Best of Both Hydrogel Worlds: Harnessing Bioactivity and Tunability by Incorporating Glycosaminoglycans in Collagen Hydrogels
Abstract
:1. Introduction
2. Collagen Alone with No Crosslinkers
3. Collagen with Crosslinkers
4. Collagen–GAG Hydrogels
4.1. Collagen–HA Hydrogels
4.2. Collagen–CS Hydrogels
4.3. Collagen-Heparin Gels
4.4. Collagen-Alginate Gels
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gorgieva, S.; Kokol, V. Collagen- vs. Gelatine-Based Biomaterials and Their Biocompatibility: Review and Perspectives. Biomater. Appl. Nanomed. 2011. [Google Scholar] [CrossRef] [Green Version]
- Lovell, C.R.; Smolenski, K.A.; Duance, V.C.; Light, N.D.; Young, S.; Dyson, M. Type I and III collagen content and fibre distribution in normal human skin during ageing. Br. J. Dermatol. 1987, 117, 419–428. [Google Scholar] [CrossRef]
- McKee, T.J.; Perlman, G.; Morris, M.; Komarova, S.V. Extracellular matrix composition of connective tissues: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 10542. [Google Scholar] [CrossRef] [Green Version]
- Boskey, A.L.; Robey, P.G. The Composition of Bone. In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism; American Society for Bone and Mineral Research: Washington, DC, USA, 2018; pp. 84–92. [Google Scholar]
- Meek, K.M. Corneal collagen-its role in maintaining corneal shape and transparency. Biophys. Rev. 2009, 1, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, J.M.; Simons, M. The extracellular matrix and blood vessel formation: Not just a scaffold. J. Cell Mol. Med. 2007, 11, 176–205. [Google Scholar] [CrossRef]
- Komuro, T. The lattice arrangement of the collagen fibres in the submucosa of the rat small intestine: Scanning electron microscopy. Cell Tissue Res. 1988, 251, 117–121. [Google Scholar] [CrossRef]
- Sharabi, M.; Wade, K.; Haj-Ali, R. The Mechanical Role of Collagen Fibers in the Intervertebral Disc. In Biomechanics of the Spine; Galbusera, F., Wilke, H.-J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 105–123. [Google Scholar]
- Ramachandran, G.N.; Kartha, G. Structure of collagen. Nature 1954, 174, 269–270. [Google Scholar] [CrossRef]
- Rich, A.; Crick, F.H. The structure of collagen. Nature 1955, 176, 915–916. [Google Scholar] [CrossRef]
- Cowan, P.M.; McGavin, S.; North, A.C. The polypeptide chain configuration of collagen. Nature 1955, 176, 1062–1064. [Google Scholar] [CrossRef]
- Pinnell, S.R.; Martin, G.R. The cross-linking of collagen and elastin: Enzymatic conversion of lysine in peptide linkage to alpha-aminoadipic-delta-semialdehyde (allysine) by an extract from bone. Proc. Natl. Acad. Sci. USA 1968, 61, 708–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyre, D.R.; Paz, M.A.; Gallop, P.M. Cross-linking in collagen and elastin. Annu. Rev. Biochem. 1984, 53, 717–748. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Raynal, N.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015, 25, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Meek, K.M.; Chapman, J.A.; Hardcastle, R.A. The staining pattern of collagen fibrils. Improved correlation with sequence data. J. Biol. Chem. 1979, 254, 10710–10714. [Google Scholar]
- Fietzek, P.P.; Kuhn, K. Information contained in the amino acid sequence of the alpha1(I)-chain of collagen and its consequences upon the formation of the triple helix, of fibrils and crosslinks. Mol. Cell Biochem. 1975, 8, 141–157. [Google Scholar] [CrossRef]
- Berg, R.A.; Prockop, D.J. The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Biochem. Biophys. Res. Commun. 1973, 52, 115–120. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [Green Version]
- Knight, C.G.; Morton, L.F.; Peachey, A.R.; Tuckwell, D.S.; Farndale, R.W.; Barnes, M.J. The collagen-binding A-domains of integrins alpha(1)beta(1) and alpha(2)beta(1) recognize the same specific amino acid sequence, GFOGER, in native (triple-helical) collagens. J. Biol. Chem. 2000, 275, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Legate, K.R.; Wickstrom, S.A.; Fassler, R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009, 23, 397–418. [Google Scholar] [CrossRef] [Green Version]
- Guan, J.L. Role of focal adhesion kinase in integrin signaling. Int. J. Biochem. Cell Biol. 1997, 29, 1085–1096. [Google Scholar] [CrossRef]
- Moraes, J.A.; Frony, A.C.; Dias, A.M.; Renovato-Martins, M.; Rodrigues, G.; Marcinkiewicz, C.; Assreuy, J.; Barja-Fidalgo, C. Alpha1beta1 and integrin-linked kinase interact and modulate angiotensin II effects in vascular smooth muscle cells. Atherosclerosis 2015, 243, 477–485. [Google Scholar] [CrossRef] [Green Version]
- Heino, J. Cellular Signaling by Collagen-Binding Integrins. In I Domain Integrins; Gullberg, D., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 143–155. [Google Scholar] [CrossRef]
- Wu, D.; Witt, R.L.; Harrington, D.A.; Farach-Carson, M.C. Dynamic Assembly of Human Salivary Stem/Progenitor Microstructures Requires Coordinated alpha1beta1 Integrin-Mediated Motility. Front. Cell Dev. Biol. 2019, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.E. Affinity of integrins for damaged extracellular matrix: Alpha v beta 3 binds to denatured collagen type I through RGD sites. Biochem. Biophys. Res. Commun. 1992, 182, 1025–1031. [Google Scholar] [CrossRef]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of cell binding to collagen and gelatin: A study of the effect of 2D and 3D architecture and surface chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapudom, J.; Rubner, S.; Martin, S.; Kurth, T.; Riedel, S.; Mierke, C.T.; Pompe, T. The phenotype of cancer cell invasion controlled by fibril diameter and pore size of 3D collagen networks. Biomaterials 2015, 52, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Roeder, B.A.; Kokini, K.; Voytik-Harbin, S.L. Fibril microstructure affects strain transmission within collagen extracellular matrices. J. Biomech. Eng. 2009, 131, 031004. [Google Scholar] [CrossRef]
- Corin, K.A.; Gibson, L.J. Cell contraction forces in scaffolds with varying pore size and cell density. Biomaterials 2010, 31, 4835–4845. [Google Scholar] [CrossRef]
- Ray, A.; Morford, R.K.; Ghaderi, N.; Odde, D.J.; Provenzano, P.P. Dynamics of 3D carcinoma cell invasion into aligned collagen. Integr. Biol. 2018, 10, 100–112. [Google Scholar] [CrossRef] [Green Version]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of collagen I hydrogels for bioengineered tissue microenvironments: Characterization of mechanics, structure, and transport. Tissue Eng. Part B Rev. 2014, 20, 683–696. [Google Scholar] [CrossRef] [Green Version]
- Parenteau-Bareil, R.; Gauvin, R.; Cliche, S.; Gariépy, C.; Germain, L.; Berthod, F. Comparative study of bovine, porcine and avian collagens for the production of a tissue engineered dermis. Acta Biomater. 2011, 7, 3757–3765. [Google Scholar] [CrossRef]
- Kreger, S.T.; Bell, B.J.; Bailey, J.; Stites, E.; Kuske, J.; Waisner, B.; Voytik-Harbin, S.L. Polymerization and matrix physical properties as important design considerations for soluble collagen formulations. Biopolymers 2010, 93, 690–707. [Google Scholar] [CrossRef]
- Bailey, J.L.; Critser, P.J.; Whittington, C.; Kuske, J.L.; Yoder, M.C.; Voytik-Harbin, S.L. Collagen oligomers modulate physical and biological properties of three-dimensional self-assembled matrices. Biopolymers 2011, 95, 77–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanujan, S.; Pluen, A.; McKee, T.D.; Brown, E.B.; Boucher, Y.; Jain, R.K. Diffusion and convection in collagen gels: Implications for transport in the tumor interstitium. Biophys. J. 2002, 83, 1650–1660. [Google Scholar] [CrossRef] [Green Version]
- Erikson, A.; Andersen, H.N.; Naess, S.N.; Sikorski, P.; Davies Cde, L. Physical and chemical modifications of collagen gels: Impact on diffusion. Biopolymers 2008, 89, 135–143. [Google Scholar] [CrossRef] [PubMed]
- McPherson, J.M.; Wallace, D.G.; Sawamura, S.J.; Conti, A.; Condell, R.A.; Wade, S.; Piez, K.A. Collagen fibrillogenesis in vitro: A characterization of fibril quality as a function of assembly conditions. Coll Relat. Res. 1985, 5, 119–135. [Google Scholar] [CrossRef]
- Raub, C.B.; Suresh, V.; Krasieva, T.; Lyubovitsky, J.; Mih, J.D.; Putnam, A.J.; Tromberg, B.J.; George, S.C. Noninvasive Assessment of Collagen Gel Microstructure and Mechanics Using Multiphoton Microscopy. Biophys. J. 2007, 92, 2212–2222. [Google Scholar] [CrossRef] [Green Version]
- Gobeaux, F.; Mosser, G.; Anglo, A.; Panine, P.; Davidson, P.; Giraud-Guille, M.M.; Belamie, E. Fibrillogenesis in Dense Collagen Solutions: A Physicochemical Study. J. Mol. Biol. 2008, 376, 1509–1522. [Google Scholar] [CrossRef]
- Naciri, M.; Kuystermans, D.; Al-Rubeai, M. Monitoring pH and dissolved oxygen in mammalian cell culture using optical sensors. Cytotechnology 2008, 57, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Millerot-Serrurot, E.; Guilbert, M.; Fourre, N.; Witkowski, W.; Said, G.; Van Gulick, L.; Terryn, C.; Zahm, J.M.; Garnotel, R.; Jeannesson, P. 3D collagen type I matrix inhibits the antimigratory effect of doxorubicin. Cancer Cell Int. 2010, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Mao, C.; Kisaalita, W.S. Characterization of 3-D collagen hydrogels for functional cell-based biosensing. Biosens. Bioelectron. 2004, 19, 1075–1088. [Google Scholar] [CrossRef]
- Buchanan, C.F.; Voigt, E.E.; Szot, C.S.; Freeman, J.W.; Vlachos, P.P.; Rylander, M.N. Three-dimensional microfluidic collagen hydrogels for investigating flow-mediated tumor-endothelial signaling and vascular organization. Tissue Eng. Part C Methods 2014, 20, 64–75. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Portalati, N.N.; Kilmer, C.E.; Panitch, A.; Liu, J.C. Characterization of Collagen Type I and II Blended Hydrogels for Articular Cartilage Tissue Engineering. Biomacromolecules 2016, 17, 3145–3152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilmer, C.; Battistoni, C.M.; Cox, A.; Breur, G.J.; Panitch, A.; Liu, J.C. Collagen Type I and II Blend Hydrogel with Autologous Mesenchymal Stem Cells as a Scaffold for Articular Cartilage Defect Repair. ACS Biomater. Sci. Eng. 2020, 6, 3464–3476. [Google Scholar] [CrossRef]
- Ren, X.; Wang, F.; Chen, C.; Gong, X.; Yin, L.; Yang, L. Engineering zonal cartilage through bioprinting collagen type II hydrogel constructs with biomimetic chondrocyte density gradient. BMC Musculoskelet. Disord. 2016, 17, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Yuan, T.; Guo, L.; Zhang, X. An in vitro study of collagen hydrogel to induce the chondrogenic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. A 2012, 100, 2717–2725. [Google Scholar] [CrossRef] [PubMed]
- Hesse, E.; Hefferan, T.E.; Tarara, J.E.; Haasper, C.; Meller, R.; Krettek, C.; Lu, L.; Yaszemski, M.J. Collagen type I hydrogel allows migration, proliferation, and osteogenic differentiation of rat bone marrow stromal cells. J. Biomed. Mater. Res. A 2010, 94, 442–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.A.; Lee, H.Y.; Lee, J.H.; Kim, T.H.; Jang, J.H.; Kim, H.W.; Wall, I. Collagen three-dimensional hydrogel matrix carrying basic fibroblast growth factor for the cultivation of mesenchymal stem cells and osteogenic differentiation. Tissue Eng. Part A 2012, 18, 1087–1100. [Google Scholar] [CrossRef]
- Noth, U.; Schupp, K.; Heymer, A.; Kall, S.; Jakob, F.; Schutze, N.; Baumann, B.; Barthel, T.; Eulert, J.; Hendrich, C. Anterior cruciate ligament constructs fabricated from human mesenchymal stem cells in a collagen type I hydrogel. Cytotherapy 2005, 7, 447–455. [Google Scholar] [CrossRef]
- Szot, C.S.; Buchanan, C.F.; Freeman, J.W.; Rylander, M.N. 3D in vitro bioengineered tumors based on collagen I hydrogels. Biomaterials 2011, 32, 7905–7912. [Google Scholar] [CrossRef] [Green Version]
- Responte, D.J.; Natoli, R.M.; Athanasiou, K.A. Collagens of articular cartilage: Structure, function, and importance in tissue engineering. Crit. Rev. Biomed. Eng. 2007, 35, 363–411. [Google Scholar] [CrossRef]
- Hassell, J.R.; Birk, D.E. The molecular basis of corneal transparency. Exp. Eye Res. 2010, 91, 326–335. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Gurkan, U.A.; Dehen, C.J.; Tate, M.P.; Hillhouse, H.W.; Simpson, G.J.; Akkus, O. An electrochemical fabrication process for the assembly of anisotropically oriented collagen bundles. Biomaterials 2008, 29, 3278–3288. [Google Scholar] [CrossRef] [PubMed]
- Kishore, V.; Bullock, W.; Sun, X.; Van Dyke, W.S.; Akkus, O. Tenogenic differentiation of human MSCs induced by the topography of electrochemically aligned collagen threads. Biomaterials 2012, 33, 2137–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Panitch, A.; Neu, C.P. Incorporation of an aggrecan mimic prevents proteolytic degradation of anisotropic cartilage analogs. Acta Biomater. 2013, 9, 4618–4625. [Google Scholar] [CrossRef] [PubMed]
- Torbet, J.; Malbouyres, M.; Builles, N.; Justin, V.; Roulet, M.; Damour, O.; Oldberg, A.; Ruggiero, F.; Hulmes, D.J. Orthogonal scaffold of magnetically aligned collagen lamellae for corneal stroma reconstruction. Biomaterials 2007, 28, 4268–4276. [Google Scholar] [CrossRef]
- Builles, N.; Janin-Manificat, H.; Malbouyres, M.; Justin, V.; Rovere, M.R.; Pellegrini, G.; Torbet, J.; Hulmes, D.J.; Burillon, C.; Damour, O.; et al. Use of magnetically oriented orthogonal collagen scaffolds for hemi-corneal reconstruction and regeneration. Biomaterials 2010, 31, 8313–8322. [Google Scholar] [CrossRef]
- Paten, J.A.; Siadat, S.M.; Susilo, M.E.; Ismail, E.N.; Stoner, J.L.; Rothstein, J.P.; Ruberti, J.W. Flow-Induced Crystallization of Collagen: A Potentially Critical Mechanism in Early Tissue Formation. ACS Nano 2016, 10, 5027–5040. [Google Scholar] [CrossRef]
- Gao, Y.; Li, B.; Kong, W.; Yuan, L.; Guo, L.; Li, C.; Fan, H.; Fan, Y.; Zhang, X. Injectable and self-crosslinkable hydrogels based on collagen type II and activated chondroitin sulfate for cell delivery. Int. J. Biol. Macromol. 2018, 118, 2014–2020. [Google Scholar] [CrossRef]
- Antman-Passig, M.; Levy, S.; Gartenberg, C.; Schori, H.; Shefi, O. Mechanically Oriented 3D Collagen Hydrogel for Directing Neurite Growth. Tissue Eng. Part A 2017, 23, 403–414. [Google Scholar] [CrossRef]
- Danielson, K.G.; Baribault, H.; Holmes, D.F.; Graham, H.; Kadler, K.E.; Iozzo, R.V. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 1997, 136, 729–743. [Google Scholar] [CrossRef] [Green Version]
- Helary, C.; Bataille, I.; Abed, A.; Illoul, C.; Anglo, A.; Louedec, L.; Letourneur, D.; Meddahi-Pelle, A.; Giraud-Guille, M.M. Concentrated collagen hydrogels as dermal substitutes. Biomaterials 2010, 31, 481–490. [Google Scholar] [CrossRef]
- Braziulis, E.; Diezi, M.; Biedermann, T.; Pontiggia, L.; Schmucki, M.; Hartmann-Fritsch, F.; Luginbuhl, J.; Schiestl, C.; Meuli, M.; Reichmann, E. Modified plastic compression of collagen hydrogels provides an ideal matrix for clinically applicable skin substitutes. Tissue Eng. Part C Methods 2012, 18, 464–474. [Google Scholar] [CrossRef] [Green Version]
- Sohutskay, D.O.; Buno, K.P.; Tholpady, S.S.; Nier, S.J.; Voytik-Harbin, S.L. Design and biofabrication of dermal regeneration scaffolds: Role of oligomeric collagen fibril density and architecture. Regen. Med. 2020, 15, 1295–1312. [Google Scholar] [CrossRef] [PubMed]
- Rýglová, Š.; Braun, M.; Suchý, T. Collagen and Its Modifications-Crucial Aspects with Concern to Its Processing and Analysis. Macromol. Mater. Eng. 2017, 302, 1600460. [Google Scholar] [CrossRef]
- Goodarzi, H.; Jadidi, K.; Pourmotabed, S.; Sharifi, E.; Aghamollaei, H. Preparation and in vitro characterization of cross-linked collagen-gelatin hydrogel using EDC/NHS for corneal tissue engineering applications. Int. J. Biol. Macromol. 2019, 126, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, Z.W.; Wang, B.H.; Wen, Z.K. Construction and biocompatibility of a thin type I/II collagen composite scaffold. Cell Tissue Bank 2018, 19, 47–59. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Larsen, J.; Krasieva, T.B.; Lyubovitsky, J.G. Effect of genipin crosslinking on the optical spectral properties and structures of collagen hydrogels. ACS Appl. Mater. Interfaces 2011, 3, 2579–2584. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Chen, X.; Yang, T.; Zhang, N.; Dong, L.; Ma, S.; Liu, X.; Zhou, M.; Li, B. The effects of different crossing-linking conditions of genipin on type I collagen scaffolds: An in vitro evaluation. Cell Tissue Bank 2014, 15, 531–541. [Google Scholar] [CrossRef]
- Sundararaghavan, H.G.; Monteiro, G.A.; Lapin, N.A.; Chabal, Y.J.; Miksan, J.R.; Shreiber, D.I. Genipin-induced changes in collagen gels: Correlation of mechanical properties to fluorescence. J. Biomed. Mater. Res. Part A 2008, 87, 308–320. [Google Scholar] [CrossRef]
- Zhou, X.; Tao, Y.; Chen, E.; Wang, J.; Fang, W.; Zhao, T.; Liang, C.; Li, F.; Chen, Q. Genipin-cross-linked type II collagen scaffold promotes the differentiation of adipose-derived stem cells into nucleus pulposus-like cells. J. Biomed. Mater. Res. Part A 2018, 106, 1258–1268. [Google Scholar] [CrossRef]
- Selling, G.W.; Woods, K.K.; Sessa, D.; Biswas, A. Electrospun zein fibers using glutaraldehyde as the crosslinking reagent: Effect of time and temperature. Macromol. Chem. Phys. 2008, 209, 1003–1011. [Google Scholar] [CrossRef]
- Goissis, G.; Marcantonio, E., Jr.; Marcantonio, R.A.; Lia, R.C.; Cancian, D.C.; de Carvalho, W.M. Biocompatibility studies of anionic collagen membranes with different degree of glutaraldehyde cross-linking. Biomaterials 1999, 20, 27–34. [Google Scholar] [CrossRef]
- Olde Damink, L.H.H.; Dijkstra, P.J.; Van Luyn, M.J.A.; Van Wachem, P.B.; Nieuwenhuis, P.; Feijen, J. Glutaraldehyde as a crosslinking agent for collagen-based biomaterials. J. Mater. Sci. Mater. Med. 1995, 6, 460–472. [Google Scholar] [CrossRef] [Green Version]
- Gough, J.E.; Scotchford, C.A.; Downes, S. Cytotoxicity of glutaraldehyde crosslinked collagen/poly(vinyl alcohol) films is by the mechanism of apoptosis. J. Biomed. Mater. Res. 2002, 61, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Speer, D.P.; Chvapil, M.; Eskelson, C.D.; Ulreich, J. Biological effects of residual glutaraldehyde in glutaraldehyde-tanned collagen biomaterials. J. Biomed. Mater. Res. 1980, 14, 753–764. [Google Scholar] [CrossRef]
- Liu, Y.; Shu, X.Z.; Gray, S.D.; Prestwich, G.D. Disulfide-crosslinked hyaluronan-gelatin sponge: Growth of fibrous tissue in vivo. J. Biomed. Mater. Res. Part A 2004, 68, 142–149. [Google Scholar] [CrossRef]
- Xu, G.; Wang, X.; Deng, C.; Teng, X.; Suuronen, E.J.; Shen, Z.; Zhong, Z. Injectable biodegradable hybrid hydrogels based on thiolated collagen and oligo(acryloyl carbonate)-poly(ethylene glycol)-oligo(acryloyl carbonate) copolymer for functional cardiac regeneration. Acta Biomater. 2015, 15, 55–64. [Google Scholar] [CrossRef]
- Singh, R.K.; Seliktar, D.; Putnam, A.J. Capillary morphogenesis in PEG-collagen hydrogels. Biomaterials 2013, 34, 9331–9340. [Google Scholar] [CrossRef] [Green Version]
- Kurimoto, A.; Tanabe, T.; Tachibana, A.; Yamauchi, K. Thiolated dermal bovine collagen as a novel support for bioactive substances—Conjugation with lysozyme. J. Biotechnol. 2001, 86, 1–8. [Google Scholar] [CrossRef]
- Pupkaite, J.; Rosenquist, J.; Hilborn, J.; Samanta, A. Injectable Shape-Holding Collagen Hydrogel for Cell Encapsulation and Delivery Cross-linked Using Thiol-Michael Addition Click Reaction. Biomacromolecules 2019, 20, 3475–3484. [Google Scholar] [CrossRef]
- Yamauchi, K.; Takeuchi, N.; Kurimoto, A.; Tanabe, T. Films of collagen crosslinked by S-S bonds: Preparation and characterization. Biomaterials 2001, 22, 855–863. [Google Scholar] [CrossRef]
- Drexler, J.W.; Powell, H.M. Dehydrothermal crosslinking of electrospun collagen. Tissue Eng. Part C Methods 2011, 17, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Weadock, K.S.; Miller, E.J.; Bellincampi, L.D.; Zawadsky, J.P.; Dunn, M.G. Physical crosslinking of collagen fibers: Comparison of ultraviolet irradiation and dehydrothermal treatment. J. Biomed. Mater. Res. 1995, 29, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Achilli, M.; Lagueux, J.; Mantovani, D. On the effects of UV-C and pH on the mechanical behavior, molecular conformation and cell viability of collagen-based scaffold for vascular tissue engineering. Macromol. Biosci. 2010, 10, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Menter, J.M.; Williamson, G.D.; Carlyle, K.; Moore, C.L.; Willis, I. Photochemistry of type I acid-soluble calf skin collagen: Dependence on excitation wavelength. Photochem. Photobiol. 1995, 62, 402–408. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kaminska, A. Thermal helix-coil transition in UV irradiated collagen from rat tail tendon. Int. J. Biol. Macromol. 1999, 24, 337–340. [Google Scholar] [CrossRef]
- Ohan, M.P.; Weadock, K.S.; Dunn, M.G. Synergistic effects of glucose and ultraviolet irradiation on the physical properties of collagen. J. Biomed. Mater. Res. 2002, 60, 384–391. [Google Scholar] [CrossRef]
- Cornwell, K.G.; Lei, P.; Andreadis, S.T.; Pins, G.D. Crosslinking of discrete self-assembled collagen threads: Effects on mechanical strength and cell-matrix interactions. J. Biomed. Mater. Res. Part A 2007, 80, 362–371. [Google Scholar] [CrossRef]
- Docherty, R.; Forrester, J.V.; Lackie, J.M.; Gregory, D.W. Glycosaminoglycans facilitate the movement of fibroblasts through three-dimensional collagen matrices. J. Cell Sci. 1989, 92, 263–270. [Google Scholar]
- Bittner, K.; Liszio, C.; Blumberg, P.; Schonherr, E.; Kresse, H. Modulation of collagen gel contraction by decorin. Biochem. J. 1996, 314, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Casale, J.; Crane, J.S. Biochemistry, Glycosaminoglycans. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Gandhi, N.S.; Mancera, R.L. The structure of glycosaminoglycans and their interactions with proteins. Chem. Biol. Drug Des. 2008, 72, 455–482. [Google Scholar] [CrossRef]
- Sasisekharan, R.; Venkataraman, G. Heparin and heparan sulfate: Biosynthesis, structure and function. Curr. Opin. Chem. Biol. 2000, 4, 626–631. [Google Scholar] [CrossRef]
- Alphonsus, C.S.; Rodseth, R.N. The endothelial glycocalyx: A review of the vascular barrier. Anaesthesia 2014, 69, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Masola, V.; Zaza, G.; Onisto, M.; Lupo, A.; Gambaro, G. Glycosaminoglycans, proteoglycans and sulodexide and the endothelium: Biological roles and pharmacological effects. Int. Angiol. 2014, 33, 243–254. [Google Scholar] [PubMed]
- Chappell, D.; Brettner, F.; Doerfler, N.; Jacob, M.; Rehm, M.; Bruegger, D.; Conzen, P.; Jacob, B.; Becker, B.F. Protection of glycocalyx decreases platelet adhesion after ischaemia/reperfusion: An animal study. Eur. J. Anaesthesiol. 2014, 31, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Glant, T.T.; Buzas, E.I.; Finnegan, A.; Negroiu, G.; Cs-Szabo, G.; Mikecz, K. Critical roles of glycosaminoglycan side chains of cartilage proteoglycan (aggrecan) in antigen recognition and presentation. J. Immunol. 1998, 160, 3812–3819. [Google Scholar] [PubMed]
- Walimbe, T.; Panitch, A.; Sivasankar, P.M. A Review of Hyaluronic Acid and Hyaluronic Acid-based Hydrogels for Vocal Fold Tissue Engineering. J. Voice 2017, 31, 416–423. [Google Scholar] [CrossRef]
- Parry, D.A.D.; Flint, M.H.; Gillard, G.C.; Craig, A.S. A role for glycosaminoglycans in the development of collagen fibrils. FEBS Lett. 1982, 149, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Eng, D.; Caplan, M.; Preul, M.; Panitch, A. Hyaluronan scaffolds: A balance between backbone functionalization and bioactivity. Acta Biomater. 2010, 6, 2407–2414. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Thones, S.; Rother, S.; Wippold, T.; Blaszkiewicz, J.; Balamurugan, K.; Moeller, S.; Ruiz-Gomez, G.; Schnabelrauch, M.; Scharnweber, D.; Saalbach, A.; et al. Hyaluronan/collagen hydrogels containing sulfated hyaluronan improve wound healing by sustained release of heparin-binding EGF-like growth factor. Acta Biomater. 2019, 86, 135–147. [Google Scholar] [CrossRef]
- Rother, S.; Galiazzo, V.D.; Kilian, D.; Fiebig, K.M.; Becher, J.; Moeller, S.; Hempel, U.; Schnabelrauch, M.; Waltenberger, J.; Scharnweber, D.; et al. Hyaluronan/Collagen Hydrogels with Sulfated Hyaluronan for Improved Repair of Vascularized Tissue Tune the Binding of Proteins and Promote Endothelial Cell Growth. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Rother, S.; Kronert, V.; Hauck, N.; Berg, A.; Moeller, S.; Schnabelrauch, M.; Thiele, J.; Scharnweber, D.; Hintze, V. Hyaluronan/collagen hydrogel matrices containing high-sulfated hyaluronan microgels for regulating transforming growth factor-beta1. J. Mater. Sci. Mater. Med. 2019, 30, 65. [Google Scholar] [CrossRef] [PubMed]
- Hempel, U.; Hintze, V.; Moller, S.; Schnabelrauch, M.; Scharnweber, D.; Dieter, P. Artificial extracellular matrices composed of collagen I and sulfated hyaluronan with adsorbed transforming growth factor beta1 promote collagen synthesis of human mesenchymal stromal cells. Acta Biomater. 2012, 8, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Hempel, U.; Moller, S.; Noack, C.; Hintze, V.; Scharnweber, D.; Schnabelrauch, M.; Dieter, P. Sulfated hyaluronan/collagen I matrices enhance the osteogenic differentiation of human mesenchymal stromal cells in vitro even in the absence of dexamethasone. Acta Biomater. 2012, 8, 4064–4072. [Google Scholar] [CrossRef]
- Kliemt, S.; Lange, C.; Otto, W.; Hintze, V.; Moller, S.; von Bergen, M.; Hempel, U.; Kalkhof, S. Sulfated hyaluronan containing collagen matrices enhance cell-matrix-interaction, endocytosis, and osteogenic differentiation of human mesenchymal stromal cells. J. Proteome Res. 2013, 12, 378–389. [Google Scholar] [CrossRef]
- Hempel, U.; Matthaus, C.; Preissler, C.; Moller, S.; Hintze, V.; Dieter, P. Artificial matrices with high-sulfated glycosaminoglycans and collagen are anti-inflammatory and pro-osteogenic for human mesenchymal stromal cells. J. Cell Biochem. 2014, 115, 1561–1571. [Google Scholar] [CrossRef]
- Shu, X.Z.; Liu, Y.; Palumbo, F.; Prestwich, G.D. Disulfide-crosslinked hyaluronan-gelatin hydrogel films: A covalent mimic of the extracellular matrix for in vitro cell growth. Biomaterials 2003, 24, 3825–3834. [Google Scholar] [CrossRef]
- Wirostko, B.; Mann, B.K.; Williams, D.L.; Prestwich, G.D. Ophthalmic Uses of a Thiol-Modified Hyaluronan-Based Hydrogel. Adv. Wound Care 2014, 3, 708–716. [Google Scholar] [CrossRef] [Green Version]
- Mazzocchi, A.; Devarasetty, M.; Huntwork, R.; Soker, S.; Skardal, A. Optimization of collagen type I-hyaluronan hybrid bioink for 3D bioprinted liver microenvironments. Biofabrication 2018, 11, 015003. [Google Scholar] [CrossRef]
- Walimbe, T.; Calve, S.; Panitch, A.; Sivasankar, M.P. Incorporation of types I and III collagen in tunable hyaluronan hydrogels for vocal fold tissue engineering. Acta Biomater. 2019, 87, 97–107. [Google Scholar] [CrossRef]
- Suri, S.; Schmidt, C.E. Photopatterned collagen-hyaluronic acid interpenetrating polymer network hydrogels. Acta Biomater. 2009, 5, 2385–2397. [Google Scholar] [CrossRef] [PubMed]
- Brigham, M.D.; Bick, A.; Lo, E.; Bendali, A.; Burdick, J.A.; Khademhosseini, A. Mechanically robust and bioadhesive collagen and photocrosslinkable hyaluronic acid semi-interpenetrating networks. Tissue Eng. Part A 2009, 15, 1645–1653. [Google Scholar] [CrossRef]
- Suri, S.; Schmidt, C.E. Cell-laden hydrogel constructs of hyaluronic acid, collagen, and laminin for neural tissue engineering. Tissue Eng. Part A 2010, 16, 1703–1716. [Google Scholar] [CrossRef] [PubMed]
- Her, G.J.; Wu, H.C.; Chen, M.H.; Chen, M.Y.; Chang, S.C.; Wang, T.W. Control of three-dimensional substrate stiffness to manipulate mesenchymal stem cell fate toward neuronal or glial lineages. Acta Biomater. 2013, 9, 5170–5180. [Google Scholar] [CrossRef]
- Murphy, C.M.; Matsiko, A.; Haugh, M.G.; Gleeson, J.P.; O’Brien, F.J. Mesenchymal stem cell fate is regulated by the composition and mechanical properties of collagen-glycosaminoglycan scaffolds. J. Mech. Behav. Biomed. Mater. 2012, 11, 53–62. [Google Scholar] [CrossRef]
- Matsiko, A.; Levingstone, T.J.; O’Brien, F.J.; Gleeson, J.P. Addition of hyaluronic acid improves cellular infiltration and promotes early-stage chondrogenesis in a collagen-based scaffold for cartilage tissue engineering. J. Mech. Behav. Biomed. Mater. 2012, 11, 41–52. [Google Scholar] [CrossRef]
- Koh, R.H.; Jin, Y.; Kang, B.J.; Hwang, N.S. Chondrogenically primed tonsil-derived mesenchymal stem cells encapsulated in riboflavin-induced photocrosslinking collagen-hyaluronic acid hydrogel for meniscus tissue repairs. Acta Biomater. 2017, 53, 318–328. [Google Scholar] [CrossRef]
- Heo, J.; Koh, R.H.; Shim, W.; Kim, H.D.; Yim, H.G.; Hwang, N.S. Riboflavin-induced photo-crosslinking of collagen hydrogel and its application in meniscus tissue engineering. Drug Deliv. Transl. Res. 2016, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, M.; Lu, W.; Zhang, X.; Ma, D.; Rong, X.; Yu, C.; Jin, Y. Cross-linked collagen-chondroitin sulfate-hyaluronic acid imitating extracellular matrix as scaffold for dermal tissue engineering. Tissue Eng. Part C Methods 2010, 16, 269–279. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, L.; Wang, Y. Crosslinked collagen-gelatin-hyaluronic acid biomimetic film for cornea tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 196–201. [Google Scholar] [CrossRef]
- Kirk, J.F.; Ritter, G.; Finger, I.; Sankar, D.; Reddy, J.D.; Talton, J.D.; Nataraj, C.; Narisawa, S.; Millan, J.L.; Cobb, R.R. Mechanical and biocompatible characterization of a cross-linked collagen-hyaluronic acid wound dressing. Biomatter 2013, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, J.; Stowers, R.; Nam, S.; Xia, Y.; Chaudhuri, O. Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials 2018, 154, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G.; Lin, P.; Schmidt, C.E. Biodegradable hydrogels composed of oxime crosslinked poly(ethylene glycol), hyaluronic acid and collagen: A tunable platform for soft tissue engineering. J. Biomater. Sci. Polym. Ed. 2015, 26, 143–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Xiao, Y.; Jiang, B.; Fan, H.; Zhang, X. Effect of adipic dihydrazide modification on the performance of collagen/hyaluronic acid scaffold. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 307–316. [Google Scholar] [CrossRef]
- Guo, Y.; Yuan, T.; Xiao, Z.; Tang, P.; Xiao, Y.; Fan, Y.; Zhang, X. Hydrogels of collagen/chondroitin sulfate/hyaluronan interpenetrating polymer network for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2012, 23, 2267–2279. [Google Scholar] [CrossRef]
- Liang, W.H.; Kienitz, B.L.; Penick, K.J.; Welter, J.F.; Zawodzinski, T.A.; Baskaran, H. Concentrated collagen-chondroitin sulfate scaffolds for tissue engineering applications. J. Biomed. Mater. Res. A 2010, 94, 1050–1060. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, K.F.; Xiao, W.Q.; Zheng, L.; Xiao, Y.M.; Fan, H.S.; Zhang, X.D. Preparation of collagen-chondroitin sulfate-hyaluronic acid hybrid hydrogel scaffolds and cell compatibility in vitro. Carbohydr. Polym. 2011, 84, 118–125. [Google Scholar] [CrossRef]
- Pieper, J.S.; Oosterhof, A.; Dijkstra, P.J.; Veerkamp, J.H.; van Kuppevelt, T.H. Preparation and characterization of porous crosslinked collagenous matrices containing bioavailable chondroitin sulphate. Biomaterials 1999, 20, 847–858. [Google Scholar] [CrossRef]
- Pieper, J.S.; van Wachem, P.B.; van Luyn, M.J.A.; Brouwer, L.A.; Hafmans, T.; Veerkamp, J.H.; van Kuppevelt, T.H. Attachment of glycosaminoglycans to collagenous matrices modulates the tissue response in rats. Biomaterials 2000, 21, 1689–1699. [Google Scholar] [CrossRef]
- Pietrucha, K. Physicochemical properties of 3D collagen-CS scaffolds for potential use in neural tissue engineering. Int. J. Biol. Macromol. 2015, 80, 732–739. [Google Scholar] [CrossRef]
- Pietrucha, K.; Szymański, J.; Drobnik, J. The Behavior of Embryonic Neural Cells within the 3D Micro-structured Collagen-Based Scaffolds. In Proceedings of the 6th European Conference of the International Federation for Medical and Biological Engineering, Dubrovnik, Croatia, 7–11 September 2014; pp. 549–552. [Google Scholar]
- Stuart, K.; Panitch, A. Influence of chondroitin sulfate on collagen gel structure and mechanical properties at physiologically relevant levels. Biopolymers 2008, 89, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Stuart, K.; Panitch, A. Characterization of gels composed of blends of collagen I, collagen III, and chondroitin sulfate. Biomacromolecules 2009, 10, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.F.; Shepherd, D.M.; West, G.B.; Stroud, S.W. Function of heparin. Nature 1955, 176, 1123. [Google Scholar] [CrossRef] [PubMed]
- Copes, F.; Chevallier, P.; Loy, C.; Pezzoli, D.; Boccafoschi, F.; Mantovani, D. Heparin-Modified Collagen Gels for Controlled Release of Pleiotrophin: Potential for Vascular Applications. Front. Bioeng. Biotechnol. 2019, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Guidry, C.; Grinnell, F. Heparin modulates the organization of hydrated collagen gels and inhibits gel contraction by fibroblasts. J. Cell Biol. 1987, 104, 1097–1103. [Google Scholar] [CrossRef] [Green Version]
- Salchert, K.; Streller, U.; Pompe, T.; Herold, N.; Grimmer, M.; Werner, C. In vitro reconstitution of fibrillar collagen type I assemblies at reactive polymer surfaces. Biomacromolecules 2004, 5, 1340–1350. [Google Scholar] [CrossRef]
- Watarai, A.; Schirmer, L.; Thones, S.; Freudenberg, U.; Werner, C.; Simon, J.C.; Anderegg, U. TGFbeta functionalized starPEG-heparin hydrogels modulate human dermal fibroblast growth and differentiation. Acta Biomater. 2015, 25, 65–75. [Google Scholar] [CrossRef]
- Fahimipour, F.; Dashtimoghadam, E.; Mahdi Hasani-Sadrabadi, M.; Vargas, J.; Vashaee, D.; Lobner, D.C.; Jafarzadeh Kashi, T.S.; Ghasemzadeh, B.; Tayebi, L. Enhancing cell seeding and osteogenesis of MSCs on 3D printed scaffolds through injectable BMP2 immobilized ECM-Mimetic gel. Dent. Mater. 2019, 35, 990–1006. [Google Scholar] [CrossRef]
- Binner, M.; Bray, L.J.; Friedrichs, J.; Freudenberg, U.; Tsurkan, M.V.; Werner, C. Cell-instructive starPEG-heparin-collagen composite matrices. Acta Biomater. 2017, 53, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Smidsrod, O.; Skjakbrk, G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990, 8, 71–78. [Google Scholar] [CrossRef]
- Liu, C.; Lewin Mejia, D.; Chiang, B.; Luker, K.E.; Luker, G.D. Hybrid collagen alginate hydrogel as a platform for 3D tumor spheroid invasion. Acta Biomater. 2018, 75, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Moxon, S.R.; Corbett, N.J.; Fisher, K.; Potjewyd, G.; Domingos, M.; Hooper, N.M. Blended alginate/collagen hydrogels promote neurogenesis and neuronal maturation. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109904. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.S.; Teply, B.A.; Stevens, M.M.; Zeitels, S.M.; Langer, R. Collagen composite hydrogels for vocal fold lamina propria restoration. Biomaterials 2006, 27, 1104–1109. [Google Scholar] [CrossRef]
- Jin, G.Z.; Kim, H.W. Efficacy of collagen and alginate hydrogels for the prevention of rat chondrocyte dedifferentiation. J. Tissue Eng. 2018, 9, 2041731418802438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahapatra, C.; Jin, G.Z.; Kim, H.W. Alginate-hyaluronic acid-collagen composite hydrogel favorable for the culture of chondrocytes and their phenotype maintenance. Tissue Eng. Regen. Med. 2016, 13, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 83, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Mei, E.C.; Li, S.K.; Song, J.W.; Xing, R.R.; Li, Z.M.; Yan, X.H. Self-assembling Collagen/Alginate hybrid hydrogels for combinatorial photothermal and immuno tumor therapy. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 577, 570–575. [Google Scholar] [CrossRef]
- Wong, F.S.; Wong, C.C.; Chan, B.P.; Lo, A.C. Sustained Delivery of Bioactive GDNF from Collagen and Alginate-Based Cell-Encapsulating Gel Promoted Photoreceptor Survival in an Inherited Retinal Degeneration Model. PLoS ONE 2016, 11, e0159342. [Google Scholar] [CrossRef] [Green Version]
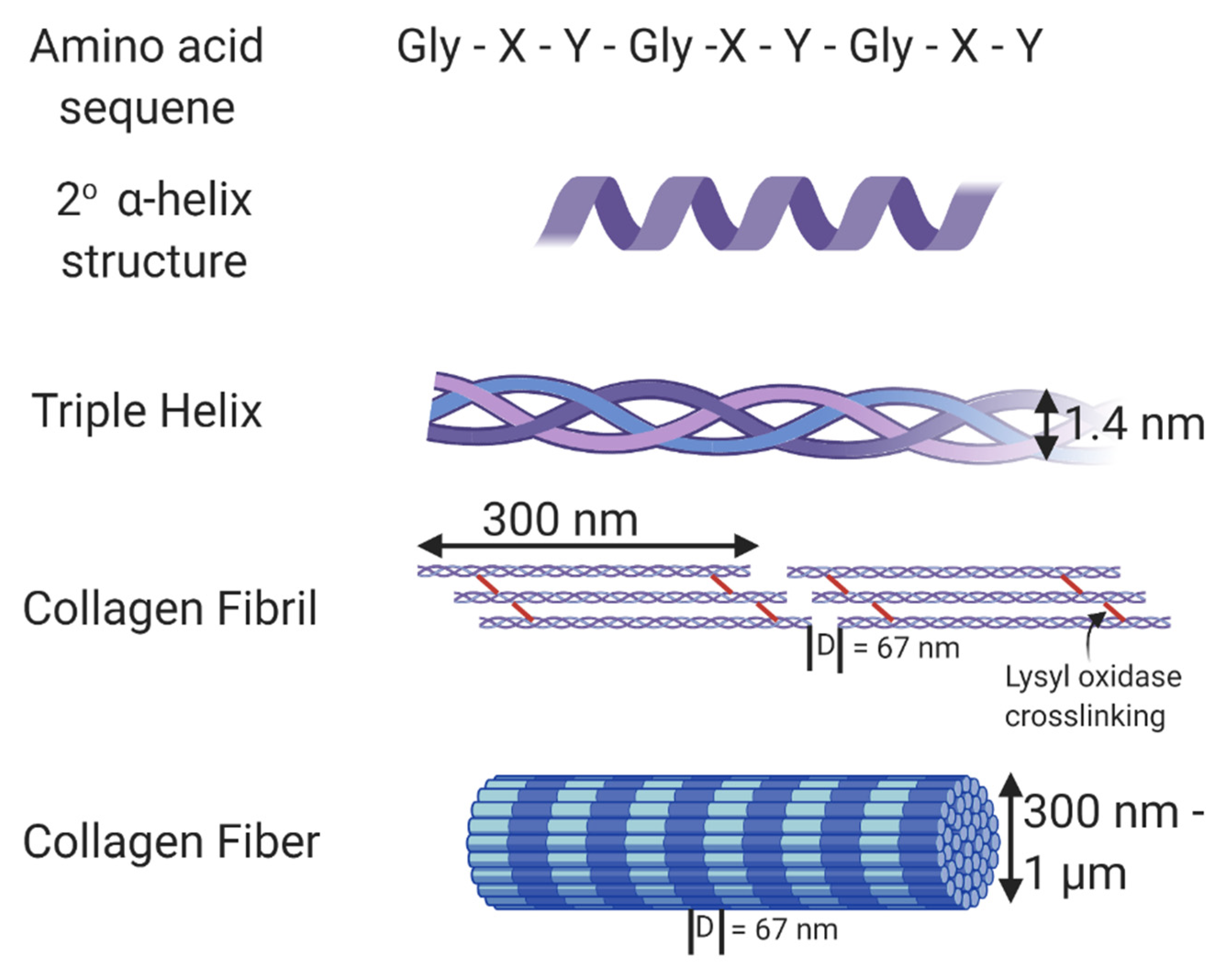

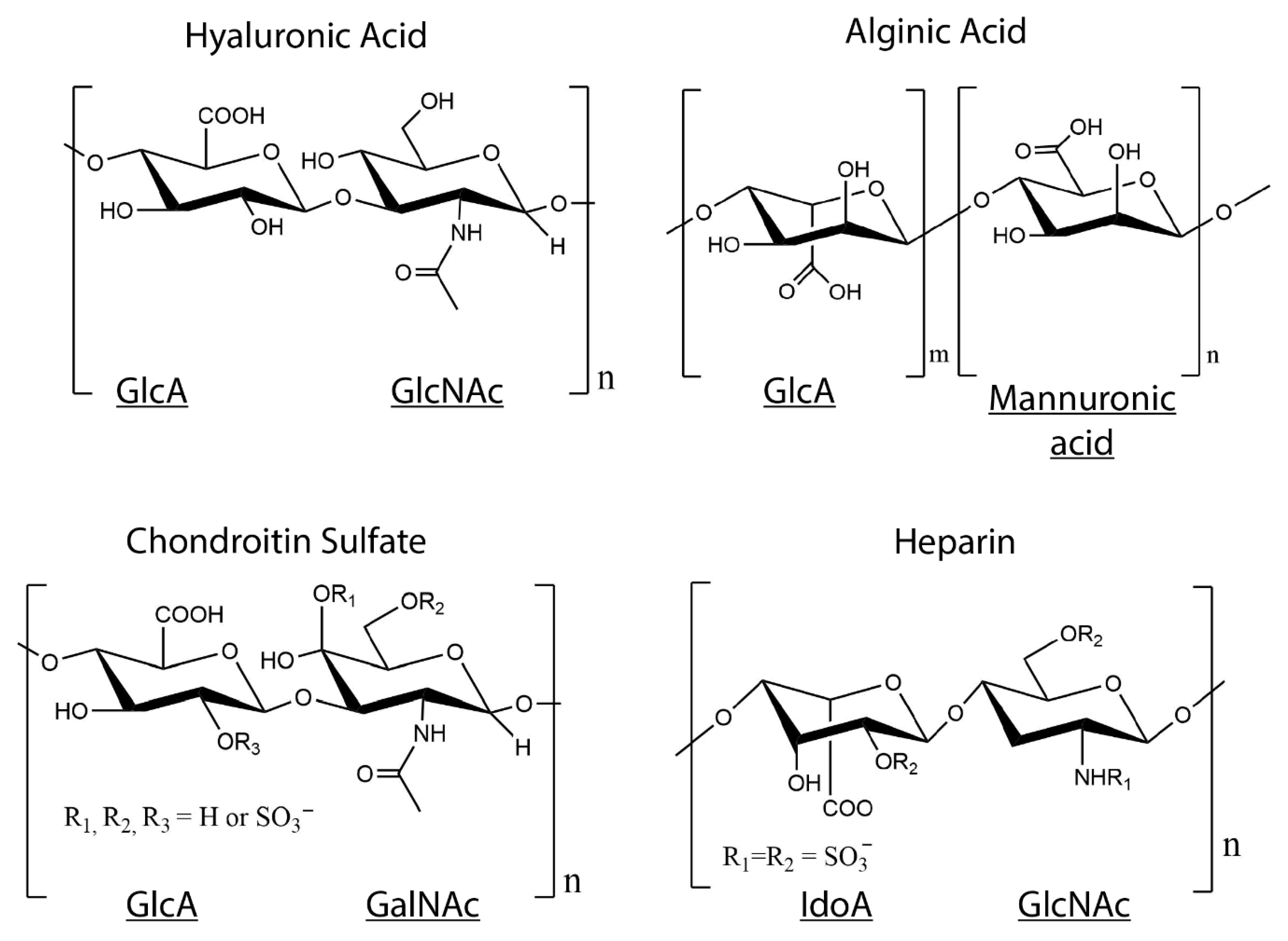
| Collagen Hydrogels with No Crosslinkers | |||
| Hydrogel | Collagen Concentration and Temperature | Application | Reference |
| Collagen type I | 3 mg/mL, 37 °C 8 mg/mL, 37 °C 7 mg/mL, 37 °C 3.45 mg/mL, 37 °C | 3D test bed for drug testing | [41] |
| 3D tumor model | [43,51] | ||
| Stem cell differentiation | [47,48,49,50] | ||
| Electrochemically or magnetically aligned collagen | 7 mg/mL, 37 °C 4 mg/mL, 37 °C 5 mg/mL, 37 °C 4 mg/mL, 30 °C 3 mg/mL, 25 °C | Tendon tissue engineering | [54,55] |
| Cartilage tissue engineering | [56] | ||
| Corneal tissue engineering | [57,58] | ||
| Neural tissue engineering | [61] | ||
| Collagen type I and/or type II | 4 mg/mL, 37 °C | Cartilage tissue engineering | [44,45,46,68] |
| Concentrated/compressed collagen | 2 mg/mL, 10 mg/mL, 15 mg/mL, 25 °C | Dermal tissue engineering | [63,64] |
| Crosslinked Collagen Hydrogels | |||
| Hydrogel | Collagen Concentration and Temperature | Application | Reference |
| EDC crosslinked collagen | 6.33 mg/mL, 4 °C | Corneal tissue engineering | [67] |
| Genipin crosslinked collagen | 2 mg/mL, 37 °C 6 mg/mL | Cartilage tissue engineering, stem cell differentiation | [69,72] |
| Dehydrothermal or UV crosslinked collagen | Unknown; Unknown | Vascular tissue engineering | [86] |
| Tendon tissue engineering | [88] | ||
| Thiol crosslinked collagen | 1% wt/v, 37 °C 3 mg/mL, 37 °C | Cardiovascular tissue engineering | [79] |
| Liver regeneration | [113] | ||
| Skin tissue engineering | [80] | ||
| Collagen HA Hydrogels | |||
| Hydrogel | Collagen Concentration and Temperature | Application | Reference |
| Sulfated HA–collagen | 1 mg/mL, 37 °C 0.5 mg/mL, 37 °C 1 mg/mL, 37 °C | Vascular tissue engineering | [105] |
| Skin tissue engineering | [106] | ||
| Bone tissue engineering | [107,108,109,110] | ||
| Thiolated HA–collagen IPN | 4 mg/mL, 37 °C | Vocal fold tissue engineering | [114] |
| HA hydrazine, HA aldehyde–collagen IPN | 2.5 mg/mL, 37 °C | Mimic in vivo microenvironment | [126] |
| Photocrosslinked HA–collagen IPN | 3 mg/mL, 37 °C 3 mg/mL, 37 °C | Regenerative medicine | [115,116] |
| Neural tissue engineering | [117] | ||
| EDC crosslinked HA–collagen | 0.5 wt%, 1 wt% 5 mg/mL, 25 °C 1 mg/mL, 37 °C 6 mg/mL, 37 °C | Stem cell differentiation | [118,119] |
| Cartilage tissue engineering | [60,120] | ||
| Dermal tissue engineering | [123,125] | ||
| Corneal tissue engineering | [124] | ||
| HA aldehyde–aminooxy PEG-collagen | 50, 100, 200 μg/mL | Neural tissue engineering | [127] |
| AAD modified HA-collagen | 8 mg/mL, 25 °C | Cartilage tissue engineering | [128] |
| Collagen CS Hydrogels | |||
| Hydrogel | Application | Reference | |
| Photocrosslinked CS–collagen IPN | 5 mg/mL, 37 °C | Cartilage tissue engineering | [129] |
| Dehydrothermal crosslinked CS–collagen | 2.6 mg/mL, 25 °C | Cartilage and dermal tissue engineering | [130] |
| Genipin crosslinked CS–collagen | 1 mg/mL, 37 °C | Cartilage tissue engineering | [131] |
| EDC crosslinked CS–collagen | 2.5 mg/mL 11, 8, 6, 4, 3 mg/mL | Dermal tissue engineering | [132,133] |
| Cartilage tissue engineering | [60] | ||
| Neural tissue engineering | [134,135] | ||
| Non crosslinked CS–collagen | 4 mg/mL, 37 °C | Cartilage tissue engineering | [137] |
| Collagen Heparin Hydrogels | |||
| Hydrogel | Collagen Concentration and Temperature | Application | Reference |
| Non crosslinked Heparin-collagen | 4 mg/mL, 25 °C | Vascular tissue engineering | [139] |
| EDC crosslinked Heparin–collagen | 2.5 mg/mL, 37 °C | Bone tissue engineering | [143] |
| starPEG–heparin–collagen | unknown | Cell instruction and differentiation | [142] |
| Collagen–Alginate hydrogels | |||
| Hydrogel | Collagen Concentration and Temperature | Application | Reference |
| CaCl2 crosslinked alginate–collagen IPN | 3 mg/mL, 37 °C 2.5 mg/mL, 37 °C 5 mg/mL, 37 °C 1 mg/mL, 37 °C 2 mg/mL, 37 °C | 3D tumor model | [146] |
| Neural tissue engineering | [147] | ||
| Vocal fold tissue engineering | [148] | ||
| Cartilage tissue engineering | [149,150,151] | ||
| Corneal tissue engineering | [153] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walimbe, T.; Panitch, A. Best of Both Hydrogel Worlds: Harnessing Bioactivity and Tunability by Incorporating Glycosaminoglycans in Collagen Hydrogels. Bioengineering 2020, 7, 156. https://doi.org/10.3390/bioengineering7040156
Walimbe T, Panitch A. Best of Both Hydrogel Worlds: Harnessing Bioactivity and Tunability by Incorporating Glycosaminoglycans in Collagen Hydrogels. Bioengineering. 2020; 7(4):156. https://doi.org/10.3390/bioengineering7040156
Chicago/Turabian StyleWalimbe, Tanaya, and Alyssa Panitch. 2020. "Best of Both Hydrogel Worlds: Harnessing Bioactivity and Tunability by Incorporating Glycosaminoglycans in Collagen Hydrogels" Bioengineering 7, no. 4: 156. https://doi.org/10.3390/bioengineering7040156
APA StyleWalimbe, T., & Panitch, A. (2020). Best of Both Hydrogel Worlds: Harnessing Bioactivity and Tunability by Incorporating Glycosaminoglycans in Collagen Hydrogels. Bioengineering, 7(4), 156. https://doi.org/10.3390/bioengineering7040156






