. 2014, e51866. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Sabourin, L.A.; Girgis-Gabardo, A.; Mansouri, A.; Gruss, P.; Rudnicki, M.A. Pax7 Is Required for the Specification of Myogenic Satellite Cells. Cell 2000, 102, 777–786. [Google Scholar] [CrossRef]
- Weintraub, H.; Davis, R.; Tapscott, S.; Thayer, M.; Krause, M.; Benezra, R.; Blackwell, T.; Turner, D.; Rupp, R.; Hollenberg, S.; et al. The myoD gene family: Nodal point during specification of the muscle cell lineage. Science 1991, 251, 761–766. [Google Scholar] [CrossRef]
- Buckingham, M. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr. Opin. Genet. Dev. 2006, 16, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Talbot, J.; Maves, L. Skeletal muscle fiber type: Using insights from muscle developmental biology to dissect targets for susceptibility and resistance to muscle disease. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 518–534. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Fiber Types in Mammalian Skeletal Muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Caiozzo, V.J.; Baker, M.J.; Huang, K.; Chou, H.; Wu, Y.Z.; Baldwin, K.M. Single-fiber myosin heavy chain polymorphism: How many patterns and what proportions? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R570–R580. [Google Scholar] [CrossRef]
- Jurdana, M.; Fumagalli, G.; Grubic, Z.; Lorenzon, P.; Mars, T.; Sciancalepore, M. Neural agrin changes the electrical properties of developing human skeletal muscle cells. Cell. Mol. Neurobiol. 2009, 29, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Greene, K.; Akanda, N.; Smith, A.; Stancescu, M.; Lambert, S.; Vandenburgh, H.; Hickman, J. In vitro Differentiation of Functional Human Skeletal Myotubes in a Defined System. Biomater. Sci. 2014, 2, 131–138. [Google Scholar] [CrossRef]
- Smith, A.S.T.; Long, C.J.; Pirozzi, K.; Najjar, S.; McAleer, C.; Vandenburgh, H.H.; Hickman, J.J. A multiplexed chip-based assay system for investigating the functional development of human skeletal myotubes in vitro. J. Biotechnol. 2014, 185, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Lavado, A.; Guo, X.; Smith, A.S.; Akanda, N.; Martin, C.; Cai, Y.; Elbrecht, D.; Tran, M.; Bryant, J.-P.; Colon, A.; et al. Evaluation of Holistic Treatment for ALS Reveals Possible Mechanism and Therapeutic Potential. Int. J. Pharm. Pharm. Res. 2017, 11, 348–374. [Google Scholar] [PubMed]
- Guo, X.; Gonzalez, M.; Stancescu, M.; Vandenburgh, H.; Hickman, J.J. Neuromuscular junction formation between human stem cell-derived motoneurons and human skeletal muscle in a defined system. Biomaterials 2011, 32, 9602–9611. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |



| RP(mV) | INa+ (pA) | IK+ (pA) | AP (mV) | Rm (MΩ) | Cm (pF) | |
|---|---|---|---|---|---|---|
| Average | −60.80 | 1128.85 | 633.20 | 107.10 | 214.25 | 196.60 |
| SEM | 1.40 | 211.91 | 100.81 | 21.85 | 18.31 | 9.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Badu-Mensah, A.; Thomas, M.C.; McAleer, C.W.; Hickman, J.J. Characterization of Functional Human Skeletal Myotubes and Neuromuscular Junction Derived—From the Same Induced Pluripotent Stem Cell Source. Bioengineering 2020, 7, 133. https://doi.org/10.3390/bioengineering7040133
Guo X, Badu-Mensah A, Thomas MC, McAleer CW, Hickman JJ. Characterization of Functional Human Skeletal Myotubes and Neuromuscular Junction Derived—From the Same Induced Pluripotent Stem Cell Source. Bioengineering. 2020; 7(4):133. https://doi.org/10.3390/bioengineering7040133
Chicago/Turabian StyleGuo, Xiufang, Agnes Badu-Mensah, Michael C. Thomas, Christopher W. McAleer, and James J. Hickman. 2020. "Characterization of Functional Human Skeletal Myotubes and Neuromuscular Junction Derived—From the Same Induced Pluripotent Stem Cell Source" Bioengineering 7, no. 4: 133. https://doi.org/10.3390/bioengineering7040133
APA StyleGuo, X., Badu-Mensah, A., Thomas, M. C., McAleer, C. W., & Hickman, J. J. (2020). Characterization of Functional Human Skeletal Myotubes and Neuromuscular Junction Derived—From the Same Induced Pluripotent Stem Cell Source. Bioengineering, 7(4), 133. https://doi.org/10.3390/bioengineering7040133




