Future Perspectives in Small-Diameter Vascular Graft Engineering
Abstract
:1. Introduction
2. Characteristics of Engineered SDVGs
3. TEVGs Derived from Synthetic Polymers
3.1. Non-Degradable Polymers
3.2. Degradable Polymers
3.3. Biopolymers
3.4. Hybrid Polymers
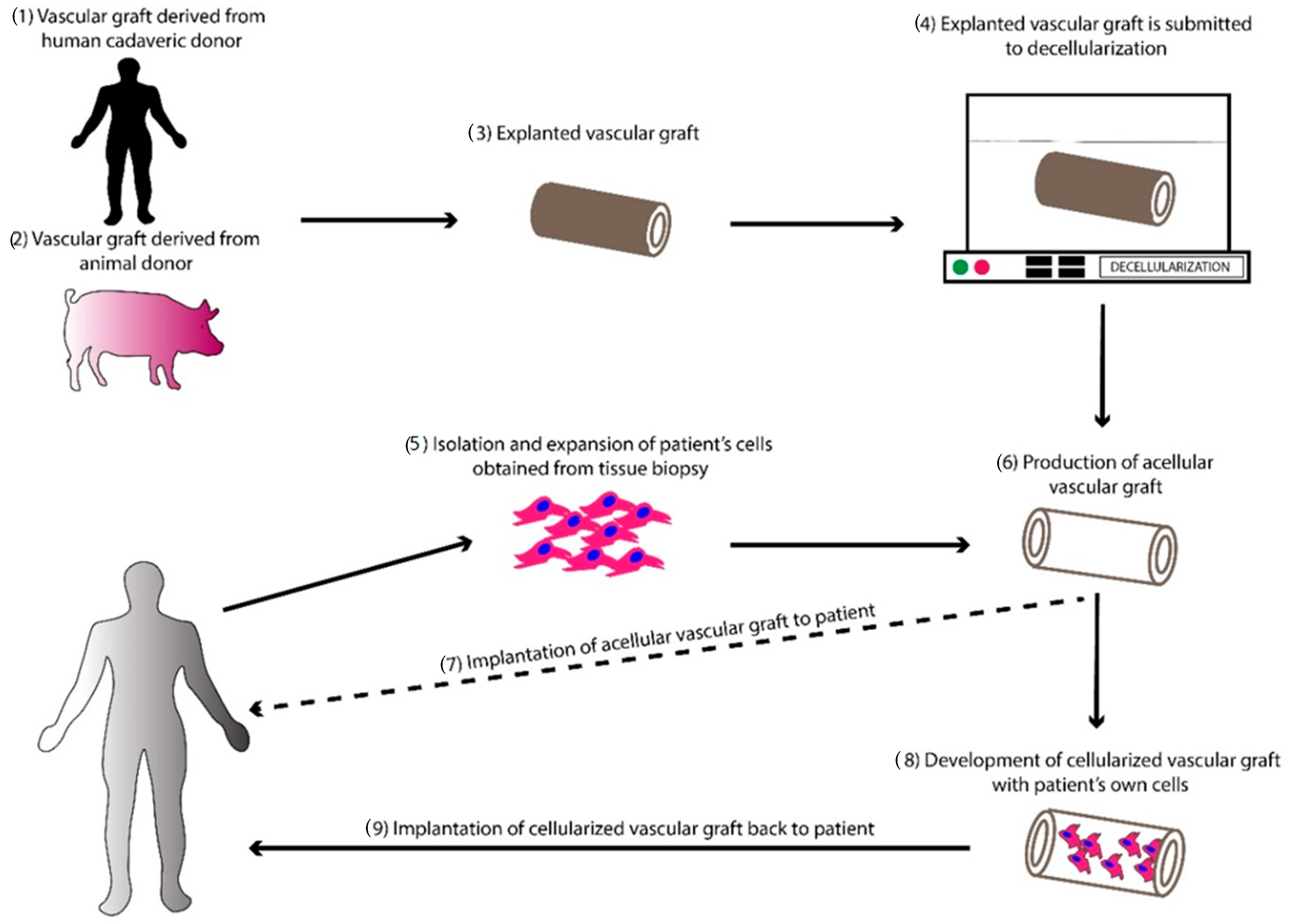
4. Decellularized Vascular Grafts
4.1. Decellularization as a Method for the Production of Vascular Grafts
4.2. Establishment of the Decellularization Approach
- <50 ng/double-stranded (ds) DNA/mg ECM dry weight
- <200 bp DNA fragmented length
- Lack of visible nuclear materials, either with 4′,6-diamidino-2-Phenylindole (DAPI) or hematoxylin and eosin (H&E)
4.3. Decellularized Animal-Derived SDVGs
4.4. Decellularized Human-Derived SDVGs
4.5. In Vivo Performance of Decellularized and Cellularized SDVGs
5. Manufacturing Methods for the Development of SDVGs
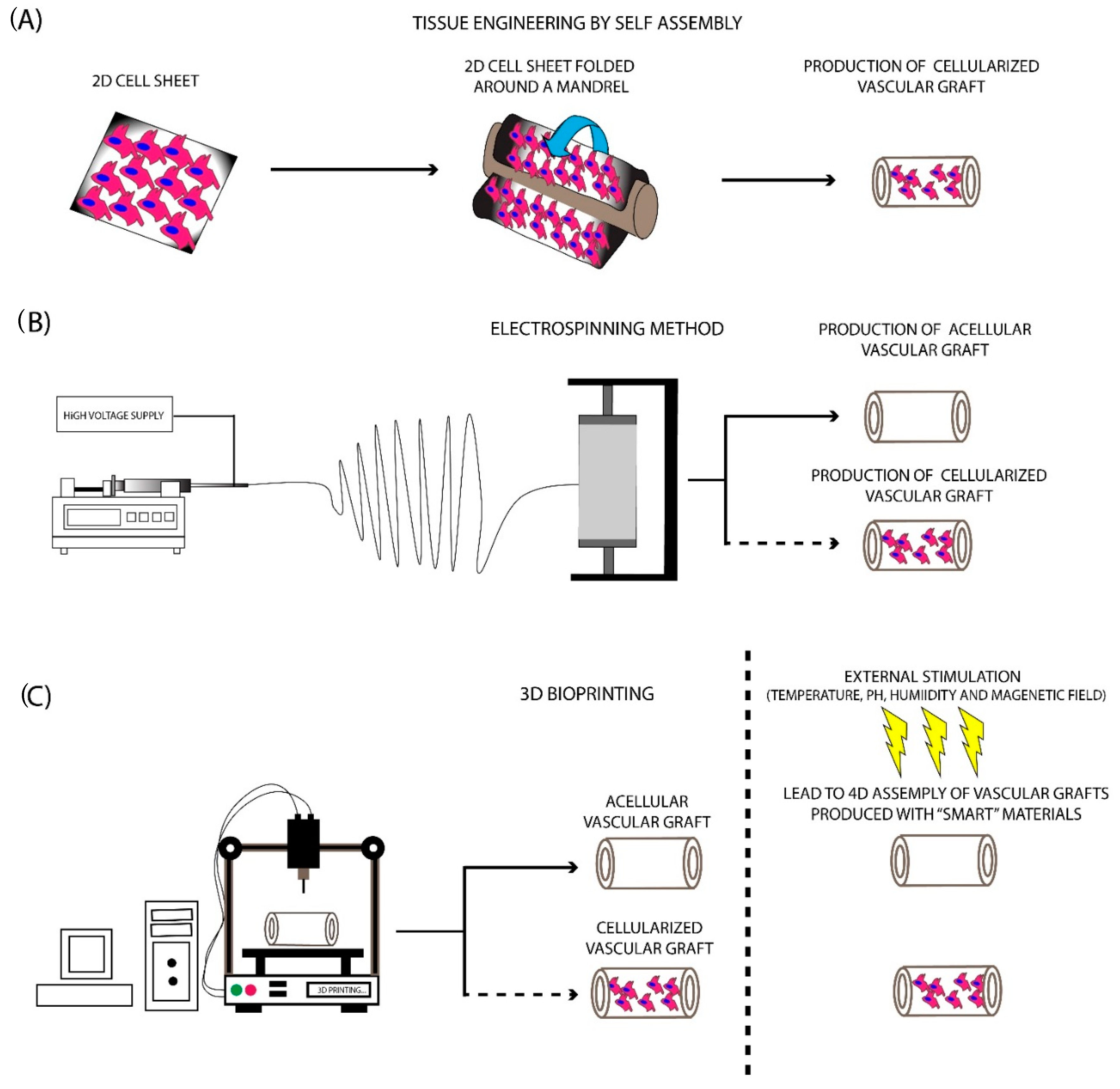
5.1. TESA Approach
5.2. Electrospinning
5.3. Three Dimensional (3D) Bioprinting
5.4. Four-Dimensional (4D) Bioprinting
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Matsuzaki, Y.; John, K.; Shoji, T.; Shinoka, T. The Evolution of Tissue Engineered Vascular Graft Technologies: From Preclinical Trials to Advancing Patient Care. Appl. Sci. 2019, 9, 1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The Tissue-Engineered Vascular Graft-Past, Present, and Future. Tissue Eng. Part B Rev. 2016, 22, 68–100. [Google Scholar] [CrossRef]
- Ong, C.S.; Zhou, X.; Huang, C.Y.; Fukunishi, T.; Zhang, H.; Hibino, N. Tissue engineered vascular grafts: Current state of the field. Expert Rev. Med. Devices 2017, 14, 383–392. [Google Scholar] [CrossRef]
- Xue, L.; Greisler, H.P. Biomaterials in the development and future of vascular grafts. J. Vasc. Surg. 2003, 37, 472–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clupac, J.; Filova, E.; Bacakova, L. Blood vessel replacement: 50 years of development and tissue engineering paradigms in vascular surgery. Physiol. Res. 2009, 58, s119–s139. [Google Scholar]
- Brewster, D.C. Current controversies in the management of aortoiliac occlusive disease. J. Vasc. Surg. 1997, 25, 365–379. [Google Scholar] [CrossRef] [Green Version]
- Hadinata, I.E.; Hayward, P.A.; Hare, D.L.; Matalanis, G.S.; Seevanayagam, S.; Rosalion, A.; Buxton, B.F. Choice of conduit for the right coronary system: 8-year analysis of Radial Artery Patency and Clinical Outcomes trial. Ann. Thorac. Surg. 2009, 88, 1404–1409. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Duncan, T.; Antman, E.; Barbosa, M.; Champagne, B.; Chen, D.; Gamra, H.; Harold, J.G.; Josephson, S.; Komajda, M.; et al. Sustainable development goals and the future of cardiovascular health: A statement from the Global Cardiovascular Disease Taskforce. Glob. Heart 2014, 9, 273–274. [Google Scholar] [CrossRef]
- Matters, C.D.; Loncar, D. Projections of Global Mortality and Burden of Disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [Green Version]
- Abdulhannan, P.; Russell, D.A.; Homer-Vanniasinkam, S. Peripheral arterial disease: A literature review. Br. Med. Bull. 2012, 104, 21–39. [Google Scholar] [CrossRef]
- European Cardiovascular Disease Statistics. Available online: http://www.ehnheart.org/cvd-statistics.html (accessed on 25 October 2020).
- Movsisyan, N.K.; Vinciguerra, M.; Medina-Inojosa, J.R.; Lopez-Jimenez, F. Cardiovascular Diseases in Central and Eastern Europe: A Call for More Surveillance and Evidence-Based Health Promotion. Ann. Glob. Health 2020, 86, 21. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.euro.who.int/en/health-topics/noncommunicable-diseases/cardiovascular-diseases (accessed on 25 October 2020).
- Mensah, G.A.; Brown, D.W. An overview of cardiovascular disease burden in the United States. Health Aff. 2007, 26, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Heart and Stroke Statistics. Available online: https://www.heart.org/en/about-us/heart-and-stroke-association-statistics (accessed on 25 October 2020).
- Thom, T.; Haase, N.; Rosamond, W.; Howard, V.J.; Rumsfeld, J.; Manolio, T.; Zheng, Z.J.; Flegal, K.; O’Donnell, C.; Kittner, S.; et al. American Heart Association Statistics, C.; Stroke Statistics, S. Heart disease and stroke statistics—2006 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2006, 113, e85–e151. [Google Scholar] [PubMed] [Green Version]
- Noly, P.E.; Ben Ali, W.; Lamarche, Y.; Carrier, M. Status, Indications, and Use of Cardiac Replacement Therapy in the Era of Multimodal Mechanical Approaches to Circulatory Support: A Scoping Review. Can. J. Cardiol. 2020, 36, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Ditano-Vazquez, P.; Torres-Pena, J.D.; Galeano-Valle, F.; Perez-Caballero, A.I.; Demelo-Rodriguez, P.; Lopez-Miranda, J.; Katsiki, N.; Delgado-Lista, J.; Alvarez-Sala-Walther, L.A. The Fluid Aspect of the Mediterranean Diet in the Prevention and Management of Cardiovascular Disease and Diabetes: The Role of Polyphenol Content in Moderate Consumption of Wine and Olive Oil. Nutrients 2019, 11, 2833. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Cardiovascular Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 25 October 2020).
- Maniadakis, N.; Kourlaba, G.; Fragoulakis, V. Self-reported prevalence of atherothrombosis in a general population sample of adults in Greece; A telephone survey. BMC Cardiovasc. Disord. 2011, 11, 16. [Google Scholar] [CrossRef] [Green Version]
- Maniadakis, N.; Kourlaba, G.; Angeli, A.; Kyriopoulos, J. The economic burden if atherothrombosis in Greece: Results from the THESIS study. Eur. J. Health Econ. 2013, 14, 655–665. [Google Scholar] [CrossRef]
- Sanchez, P.F.; Brey, E.M.; Briceno, J.C. Endothelialization mechanisms in vascular grafts. J. Tissue Eng. Regen. Med. 2018, 12, 2164–2178. [Google Scholar] [CrossRef]
- Cheng, D.; Allen, K.; Cohn, W.; Connolly, M.; Edgerton, J.; Falk, V.; Martin, J.; Ohtsuka, T.; Vitali, R. Endoscopic vascular harvest in coronary artery bypass grafting surgery: A meta-analysis of randomized trials and controlled trials. Innovations 2005, 1, 61–74. [Google Scholar] [CrossRef]
- Björk, V.O.; Ekeström, S.; Henze, A.; Ivert, T.; Landou, C. Early and Late Patency of Aortocoronary Vein Grafts. Scand. J. Thorac. Cardiovasc. Surg. 1981, 15, 11–21. [Google Scholar] [CrossRef]
- Widimsky, P.; Straka, Z.; Stros, P.; Jirasek, K.; Dvorak, J.; Votava, J.; Lisa, L.; Budesinsky, T.; Kolesar, M.; Vanek, T.; et al. One-Year Coronary Bypass Graft Patency. Circulation 2004, 110, 3418–3423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgibbon, G.M.; Kafka, H.P.; Leach, A.J.; Keon, W.J.; Hooper, G.D.; Burton, J.R. Coronary bypass graft fate and patient outcome: Angiographic follow-up of 5065 grafts related to survival and reoperation in 1,388 patients during 25 years. J. Am. Coll. Cardiol. 1996, 28, 616–626. [Google Scholar] [CrossRef] [Green Version]
- Gaudino, M.; Benedetto, U.; Fremes, S.; Biondi-Zoccai, G.; Sedrakyan, A.; Puskas, J.D.; Angelini, G.D.; Buxton, B.; Frati, G.; Hare, D.L.; et al. Radial-Artery or Saphenous-Vein Grafts in Coronary-Artery Bypass Surgery. N. Engl. J. Med. 2018, 378, 2069–2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menzoian, J.O.; Koshar, A.L.; Rodrigues, N. Alexis Carrel, Rene Leriche, Jean Kunlin, and the history of bypass surgery. J. Vasc. Surg. 2011, 54, 571–574. [Google Scholar] [CrossRef] [Green Version]
- Shah, P.J.; Bui, K.; Blackmore, S.; Gordon, I.; Hare, D.L.; Fuller, J.; Seevanayagam, S.; Buxton, B.F. Has the in situ right internal thoracic artery been overlooked? An angiographic study of the radial artery, internal thoracic arteries and saphenous vein graft patencies in symptomatic patients. Eur. J. Cardio-Thorac. Surg. 2005, 27, 870–875. [Google Scholar] [CrossRef]
- Chard, R.B.; Johnson, D.C.; Nunn, G.R.; Cartmill, T.B. Aorta-coronary bypass grafting with polytetrafluoroethylene conduits. Early and late outcome in eight patients. J. Thorac. Cardiovasc. Surg. 1987, 94, 132–134. [Google Scholar] [CrossRef]
- Popov, G.; Vavilov, V.; Popryaduhin, P. Is it Possible to Create Readily Available Tissue-Engineered Vascular Grafts Without Using Cells? Eur. J. Vasc. Endovasc. Surg. 2019, 58, e190–e191. [Google Scholar] [CrossRef]
- Patterson, J.; Gilliland, T.; Maxfield, M.; Church, S.; Naito, Y.; Shinoka, T.; Breuer, C.K. Tissue-engineered vascular grafts for use in the treatment of congenital heart disease: From the bench to the clinic and back again. Reg. Med. 2012, 7, 409–419. [Google Scholar] [CrossRef] [Green Version]
- Weinberg, C.B.; Bell, E. A blood vessel model constructed from collagen and cultured vascular cells. Science 1986, 231, 397–400. [Google Scholar] [CrossRef]
- Carrabba, M.; Madeddu, P. Current Strategies for the Manufacture of Small Size Tissue Engineering Vascular Grafts. Front. Bioeng. Biotechnol. 2018, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Mirensky, T.L.; Hibino, N.; Sawh-Martinez, R.F.; Yi, T.; Villalona, G.; Shinoka, T.; Breuer, C.K. Tissue-engineered vascular grafts: Does cell seeding matter? J. Pediatric Surg. 2010, 45, 1299–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.H.; Hsia, K.; Ma, H.; Lee, H.; Lu, J.H. In Vivo Performance of Decellularized Vascular Grafts: A Review Article. Int. J. Mol. Sci. 2018, 19, 2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michiels, C. Endothelial cell functions. J. Cell. Physiol. 2003, 196, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Feletou, M. The Endothelium: Part 1: Multiple Functions of the Endothelial Cells—Focus on Endothelium-Derived Vasoactive Mediators. Morgan Claypool Life Sci. 2011, 3, 1–306. [Google Scholar]
- Mallis, P.; Papapanagiotou, A.; Katsimpoulas, M.; Kostakis, A.; Siasos, G.; Kassi, E.; Stavropoulos-Giokas, C.; Michalopoulos, E. Efficient differentiation of vascular smooth muscle cells from Wharton’s Jelly mesenchymal stromal cells using human platelet lysate: A potential cell source for small blood vessel engineering. World J. Stem Cells 2020, 12, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Liu, W.; Cui, L.; Cao, Y. Tissue engineering of blood vessel. J. Cell. Mol. Med. 2007, 11, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.K.; Gillies, E.R.; Mequanint, K. Vascular Grafting Strategies in Coronary Intervention. Front. Mater. 2014, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Hashi, C.K.; Derugin, N.; Janairo, R.R.; Lee, R.; Schultz, D.; Lotz, J.; Li, S. Antithrombogenic modification of small-diameter microfibrous vascular grafts. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1621–1627. [Google Scholar] [CrossRef] [Green Version]
- Radke, D.; Jia, W.; Sharma, D.; Fena, K.; Wang, G.; Goldman, J.; Zhao, F. Tissue Engineering at the Blood-Contacting Surface: A Review of Challenges and Strategies in Vascular Graft Development. Adv. Healthc. Mater. 2018, 7, e1701461. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, C.; Liu, Y.; Lu, T.; Wu, Z. Strategies in cell-free tissue-engineered vascular grafts. J. Biomed. Mater. Res. Part A 2020, 108, 426–445. [Google Scholar] [CrossRef]
- Ku, D.N.; Han, H.-C. Assessment of Function in Tissue-Engineered Vascular Grafts. In Functional Tissue Engineering; Guilak, F., Butler, D.L., Goldstein, S.A., Mooney, D.J., Eds.; Springer: New York, NY, USA, 2003; pp. 258–267. [Google Scholar]
- Atlan, M.; Simon-Yarza, T.; Ino, J.M.; Hunsinger, V.; Corte, L.; Ou, P.; Aid-Launais, R.; Chaouat, M.; Letourneur, D. Design, characterization and in vivo performance of synthetic 2 mm-diameter vessel grafts made of PVA-gelatin blends. Sci. Rep. 2018, 8, 7417. [Google Scholar] [CrossRef] [Green Version]
- Ravi, S.; Qu, Z.; Chaikof, E.L. Polymeric materials for tissue engineering of arterial substitutes. Vascular 2009, 17 (Suppl. 1), S45–S54. [Google Scholar] [CrossRef] [Green Version]
- Ravi, S.; Chaikof, E.L. Biomaterials for vascular tissue engineering. Regen. Med. 2010, 5, 107–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, C.D.; Brooks, D.H.; Webster, M.W.; Bahnson, H.T. The use of expanded microporous polytetrafluoroethylene for limb salvage: A preliminary report. Surgery 1976, 79, 485–491. [Google Scholar] [PubMed]
- McAuley, C.E.; Steed, D.L.; Webster, M.W. Seven-year follow-up of expanded polytetrafluoroethylene (PTFE) femoropopliteal bypass grafts. Ann. Surg. 1984, 199, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.; McCollum, C.N.; Hawker, R.J.; Drolc, Z.; Slaney, G. Dacron arterial grafts: The influence of porosity, velour, and maturity on thrombogenicity. Surgery 1982, 92, 947–952. [Google Scholar] [PubMed]
- Lodi, M.; Cavallini, G.; Susa, A.; Lanfredi, M. Biomaterials and immune system: Cellular reactivity towards PTFE and Dacron vascular substitutes pointed out by the leukocyte adherence inhibition (LAI) test. Int. Angiol. 1988, 7, 344–348. [Google Scholar]
- Mitchell, R.N. Graft vascular disease: Immune response meets the vessel wall. Annu. Rev. Pathol. 2009, 4, 19–47. [Google Scholar] [CrossRef] [PubMed]
- Antonova, L.V.; Silnikov, V.N.; Sevostyanova, V.V.; Yuzhalin, A.E.; Koroleva, L.S.; Velikanova, E.A.; Mironov, A.V.; Godovikova, T.S.; Kutikhin, A.G.; Glushkova, T.V.; et al. Biocompatibility of Small-Diameter Vascular Grafts in Different Modes of RGD Modification. Polymers 2019, 11, 174. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Hill, A.; Imran, M. In vitro and in vivo studies of ePTFE vascular grafts treated with P15 peptide. J. Biomater. Sci. Polym. Ed. 2005, 16, 875–891. [Google Scholar] [CrossRef]
- Heidenhain, C.; Veeravoorn, A.; Vachkov, B.; Weichert, W.; Schmidmaier, G.; Wildemann, B.; Neuhaus, P.; Heise, M. Fibroblast and vascular endothelial growth factor coating of decellularized vascular grafts stimulates undesired giant cells and graft encapsulation in a rat model. Artif. Organs 2011, 35, E1–E10. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, M.; Blomberg, P.; Baliulis, G.; Carlsson, F.; Khamis, H.; Zemgulis, V. In vivo h-VEGF165 gene transfer improves early endothelialisation and patency in synthetic vascular grafts. Eur. J. Cardio-Thorac. Surg. 2007, 31, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClure, M.J.; Wolfe, P.S.; Rodriguez, I.A.; Bowlin, G.L. Bioengineered vascular grafts: Improving vascular tissue engineering through scaffold design. J. Drug Deliv. Sci. Technol. 2011, 21, 211–227. [Google Scholar] [CrossRef]
- Randone, B.; Cavallaro, G.; Polistena, A.; Cucina, A.; Coluccia, P.; Graziano, P.; Cavallaro, A. Dual role of VEGF in pretreated experimental ePTFE arterial grafts. J. Surg. Res. 2005, 127, 70–79. [Google Scholar] [CrossRef]
- Suzuki, Y.; Montagne, K.; Nishihara, A.; Watabe, T.; Miyazono, K. BMPs promote proliferation and migration of endothelial cells via stimulation of VEGF-A/VEGFR2 and angiopoietin-1/Tie2 signalling. J. Biochem. 2008, 143, 199–206. [Google Scholar] [CrossRef]
- Milliat, F.; Francois, A.; Isoir, M.; Deutsch, E.; Tamarat, R.; Tarlet, G.; Atfi, A.; Validire, P.; Bourhis, J.; Sabourin, J.C.; et al. Influence of endothelial cells on vascular smooth muscle cells phenotype after irradiation: Implication in radiation-induced vascular damages. Am. J. Pathol. 2006, 169, 1484–1495. [Google Scholar] [CrossRef] [Green Version]
- Kakisis, J.D.; Liapis, C.D.; Breuer, C.; Sumpio, B.E. Artificial blood vessel: The Holy Grail of peripheral vascular surgery. J. Vasc. Surg. 2005, 41, 349–354. [Google Scholar] [CrossRef] [Green Version]
- Hamilos, M.; Petousis, S.; Parthenakis, F. Interaction between platelets and endothelium: From pathophysiology to new therapeutic options. Cardiovasc. Diagn. Ther. 2018, 8, 568–580. [Google Scholar] [CrossRef]
- Hoshi, R.A.; Van Lith, R.; Jen, M.C.; Allen, J.B.; Lapidos, K.A.; Ameer, G. The blood and vascular cell compatibility of heparin-modified ePTFE vascular grafts. Biomaterials 2013, 34, 30–41. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, Y.; Miyata, T.; Sato, O.; Kimura, H.; Namba, T.; Makuuchi, M. Rapid postincubation endothelial retention by Dacron grafts. J. Surg. Res. 1997, 67, 132–136. [Google Scholar] [CrossRef]
- Phaneuf, M.D.; Dempsey, D.J.; Bide, M.J.; Quist, W.C.; LoGerfo, F.W. Coating of Dacron vascular grafts with an ionic polyurethane: A novel sealant with protein binding properties. Biomterilas 2001, 22, 463–469. [Google Scholar] [CrossRef]
- Hytonen, J.P.; Leppanen, O.; Taavitsainen, J.; Korpisalo, P.; Laidinen, S.; Alitalo, K.; Wadstrom, J.; Rissanen, T.T.; Yla-Herttuala, S. Improved endothelialization of small-diameter ePTFE vascular grafts through growth factor therapy. Vasc. Biol. 2019, 1, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mall, J.W.; Philipp, A.W.; Rademacher, A.; Paulitschke, M.; Buttemeyer, R. Re-endothelialization of punctured ePTFE graft: An in vitro study under pulsed perfusion conditions. Nephrol. Dial. Transplant. 2004, 19, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Sipehia, R.; Liszkowski, M.; Lu, A. In vivo evaluation of ammonia plasma modified ePTFE grafts for small diameter blood vessels replacement. A preliminary report. J. Cardiovasc. Surg. 2001, 42, 537–542. [Google Scholar]
- Zhang, Z.; Wang, Z.; Liu, S.; Kodama, M. Pore size, tissue ingrowth, and endothelialization of small-diameter microporous polyurethane vascular prostheses. Biomaterials 2004, 25, 177–187. [Google Scholar] [CrossRef]
- Chandy, T.; Das, G.S.; Wilson, R.F.; Rao, G.H. Use of plasma glow for surface-engineering biomolecules to enhance bloodcompatibility of Dacron and PTFE vascular prosthesis. Biomaterials 2000, 21, 699–712. [Google Scholar] [CrossRef]
- Hirko, M.K.; Schmidt, S.P.; Hunter, T.J.; Evancho, M.M.; Sharp, W.V.; Donovan, D.L. Endothelial cell seeding improves 4 mm PTFE vascular graft performance in antiplatelet medicated dogs. Artery 1987, 14, 137–153. [Google Scholar]
- Lewitus, D.Y.; Rios, F.; Rojas, R.; Kohn, J. Molecular design and evaluation of biodegradable polymers using a statistical approach. J. Mater. Sci. Mater. Med. 2013, 24, 2529–2535. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, R.K.; Pani, K.C.; Neuman, C.; Leonard, F. Polylactic acid for surgical implants. Arch. Surg. 1966, 93, 839–843. [Google Scholar] [CrossRef]
- Tallawi, M.; Rosellini, E.; Barbani, N.; Cascone, M.G.; Rai, R.; Saint-Pierre, G.; Boccaccini, A.R. Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: A review. J. R. Soc. Interface 2015, 12, 20150254. [Google Scholar] [CrossRef]
- He, W.; Yong, T.; Teo, W.E.; Ma, Z.; Ramakrishna, S. Fabrication and endothelialization of collagen-blended biodegradable polymer nanofibers: Potential vascular graft for blood vessel tissue engineering. Tissue Eng. 2005, 11, 1574–1588. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Yin, G.; Wang, H.; Dong, Z. Electrospun polylactide/silk fibroin-gelatin composite tubular scaffolds for small-diameter tissue engineering blood vessels. J. Appl. Polym. Sci. 2009, 113, 2675–2682. [Google Scholar] [CrossRef]
- Quint, C.; Arief, M.; Muto, A.; Dardik, A.; Niklason, L.E. Allogeneic human tissue-engineered blood vessel. J. Vasc. Surg. 2012, 55, 790–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, J.H.; Glickman, M.H.; Ilzecki, M.; Jakimowicz, T.; Jaroszynski, A.; Peden, E.K.; Pilgrim, A.J.; Prichard, H.L.; Guziewicz, M.; Przywara, S.; et al. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: Two phase 2 single-arm trials. Lancet 2016, 387, 2026–2034. [Google Scholar] [CrossRef] [Green Version]
- Antonova, L.V.; Mironov, A.V.; Yuzhalin, A.E.; Krivkina, E.O.; Shabaev, A.R.; Rezvova, M.A.; Tkachenko, V.O.; Khanova, M.Y.; Sergeeva, T.Y.; Krutitskiy, S.S.; et al. A Brief Report on an Implantation of Small-Caliber Biodegradable Vascular Grafts in a Carotid Artery of the Sheep. Pharmaceuticals 2020, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.H.P.; Tan, R.P.; Michael, P.L.; Lee, B.S.L.; Vanags, L.Z.; Ng, M.K.C.; Bursill, C.A.; Wise, S.G. Evaluation of synthetic vascular grafts in a mouse carotid grafting model. PLoS ONE 2017, 12, e0174773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercado-Pagan, A.E.; Stahl, A.M.; Ramseier, M.L.; Behn, A.W.; Yang, Y. Synthesis and characterization of polycaprolactone urethane hollow fiber membranes as small diameter vascular grafts. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 64, 61–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.Y.; Swartz, D.D.; Peng, H.F.; Gugino, S.F.; Russell, J.A.; Andreadis, S.T. Functional tissue-engineered blood vessels from bone marrow progenitor cells. Cardiovasc. Res. 2007, 75, 618–628. [Google Scholar] [CrossRef] [Green Version]
- Motlagh, D.; Allen, J.; Hoshi, R.; Yang, J.; Lui, K.; Ameer, G. Hemocompatibility evaluation of poly(diol citrate) in vitro for vascular tissue engineering. J. Biomed. Mater. Res. Part A 2007, 82, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Hashi, C.K.; Zhu, Y.; Yang, G.Y.; Young, W.L.; Hsiao, B.S.; Wang, K.; Chu, B.; Li, S. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc. Natl. Acad. Sci. USA 2007, 104, 11915–11920. [Google Scholar] [CrossRef] [Green Version]
- Niklason, L.E.; Langer, R.S. Advances in tissue engineering of blood vessels and other tissues. Transpl. Immunol. 1997, 5, 303–306. [Google Scholar] [CrossRef]
- Dahl, S.L.; Kypson, A.P.; Lawson, J.H.; Blum, J.L.; Strader, J.T.; Li, Y.; Manson, R.J.; Tente, W.E.; DiBernardo, L.; Hensley, M.T.; et al. Readily available tissue-engineered vascular grafts. Sci. Transl. Med. 2011, 3, 68ra9. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Niklason, L.E. Vascular Tissue Engineering: Building Perfusable Vasculature for Implantation. Curr. Opin. Chem. Eng. 2013, 3, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Habermehl, J.; Skopinska, J.; Boccafoschi, F.; Sionkowska, A.; Kaczmarek, H.; Laroche, G.; Mantovani, D. Preparation of ready-to-use, stockable and reconstituted collagen. Macromol. Biosci. 2005, 5, 821–828. [Google Scholar] [CrossRef]
- Cen, L.; Liu, W.; Cui, L.; Zhang, W.; Cao, Y. Collagen Tissue Engineering: Development of Novel Biomaterials and Applications. Pediatric Res. 2008, 63, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Long, T.; Yang, J.; Shi, S.S.; Guo, Y.P.; Ke, Q.F.; Zhu, Z.A. Fabrication of three-dimensional porous scaffold based on collagen fiber and bioglass for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1455–1464. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [Green Version]
- Villa, M.M.; Wang, L.; Huang, J.; Rowe, D.W.; Wei, M. Bone tissue engineering with a collagen-hydroxyapatite scaffold and culture expanded bone marrow stromal cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 243–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.H.; Wan, R.; Chiu, L.H.; Tsai, Y.H.; Fang, C.L.; Bowley, J.F.; Chen, K.C.; Shih, H.N.; Lai, W.T. Effects of collagen matrix and bioreactor cultivation on cartilage regeneration of a full-thickness critical-size knee joint cartilage defects with subchondral bone damage in a rabbit model. PLoS ONE 2018, 13, e0196779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panduranga Rao, K. Recent developments of collagen-based materials for medical applications and drug delivery systems. J. Biomater. Sci. Polym. Ed. 1996, 7, 623–645. [Google Scholar] [CrossRef] [PubMed]
- Copes, F.; Pien, N.; Van Vlierberghe, S.; Boccafoschi, F.; Mantovani, D. Collagen-Based Tissue Engineering Strategies for Vascular Medicine. Front. Bioeng. Biotechnol. 2019, 7, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konrad, P.; Dougan, P.; Bergqvist, D. Acute thrombogenicity of collagen coating of dacron grafts: An experimental study in sheep. Eur. J. Vasc. Surg. 1992, 6, 67–72. [Google Scholar] [CrossRef]
- Udelsman, B.V.; Khosravi, R.; Miller, K.S.; Dean, E.W.; Bersi, M.R.; Rocco, K.; Yi, T.; Humphrey, J.D.; Breuer, C.K. Characterization of evolving biomechanical properties of tissue engineered vascular grafts in the arterial circulation. J. Biomech. 2014, 47, 2070–2079. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Gu, Y.; Cheng, J.; Li, J.; Xu, Z.; Xing, Y.; Wang, C.; Wang, Z. Decellularization, cross-linking and heparin immobilization of porcine carotid arteries for tissue engineering vascular grafts. Cell Tissue Bank 2019, 20, 569–578. [Google Scholar] [CrossRef]
- Gu, L.; Shan, T.; Ma, Y.-X.; Tay, F.R.; Niu, L. Novel Biomedical Applications of Crosslinked Collagen. Trends Biotechnol. 2019, 37, 464–491. [Google Scholar] [CrossRef]
- Gough, J.E.; Scotchford, C.A.; Downes, S. Cytotoxicity of glutaraldehyde crosslinked collagen/poly(vinyl alcohol) films is by the mechanism of apoptosis. J. Biomed. Mater. Res. 2002, 61, 121–130. [Google Scholar] [CrossRef]
- Additive Manufacturing of Vascular Grafts and Vascularized Tissue Constructs. Tissue Eng. Part B Rev. 2017, 23, 436–450. [CrossRef]
- Brinkman, W.T.; Nagapudi, K.; Thomas, B.S.; Chaikof, E.L. Photo-Cross-Linking of Type I Collagen Gels in the Presence of Smooth Muscle Cells: Mechanical Properties, Cell Viability, and Function. Biomacromolecules 2003, 4, 890–895. [Google Scholar] [CrossRef]
- Van Wachem, P.B.; Plantinga, J.A.; Wissink, M.J.B.; Beernink, R.; Poot, A.A.; Engbers, G.H.M.; Beugeling, T.; van Aken, W.G.; Feijen, J.; van Luyn, M.J.A. In vivo biocompatibility of carbodiimide-crosslinked collagen matrices: Effects of crosslink density, heparin immobilization, and bFGF loading. J. Biomed. Mater. Res. 2001, 55, 368–378. [Google Scholar] [CrossRef]
- Alessandrino, A.; Chiarini, A.; Biagiotti, M.; Dal Prà, I.; Bassani, G.A.; Vincoli, V.; Settembrini, P.; Pierimarchi, P.; Freddi, G.; Armato, U. Three-Layered Silk Fibroin Tubular Scaffold for the Repair and Regeneration of Small Caliber Blood Vessels: From Design to in vivo Pilot Tests. Front. Bioeng. Biotechnol. 2019, 7, 356. [Google Scholar] [CrossRef] [PubMed]
- Asakura, T.; Tanaka, T.; Tanaka, R. Advanced Silk Fibroin Biomaterials and Application to Small-Diameter Silk Vascular Grafts. ACS Biomater. Sci. Eng. 2019, 5, 5561–5577. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [PubMed]
- Sericin Removal from Raw Bombyx mori Silk Scaffolds of High Hierarchical Order. Tissue Eng. Part C Methods 2014, 20, 431–439. [CrossRef] [PubMed]
- Kunz, R.I.; Brancalhão, R.M.C.; Ribeiro, L.D.F.C.; Natali, M.R.M. Silkworm Sericin: Properties and Biomedical Applications. BioMed Res. Int. 2016, 2016, 8175701. [Google Scholar] [CrossRef] [Green Version]
- Puerta, M.; Montoya, Y.; Bustamante, J.; Restrepo-Osorio, A. Potential Applications of Silk Fibroin as Vascular Implants: A Review. Crit. Rev.™ Biomed. Eng. 2019, 47, 365–378. [Google Scholar] [CrossRef]
- Enomoto, S.; Sumi, M.; Kajimoto, K.; Nakazawa, Y.; Takahashi, R.; Takabayashi, C.; Asakura, T.; Sata, M. Long-term patency of small-diameter vascular graft made from fibroin, a silk-based biodegradable material. J. Vasc. Surg. 2010, 51, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Aper, T.; Teebken, O.E.; Steinhoff, G.; Haverich, A. Use of a Fibrin Preparation in the Engineering of a Vascular Graft Model. Eur. J. Vasc. Endovasc. Surg. 2004, 28, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Weisel, J.W.; Litvinov, R.I. Fibrin Formation, Structure and Properties. Subcell. Biochem. 2017, 82, 405–456. [Google Scholar]
- Wolberg, A.S.; Campbell, R.A. Thrombin generation, fibrin clot formation and hemostasis. Transfus. Apher. Sci. 2008, 38, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Ha, Y.; Kang, N.H. Effects of Growth Factors From Platelet-Rich Fibrin on the Bone Regeneration. J. Craniofacial Surg. 2017, 28, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Mallis, P.; Gontika, I.; Dimou, Z.; Panagouli, E.; Zoidakis, J.; Makridakis, M.; Vlahou, A.; Georgiou, E.; Gkioka, V.; Stavropoulos-Giokas, C.; et al. Short Term Results of Fibrin Gel Obtained from Cord Blood Units: A Preliminary in Vitro Study. Bioengineering 2019, 6, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, C.B.; Mahindra, U.R.; Kini, Y.K.; Bakshi, M.K. Use of Platelet-Rich Fibrin over Skin Wounds: Modified Secondary Intention Healing. J. Cutan. Aesthet. Surg. 2013, 6, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Gelmetti, A.; Greppi, N.; Guez, S.; Grassi, F.; Rebulla, P.; Tadini, G. Cord blood platelet gel for the treatment of inherited epidermolysis bullosa. Transfus. Apher. Sci. 2018, 57, 370–373. [Google Scholar] [CrossRef]
- Rebulla, P.; Pupella, S.; Santodirocco, M.; Greppi, N.; Villanova, I.; Buzzi, M.; De Fazio, N.; Grazzini, G. Multicentre standardisation of a clinical grade procedure for the preparation of allogeneic platelet concentrates from umbilical cord blood. Blood Transfus. 2016, 14, 73–79. [Google Scholar] [PubMed]
- Singh, G.; Cordero, J.; Wiles, B.; Tembelis, M.N.; Liang, K.-L.; Rafailovich, M.; Simon, M.; Khan, S.U.; Bui, D.T.; Dagum, A.B. Development of In Vitro Bioengineered Vascular Grafts for Microsurgery and Vascular Surgery Applications. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2264. [Google Scholar] [CrossRef]
- Koch, S.; Flanagan, T.C.; Sachweh, J.S.; Tanios, F.; Schnoering, H.; Deichmann, T.; Ellä, V.; Kellomäki, M.; Gronloh, N.; Gries, T.; et al. Fibrin-polylactide-based tissue-engineered vascular graft in the arterial circulation. Biomaterials 2010, 31, 4731–4739. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Tschoeke, B.; Deichmann, T.; Ella, V.; Gronloh, N.; Gries, T.; Tolba, R.; Kellomäki, M.; Schmitz-Rode, T.; Jockenhoevel, S. Fibrin-based tissue engineered vascular graft in carotid artery position – the first in vivo experiences. Thorac. Cardiovasc. Surg. 2010, 58, MP25. [Google Scholar] [CrossRef]
- Swartz, D.D.; Russell, J.A.; Andreadis, S.T. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1451–H1460. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Wang, D.; Mu, S.; Lv, W.; Hao, Y.; Lu, X.; Zhang, G.; Nan, W.; Chen, H.; et al. Improved mechanical properties by modifying fibrin scaffold with PCL and its biocompatibility evaluation. J. Biomater. Sci. Polym. Ed. 2020, 31, 658–678. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, C.; Feng, Y.; Yang, Y.; Wei, Z.; Zhao, W.; Zhao, C. A chitosan modified asymmetric small-diameter vascular graft with anti-thrombotic and anti-bacterial functions for vascular tissue engineering. J. Mater. Chem. B 2020, 8, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, J.; Cui, Y.; Xu, R.; Wang, Z.; Zhang, J.; Wang, K.; Li, Y.; Zhao, Q.; Kong, D. Effect of sustained heparin release from PCL/chitosan hybrid small-diameter vascular grafts on anti-thrombogenic property and endothelialization. Acta Biomater. 2014, 10, 2739–2749. [Google Scholar] [CrossRef]
- Benhabiles, M.S.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012, 29, 48–56. [Google Scholar] [CrossRef]
- Huynh, T.N.; Tranquillo, R.T. Fusion of Concentrically Layered Tubular Tissue Constructs Increases Burst Strength. Ann. Biomed. Eng. 2010, 38, 2226–2236. [Google Scholar] [CrossRef]
- Syedain, Z.H.; Graham, M.L. A completely biological “off-the-shelf” arteriovenous graft that recellularizes in baboons. Sci. Transl. Med. 2017, 9, eaan4209. [Google Scholar] [CrossRef] [Green Version]
- Syedain, Z.; Reimer, J.; Lahti, M.; Berry, J.; Johnson, S.; Tranquillo, R.T. Tissue engineering of acellular vascular grafts capable of somatic growth in young lambs. Nat. Commun. 2016, 7, 12951. [Google Scholar] [CrossRef] [Green Version]
- Cummings, C.L.; Gawlitta, D.; Nerem, R.M.; Stegemann, J.P. Properties of engineered vascular constructs made from collagen, fibrin, and collagen–fibrin mixtures. Biomaterials 2004, 25, 3699–3706. [Google Scholar] [CrossRef]
- Arrigoni, C.; Chittò, A.; Mantero, S.; Remuzzi, A. Rotating versus perfusion bioreactor for the culture of engineered vascular constructs based on hyaluronic acid. Biotechnol. Bioeng. 2008, 100, 988–997. [Google Scholar] [CrossRef]
- Lovett, M.; Eng, G.; Kluge, J.A.; Cannizzaro, C.; Vunjak-Novakovic, G.; Kaplan, D.L. Tubular silk scaffolds for small diameter vascular grafts. Organogenesis 2010, 6, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Xu, J.; Bartolák-Suki, E.; Jiang, J.; Tien, J. Evaluation of 1-mm-diameter endothelialized dense collagen tubes in vascular microsurgery. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ao, Q.; Wang, A.; Lu, G.; Kong, L.; Gong, Y.; Zhao, N.; Zhang, X. A sandwich tubular scaffold derived from chitosan for blood vessel tissue engineering. J. Biomed. Mater. Res. Part A 2006, 77A, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Skovrind, I.; Harvald, E.B.; Juul Belling, H.; Jørgensen, C.D.; Lindholt, J.S.; Andersen, D.C. Concise Review: Patency of Small-Diameter Tissue-Engineered Vascular Grafts: A Meta-Analysis of Preclinical Trials. Stem Cells Transl. Med. 2019, 8, 671–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Chen, X.; Pan, Y.; Cui, Y.; Zhou, X.; Kong, D.; Zhao, Q. Enhanced Vascularization in Hybrid PCL/Gelatin Fibrous Scaffolds with Sustained Release of VEGF. BioMed Res. Int. 2015, 2015, 865076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Chung, J.J.; Jung, Y.; Kim, S.H. The effect of Substance P/Heparin conjugated PLCL polymer coating of bioinert ePTFE vascular grafts on the recruitment of both ECs and SMCs for accelerated regeneration. Sci. Rep. 2019, 9, 17083. [Google Scholar] [CrossRef]
- Manske, M.; Bade, E.G. Growth Factor-Induced Cell Migration: Biology and Methods of Analysis. In International Review of Cytology; Jeon, K.W., Jarvik, J., Eds.; Academic Press: Cambridge, MA, USA, 1994; Volume 155, pp. 49–96. [Google Scholar]
- Tillman, B.W.; Yazdani, S.K.; Lee, S.J.; Geary, R.L.; Atala, A.; Yoo, J.J. The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomaterials 2009, 30, 583–588. [Google Scholar] [CrossRef]
- Wise, S.G.; Byrom, M.J.; Waterhouse, A.; Bannon, P.G.; Ng, M.K.C.; Weiss, A.S. A multilayered synthetic human elastin/polycaprolactone hybrid vascular graft with tailored mechanical properties. Acta Biomater. 2011, 7, 295–303. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Xu, T.; Zhang, L. Acellular Small-Diameter Tissue-Engineered Vascular Grafts. Appl. Sci. 2019, 9, 2864. [Google Scholar] [CrossRef] [Green Version]
- Berglund, J.D.; Mohseni, M.M.; Nerem, R.M.; Sambanis, A. A biological hybrid model for collagen-based tissue engineered vascular constructs. Biomaterials 2003, 24, 1241–1254. [Google Scholar] [CrossRef]
- Gong, W.; Lei, D.; Li, S.; Huang, P.; Qi, Q.; Sun, Y.; Zhang, Y.; Wang, Z.; You, Z.; Ye, X.; et al. Hybrid small-diameter vascular grafts: Anti-expansion effect of electrospun poly ε-caprolactone on heparin-coated decellularized matrices. Biomaterials 2016, 76, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.V.; Nair, P.D. Influence of Mechanical Stimulation in the Development of a Medial Equivalent Tissue-Engineered Vascular Construct using a Gelatin-g-Vinyl Acetate Co-Polymer Scaffold. J. Biomater. Sci. Polym. Ed. 2012, 23, 2069–2087. [Google Scholar] [CrossRef] [PubMed]
- Mun, C.H.; Jung, Y.; Kim, S.-H.; Kim, H.C.; Kim, S.H. Effects of Pulsatile Bioreactor Culture on Vascular Smooth Muscle Cells Seeded on Electrospun Poly (lactide-co-ε-caprolactone) Scaffold. Artif. Organs 2013, 37, E168–E178. [Google Scholar] [CrossRef]
- Jirofti, N.; Mohebbi-Kalhori, D.; Samimi, A.; Hadjizadeh, A.; Kazemzadeh, G.H. Fabrication and characterization of a novel compliant small-diameter PET/PU/PCL triad-hybrid vascular graft. Biomed. Mater. 2020, 15, 055004. [Google Scholar] [CrossRef]
- Khodadoust, M.; Mohebbi-Kalhori, D.; Jirofti, N. Fabrication and Characterization of Electrospun Bi-Hybrid PU/PET Scaffolds for Small-Diameter Vascular Grafts Applications. Cardiovasc. Eng. Technol. 2018, 9, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Padalhin, A.R.; Seo, H.S.; Lee, B.T. A hybrid electrospun PU/PCL scaffold satisfied the requirements of blood vessel prosthesis in terms of mechanical properties, pore size, and biocompatibility. J. Biomater. Sci. Polym. Ed. 2013, 24, 1692–1706. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Cui, S.J.; Geng, X.; Ye, L.; Chen, B.; Feng, Z.G.; Zhang, J.; Li, Z.Z. Design and preparation of polyurethane-collagen/heparin-conjugated polycaprolactone double-layer bionic small-diameter vascular graft and its preliminary animal tests. Chin. Med. J. 2013, 126, 1310–1316. [Google Scholar]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. BioMed Res. Int. 2017, 2017, 9831534. [Google Scholar] [CrossRef] [Green Version]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-G.; Ugwu, F.; Li, W.-C.; Caplice, N.M.; Petcu, E.; Yip, S.P.; Huang, C.-L. Vascular Tissue Engineering: Advanced Techniques and Gene Editing in Stem Cells for Graft Generation. Tissue Eng. Part B Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Huai, G.; Qi, P.; Yang, H.; Wang, Y. Characteristics of α-Gal epitope, anti-Gal antibody, α1,3 galactosyltransferase and its clinical exploitation (Review). Int. J. Mol. Med. 2016, 37, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Galili, U. α1,3Galactosyltransferase knockout pigs produce the natural anti-Gal antibody and simulate the evolutionary appearance of this antibody in primates. Xenotransplantation 2013, 20, 267–276. [Google Scholar] [CrossRef]
- Galili, U. Significance of the evolutionary α1,3-galactosyltransferase (GGTA1) gene inactivation in preventing extinction of apes and old world monkeys. J. Mol. Evol. 2015, 80, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-C.; Kuo, Y.-J.; Sun, F.-W.; Chen, C.-H.; Chiang, C.-J.; Weng, P.-W.; Tsuang, Y.-H.; Huang, Y.-Y. Optimized decellularization protocol including α-Gal epitope reduction for fabrication of an acellular porcine annulus fibrosus scaffold. Cell Tissue Bank 2017, 18, 383–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macher, B.A.; Galili, U. The Galalpha1,3Galbeta1,4GlcNAc-R (alpha-Gal) epitope: A carbohydrate of unique evolution and clinical relevance. Biochim. Biophys. Acta 2008, 1780, 75–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Wu, Z. Genome Editing of Pigs for Agriculture and Biomedicine. Front. Genet. 2018, 9, 360. [Google Scholar] [CrossRef]
- Available online: https://www.organdonor.gov/ (accessed on 25 October 2020).
- Kim, D.H.; Sohn, S.K.; Kim, J.G.; Suh, J.S.; Lee, K.S.; Lee, K.B. Clinical impact of hyperacute graft-versus-host disease on results of allogeneic stem cell transplantation. Bone Marrow Transplant. 2004, 33, 1025–1030. [Google Scholar] [CrossRef]
- Chinen, J.; Buckley, R.H. Transplantation immunology: Solid organ and bone marrow. J. Allergy Clin. Immunol. 2010, 125 (Suppl. 2), S324–S335. [Google Scholar] [CrossRef] [Green Version]
- Keane, T.J.; Badylak, S.F. Biomaterials for tissue engineering applications. Semin. Pediatric Surg. 2014, 23, 112–118. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Freund, J.M.; Badylak, S.F. Quantification of DNA in biologic scaffold materials. J. Surg. Res. 2009, 152, 135–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Wang, Y.; Zhang, K.; Cao, N.; Yang, R.; Huang, J.; Zhao, W.; Rahman, M.; Liao, H.; Fu, Q. The Fabrication and Evaluation of a Potential Biomaterial Produced with Stem Cell Sheet Technology for Future Regenerative Medicine. Stem Cells Int. 2020, 2020, 9567362. [Google Scholar] [CrossRef] [PubMed]
- Gerli, M.F.M.; Guyette, J.P.; Evangelista-Leite, D.; Ghoshhajra, B.B.; Ott, H.C. Perfusion decellularization of a human limb: A novel platform for composite tissue engineering and reconstructive surgery. PLoS ONE 2018, 13, e0191497. [Google Scholar] [CrossRef] [Green Version]
- Balestrini, J.L.; Gard, A.L.; Liu, A.; Leiby, K.L.; Schwan, J.; Kunkemoeller, B.; Calle, E.A.; Sivarapatna, A.; Lin, T.; Dimitrievska, S.; et al. Production of decellularized porcine lung scaffolds for use in tissue engineering. Integr. Biol. 2015, 7, 1598–1610. [Google Scholar] [CrossRef] [Green Version]
- Mallis, P.; Katsimpoulas, M.; Kostakis, A.; Dipresa, D.; Korossis, S.; Papapanagiotou, A.; Kassi, E.; Stavropoulos-Giokas, C.; Michalopoulos, E. Vitrified Human Umbilical Arteries as Potential Grafts for Vascular Tissue Engineering. Tissue Eng. Regen. Med. 2020, 17, 285–299. [Google Scholar] [CrossRef]
- Bakbak, S.; Kayacan, R.; Akkuş, O. Effect of collagen fiber orientation on mechanical properties of cortical bone. J. Biomech. 2011, 44, 11. [Google Scholar] [CrossRef]
- Sokolis, D.P. Passive mechanical properties and structure of the aorta: Segmental analysis. Acta Physiol. 2007, 190, 277–289. [Google Scholar] [CrossRef]
- Sokolis, D.P. Passive mechanical properties and constitutive modeling of blood vessels in relation to microstructure. Med. Biol. Eng. Comput. 2008, 46, 1187–1199. [Google Scholar] [CrossRef]
- Rosenberg, N.; Martinez, A.; Sawyer, P.N.; Wesolowski, S.A.; Postlethwait, R.W.; Dillon, M.L., Jr. Tanned collagen arterial prosthesis of bovine carotid origin in man. Preliminary studies of enzyme-treated heterografts. Ann. Surg. 1966, 164, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Guler, S.; Aydin, H.M.; Lü, L.-X.; Yang, Y. Improvement of Decellularization Efficiency of Porcine Aorta Using Dimethyl Sulfoxide as a Penetration Enhancer. Artif. Organs 2018, 42, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Liao, J.; Joyce, E.M.; Wang, B.; Leach, J.B.; Sacks, M.S.; Wong, J.Y. Altered structural and mechanical properties in decellularized rabbit carotid arteries. Acta Biomater. 2009, 5, 993–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajbafzadeh, A.-M.; Khorramirouz, R.; Kameli, S.M.; Hashemi, J.; Bagheri, A. Decellularization of Human Internal Mammary Artery: Biomechanical Properties and Histopathological Evaluation. Biores. Open Access 2017, 6, 74–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.H.; Hsia, K.; Tsai, C.H.; Ma, H.; Lu, J.H.; Tsay, R.Y. Decellularized porcine coronary artery with adipose stem cells for vascular tissue engineering. Biomed. Mater. 2019, 14, 045014. [Google Scholar] [CrossRef]
- Singh, C.; Wong, C.S.; Wang, X. Medical Textiles as Vascular Implants and Their Success to Mimic Natural Arteries. J. Funct. Biomater. 2015, 6, 500. [Google Scholar] [CrossRef] [Green Version]
- Pennel, T.; Fercana, G.; Bezuidenhout, D.; Simionescu, A.; Chuang, T.-H.; Zilla, P.; Simionescu, D. The performance of cross-linked acellular arterial scaffolds as vascular grafts; pre-clinical testing in direct and isolation loop circulatory models. Biomaterials 2014, 35, 6311–6322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, P.; Li, X.; Fang, Q.; Wang, F.; Ao, Q.; Wang, X.; Tian, X.; Tong, H.; Bai, S.; Fan, J. Surface modification of small intestine submucosa in tissue engineering. Regen. Biomater. 2020, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Parmaksiz, M.; Elçin, A.E.; Elçin, Y.M. Decellularization of Bovine Small Intestinal Submucosa. Methods Mol. Biol. 2018, 1577, 129–138. [Google Scholar] [PubMed]
- Hussein, K.H.; Park, K.M.; Lee, Y.S.; Woo, J.S.; Kang, B.J.; Choi, K.Y.; Kang, K.S.; Woo, H.M. New insights into the pros and cons of cross-linking decellularized bioartificial organs. Int. J. Artif. Organs 2017, 40, 136–141. [Google Scholar] [CrossRef]
- Daugs, A.; Hutzler, B.; Meinke, M.; Schmitz, C.; Lehmann, N.; Markhoff, A.; Bloch, O. Detergent-Based Decellularization of Bovine Carotid Arteries for Vascular Tissue Engineering. Ann. Biomed. Eng. 2017, 45, 2683–2692. [Google Scholar] [CrossRef]
- Mancuso, L.; Gualerzi, A.; Boschetti, F.; Loy, F.; Cao, G. Decellularized ovine arteries as small-diameter vascular grafts. Biomed. Mater. 2014, 9, 045011. [Google Scholar] [CrossRef]
- Sandusky, G.E.; Lantz, G.C.; Badylak, S.F. Healing comparison of small intestine submucosa and ePTFE grafts in the canine carotid artery. J. Surg. Res. 1995, 58, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Chemla, E.S.; Morsy, M. Randomized clinical trial comparing decellularized bovine ureter with expanded polytetrafluoroethylene for vascular access. Br. J. Surg. 2009, 96, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Katzman, H.E.; Glickman, M.H.; Schild, A.F.; Fujitani, R.M.; Lawson, J.H. Multicenter evaluation of the bovine mesenteric vein bioprostheses for hemodialysis access in patients with an earlier failed prosthetic graft. J. Am. Coll. Surg. 2005, 201, 223–230. [Google Scholar] [CrossRef]
- Cho, S.W.; Lim, S.H.; Kim, I.K.; Hong, Y.S.; Kim, S.S.; Yoo, K.J.; Park, H.Y.; Jang, Y.; Chang, B.C.; Choi, C.Y.; et al. Small-diameter blood vessels engineered with bone marrow-derived cells. Ann. Surg. 2005, 241, 506–515. [Google Scholar] [CrossRef]
- Porzionato, A.; Stocco, E.; Barbon, S.; Grandi, F.; Macchi, V.; De Caro, R. Tissue-Engineered Grafts from Human Decellularized Extracellular Matrices: A Systematic Review and Future Perspectives. Int. J. Mol. Sci. 2018, 19, 4117. [Google Scholar] [CrossRef] [Green Version]
- Development and Characterization of Acellular Allogeneic Arterial Matrices. Tissue Eng. Part A 2012, 18, 471–483. [CrossRef] [PubMed]
- Teebken, O.E.; Puschmann, C.; Rohde, B.; Burgwitz, K.; Winkler, M.; Pichlmaier, A.M.; Weidemann, J.; Haverich, A. Human iliac vein replacement with a tissue-engineered graft. VASA 2009, 38, 60–65. [Google Scholar] [CrossRef]
- Olausson, M.; Patil, P.B.; Kuna, V.K.; Chougule, P.; Hernandez, N.; Methe, K.; Kullberg-Lindh, C.; Borg, H.; Ejnell, H.; Sumitran-Holgersson, S. Transplantation of an allogeneic vein bioengineered with autologous stem cells: A proof-of-concept study. Lancet 2012, 380, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Rodríguez, V.E.; Martínez-González, B.; Quiroga-Garza, A.; Reyes-Hernández, C.G.; de la Fuente-Villarreal, D.; de la Garza-Castro, O.; Guzmán-López, S.; Elizondo-Omaña, R.E. Human Umbilical Vessels: Choosing the Optimal Decellularization Method. ASAIO J. 2018, 64, 575–580. [Google Scholar] [CrossRef]
- Velarde, F.; Castañeda, V.; Morales, E.; Ortega, M.; Ocaña, E.; Álvarez-Barreto, J.; Grunauer, M.; Eguiguren, L.; Caicedo, A. Use of Human Umbilical Cord and Its Byproducts in Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 117. [Google Scholar] [CrossRef]
- Asmussen, I.; Kjeldsen, K. Intimal ultrastructure of human umbilical arteries. Observations on arteries from newborn children of smoking and nonsmoking mothers. Circ. Res. 1975, 36, 579–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, L.D.; Reynolds, L.P. Some historical aspects of understanding placental development, structure and function. Int. J. Dev. Biol. 2010, 54, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Oblath, R.W.; Buckley, F.O., Jr.; Donnelly, W.A.; Green, R.M.; Deweese, J.A. Human umbilical veins and autogenous veins as canine arterial bypass grafts. Ann. Surg. 1978, 188, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.I.; Nielsen, O.M.; Buchardt Hansen, H.J. Umbilical vein bypass in patients with severe lower limb ischemia: A report of 121 consecutive cases. Surgery 1985, 97, 294–299. [Google Scholar] [PubMed]
- Sato, O.; Okamoto, H.; Takagi, A.; Miyata, T.; Takayama, Y. Biodegradation of glutaraldehyde-tanned human umbilical vein grafts. Surg. Today 1995, 25, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Klinkert, P.; Post, P.N.; Breslau, P.J.; van Bockel, J.H. Saphenous vein versus PTFE for above-knee femoropopliteal bypass. A review of the literature. Eur. J. Vasc. Endovasc. Surg. 2004, 27, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Aalders, G.J.; van Vroonhoven, T.J.M.V. Polytetrafluoroethylene versus human umbilical vein in above-knee femoropopliteal bypass: Six-year results of a randomized clinical trial. J. Vasc. Surg. 1992, 16, 816–824. [Google Scholar] [CrossRef] [Green Version]
- Neufang, A.; Espinola-Klein, C.; Dorweiler, B.; Messow, C.M.; Schmiedt, W.; Vahl, C.F. Femoropopliteal prosthetic bypass with glutaraldehyde stabilized human umbilical vein (HUV). J. Vasc. Surg. 2007, 46, 280–288. [Google Scholar] [CrossRef] [Green Version]
- Kerdjoudj, H.; Berthelemy, N.; Rinckenbach, S.; Kearney-Schwartz, A.; Montagne, K.; Schaaf, P.; Lacolley, P.; Stoltz, J.F.; Voegel, J.C.; Menu, P. Small vessel replacement by human umbilical arteries with polyelectrolyte film-treated arteries: In vivo behavior. J. Am. Coll. Cardiol. 2008, 52, 1589–1597. [Google Scholar] [CrossRef] [Green Version]
- Kerdjoudj, H.; Boura, C.; Marchal, L.; Dumas, D.; Schaff, P.; Voegel, J.C.; Stoltz, J.F.; Menu, P. Decellularized umbilical artery treated with thin polyelectrolyte multilayer films: Potential use in vascular engineering. Bio-Med. Mater. Eng. 2006, 16 (Suppl. 4), S123–S129. [Google Scholar]
- Gui, L.; Muto, A.; Chan, S.A.; Breuer, C.K.; Niklason, L.E. Development of decellularized human umbilical arteries as small-diameter vascular grafts. Tissue Eng. Part A 2009, 15, 2665–2676. [Google Scholar] [CrossRef] [PubMed]
- Mallis, P.; Gontika, I.; Poulogiannopoulos, T.; Zoidakis, J.; Vlahou, A.; Michalopoulos, E.; Chatzistamatiou, T.; Papassavas, A.; Stavropoulos-Giokas, C. Evaluation of decellularization in umbilical cord artery. Transplant. Proc. 2014, 46, 3232–3239. [Google Scholar] [CrossRef] [PubMed]
- Tuan-Mu, H.Y.; Chang, Y.H.; Hu, J.J. Removal of an abluminal lining improves decellularization of human umbilical arteries. Sci. Rep. 2020, 10, 10556. [Google Scholar] [CrossRef] [PubMed]
- Chakhunashvili, K.; Kiladze, M.G.; Chakhunashvili, D.; Karalashvili, L.; Kakabadze, Z. A three-dimensional scaffold from decellularized human umbilical artery for bile duct reconstruction. Ann. Ital. Chir. 2019, 90, 165–173. [Google Scholar] [PubMed]
- Mallis, P.; Sokolis, D.P.; Makridakis, M.; Zoidakis, J. Insights into Biomechanical and Proteomic Characteristics of Small Diameter Vascular Grafts Utilizing the Human Umbilical Artery. Biomedicines 2020, 8, 280. [Google Scholar] [CrossRef]
- Madden, R.L.; Lipkowitz, G.S.; Browne, B.J.; Kurbanov, A. A comparison of cryopreserved vein allografts and prosthetic grafts for hemodialysis access. Ann. Vasc. Surg. 2005, 19, 686–691. [Google Scholar] [CrossRef]
- Jarrett, F.; Mahood, B.A. Long-term results of femoropopliteal bypass with stabilized human umbilical vein. Am. J. Surg. 1994, 168, 111–114. [Google Scholar] [CrossRef]
- Hoenicka, M.; Schrammel, S.; Bursa, J.; Huber, G.; Bronger, H.; Schmid, C.; Birnbaum, D.E. Development of endothelium-denuded human umbilical veins as living scaffolds for tissue-engineered small-calibre vascular grafts. J. Tissue Eng. Regen. Med. 2013, 7, 324–336. [Google Scholar] [CrossRef]
- Katsimpoulas, M.; Morticelli, L.; Gontika, I.; Kouvaka, A.; Mallis, P.; Dipresa, D.; Böer, U.; Soudah, B.; Haverich, A.; Michalopoulos, E.; et al. Biocompatibility and immunogenecity of decellularized allogeneic aorta in the orthotopic rat model. Tissue Eng. Part A. 2019, 25, 399–415. [Google Scholar] [CrossRef]
- Nuyttens, B.P.; Thijs, T.; Deckmyn, H.; Broos, K. Platelet adhesion to collagen. Thromb. Res. 2011, 127, S26–S29. [Google Scholar] [CrossRef]
- Kumar, R.A.; Dong, J.-F.; Thaggard, J.A.; Cruz, M.A.; López, J.A.; McIntire, L.V. Kinetics of GPIbalpha-vWF-A1 tether bond under flow: Effect of GPIbalpha mutations on the association and dissociation rates. Biophys. J. 2003, 85, 4099–4109. [Google Scholar] [CrossRef] [Green Version]
- Pugh, N.; Simpson, A.M.; Smethurst, P.A.; de Groot, P.G.; Raynal, N.; Farndale, R.W. Synergism between platelet collagen receptors defined using receptor-specific collagen-mimetic peptide substrata in flowing blood. Blood 2010, 115, 5069–5079. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, Z.; Liu, C.; Jiang, X.; Wei, Z.; Qiao, W.; Ran, F.; Wang, W.; Qiao, T.; Liu, C. Tissue engineering of small-diameter vascular grafts by endothelial progenitor cells seeding heparin-coated decellularized scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, S.; Amiel, G.E.; Guleserian, K.J.; Shapira, O.M.; Perry, T.; Sutherland, F.W.; Rabkin, E.; Moran, A.M.; Schoen, F.J.; Atala, A.; et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat. Med. 2001, 7, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; He, Z.; Li, L.; Liu, G.; Li, Q.; Yang, D.; Zhang, Y.; Li, N. Development and in vivo validation of tissue-engineered, small-diameter vascular grafts from decellularized aortae of fetal pigs and canine vascular endothelial cells. J. Cardiothorac. Surg. 2017, 12, 101. [Google Scholar] [CrossRef] [Green Version]
- Row, S.; Peng, H.; Schlaich, E.M.; Koenigsknecht, C.; Andreadis, S.T.; Swartz, D.D. Arterial grafts exhibiting unprecedented cellular infiltration and remodeling in vivo: The role of cells in the vascular wall. Biomaterials 2015, 50, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Peck, M.; Dusserre, N.; McAllister, T.N.; L’Heureux, N. Tissue engineering by self-assembly. Mater. Today 2011, 14, 218–224. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.-S.; Edwards, J.R.; Jeon, H. Electrospun Fibrous Scaffolds for Tissue Engineering: Viewpoints on Architecture and Fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef] [Green Version]
- Papaioannou, T.G.; Manolesou, D.; Dimakakos, E.; Tsoucalas, G.; Vavuranakis, M.; Tousoulis, D. 3D Bioprinting Methods and Techniques: Applications on Artificial Blood Vessel Fabrication. Acta Cardiol. Sin. 2019, 35, 284–289. [Google Scholar]
- Vacanti, J.P.; Langer, R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 1999, 354 (Suppl. 1), Si32–Si34. [Google Scholar] [CrossRef]
- L’Heureux, N.; Dusserre, N.; Konig, G.; Victor, B.; Keire, P.; Wight, T.N.; Chronos, N.A.; Kyles, A.E.; Gregory, C.R.; Hoyt, G.; et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat. Med. 2006, 12, 361–365. [Google Scholar] [CrossRef] [PubMed]
- L’Heureux, N.; Pâquet, S.; Labbé, R.; Germain, L.; Auger, F.A. A completely biological tissue-engineered human blood vessel. FASEB J. 1998, 12, 47–56. [Google Scholar]
- McAllister, T.N.; Maruszewski, M.; Garrido, S.A.; Wystrychowski, W.; Dusserre, N.; Marini, A.; Zagalski, K.; Fiorillo, A.; Avila, H.; Manglano, X.; et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: A multicentre cohort study. Lancet 2009, 373, 1440–1446. [Google Scholar] [CrossRef]
- Niu, H.; Zhou, H.; Wang, H. Electrospinning: An Advanced Nanofiber Production Technology. In Energy Harvesting Properties of Electrospun Nanofibers; IOP Publishing: Bristol, UK, 2019; pp. 1-1–1-44. [Google Scholar]
- Mirjalili, M.; Zohoori, S. Review for application of electrospinning and electrospun nanofibers technology in textile industry. J. NanoStruct. Chem. 2016, 6, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wang, C. Effects of Working Parameters on Electrospinning. In One-Dimensional Nanostructures: Electrospinning Technique and Unique Nanofibers; Li, Z., Wang, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 15–28. [Google Scholar]
- Hasan, A.; Memic, A.; Annabi, N.; Hossain, M.; Paul, A.; Dokmeci, M.R.; Dehghani, F.; Khademhosseini, A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014, 10, 11–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of cell binding to collagen and gelatin: A study of the effect of 2D and 3D architecture and surface chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef] [Green Version]
- Soletti, L.; Nieponice, A.; Hong, Y.; Ye, S.H.; Stankus, J.J.; Wagner, W.R.; Vorp, D.A. In vivo performance of a phospholipid-coated bioerodable elastomeric graft for small-diameter vascular applications. J. Biomed. Mater. Res. Part A 2011, 96, 436–448. [Google Scholar] [CrossRef] [Green Version]
- Ju, Y.M.; Ahn, H.; Arenas-Herrera, J.; Kim, C.; Abolbashari, M.; Atala, A.; Yoo, J.J.; Lee, S.J. Electrospun vascular scaffold for cellularized small diameter blood vessels: A preclinical large animal study. Acta Biomater. 2017, 59, 58–67. [Google Scholar] [CrossRef]
- Du, F.; Wang, H.; Zhao, W.; Li, D.; Kong, D.; Yang, J.; Zhang, Y. Gradient nanofibrous chitosan/poly ε-caprolactone scaffolds as extracellular microenvironments for vascular tissue engineering. Biomaterials 2012, 33, 762–770. [Google Scholar] [CrossRef]
- Gu, B.K.; Choi, D.J.; Park, S.J.; Kim, M.S.; Kang, C.M.; Kim, C.-H. 3-dimensional bioprinting for tissue engineering applications. Biomater. Res. 2016, 20, 12. [Google Scholar] [CrossRef] [Green Version]
- Tamay, D.G.; Dursun Usal, T.; Alagoz, A.S.; Yucel, D.; Hasirci, N.; Hasirci, V. 3D and 4D Printing of Polymers for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2019, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Abaci, A.; Guvendiren, M. Designing Decellularized Extracellular Matrix-Based Bioinks for 3D Bioprinting. Adv. Healthc. Mater. 2020, e2000734. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Park, H.-J.; Kim, S.-W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H.; et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosadegh, B.; Xiong, G.; Dunham, S.; Min, J.K. Current progress in 3D printing for cardiovascular tissue engineering. Biomed. Mater. 2015, 10, 034002. [Google Scholar] [CrossRef]
- Freeman, S.; Ramos, R.; Alexis Chando, P.; Zhou, L.; Reeser, K.; Jin, S.; Soman, P.; Ye, K. A bioink blend for rotary 3D bioprinting tissue engineered small-diameter vascular constructs. Acta Biomater. 2019, 95, 152–164. [Google Scholar] [CrossRef]
- Jia, W.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.; Yang, Q.; Zhao, X.; Jin, G.; Ma, Y.; Xu, F. 4D Bioprinting for Biomedical Applications. Trends Biotechnol. 2016, 34, 746–756. [Google Scholar] [CrossRef]
- Rastogi, P.; Kandasubramanian, B. Breakthrough in the printing tactics for stimuli-responsive materials: 4D printing. Chem. Eng. J. 2019, 366, 264–304. [Google Scholar] [CrossRef]
- Castro, N.J.; Meinert, C.; Levett, P.; Hutmacher, D.W. Current developments in multifunctional smart materials for 3D/4D bioprinting. Curr. Opin. Biomed. Eng. 2017, 2, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Suntornnond, R.; An, J.; Chua, C.K. Bioprinting of Thermoresponsive Hydrogels for Next Generation Tissue Engineering: A Review. Macromol. Mater. Eng. 2017, 302, 1600266. [Google Scholar] [CrossRef]
- De Souza Ferreira, S.B.; Moço, T.D.; Borghi-Pangoni, F.B.; Junqueira, M.V.; Bruschi, M.L. Rheological, mucoadhesive and textural properties of thermoresponsive polymer blends for biomedical applications. J. Mech. Behav. Biomed. Mater. 2016, 55, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Ortega, F. 3-pH-responsive polymers: Properties, synthesis and applications. In Smart Polymers and their Applications; Aguilar, M.R., San Román, J., Eds.; Woodhead Publishing: Southston, UK, 2014; pp. 45–92. [Google Scholar]
- Ratemi, E. 5-pH-responsive polymers for drug delivery applications. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Makhlouf, A.S.H., Abu-Thabit, N.Y., Eds.; Woodhead Publishing: Southston, UK, 2018; Volume 1, pp. 121–141. [Google Scholar]
- Adedoyin, A.A.; Ekenseair, A.K. Biomedical applications of magneto-responsive scaffolds. Nano Res. 2018, 11, 5049–5064. [Google Scholar] [CrossRef]
- Lv, C.; Sun, X.-C.; Xia, H.; Yu, Y.-H.; Wang, G.; Cao, X.-W.; Li, S.-X.; Wang, Y.-S.; Chen, Q.-D.; Yu, Y.-D.; et al. Humidity-responsive actuation of programmable hydrogel microstructures based on 3D printing. Sens. Actuators B Chem. 2018, 259, 736–744. [Google Scholar] [CrossRef]
- Bobis, S.; Jarocha, D.; Majka, M. Mesenchymal stem cells: Characteristics and clinical applications. Folia Histochem. Cytobiol. 2006, 44, 215–230. [Google Scholar] [PubMed]
- Gimble, J.M.; Bunnell, B.A.; Chiu, E.S.; Guilak, F. Concise review: Adipose-derived stromal vascular fraction cells and stem cells: Let’s not get lost in translation. Stem Cells 2011, 29, 749–754. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®;) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar]
- Xiao, X.; Li, N.; Zhang, D.; Yang, B.; Guo, H.; Li, Y. Generation of Induced Pluripotent Stem Cells with Substitutes for Yamanaka’s Four Transcription Factors. Cellular Reprogram. 2016, 18, 281–297. [Google Scholar] [CrossRef]
- Peng, G.-Y.; Lin, Y.; Li, J.-J.; Wang, Y.; Huang, H.-Y.; Shen, Z.-Y. The Application of Induced Pluripotent Stem Cells in Pathogenesis Study and Gene Therapy for Vascular Disorders: Current Progress and Future Challenges. Stem Cells Int. 2019, 2019, 9613258. [Google Scholar] [CrossRef]
- Wiegand, C.; Banerjee, I. Recent advances in the applications of iPSC technology. Curr. Opin. Biotechnol. 2019, 60, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, D.G.; Nelson, T.J.; Mueller, P.S.; Hook, C.C. The science and ethics of induced pluripotency: What will become of embryonic stem cells? Mayo Clin. Proc. 2011, 86, 634–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendicino, M.; Fan, Y.; Griffin, D.; Gunter, K.C.; Nichols, K. Current state of U.S. Food and Drug Administration regulation for cellular and gene therapy products: Potential cures on the horizon. Cytotherapy 2019, 21, 699–724. [Google Scholar] [CrossRef]
- Badylak, S.F.; Gilbert, T.W. Immune response to biologic scaffold materials. Semin. Immunol. 2008, 20, 109–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, M.B.; Ginn, B.; Fukunishi, T.; Bedja, D.; Suresh, A.; Chen, T.; Inoue, T.; Dietz, H.C.; Santhanam, L.; Mao, H.-Q.; et al. Regenerative and durable small-diameter graft as an arterial conduit. Proc. Natl. Acad. Sci. USA 2019, 116, 12710–12719. [Google Scholar] [CrossRef] [Green Version]

| Material Composition | Application | Comments | Research Team |
|---|---|---|---|
| Dacron | In vitro | Successful EC seeding in Dacron vessel conduits using either collagen-coated Dacron or fibronectin-coating ePTFE grafts | Sugawara et al. [65] |
| Dacron | In vitro | Coating of Dacron-based vascular graft with polyurethane. Increased porosity to the inner surface of the graft. Improved cell attachment properties | Phaneuf et al. [66] |
| ePTFE | Implantation in rabbits | ePTFE grafts were used as carotid artery interposition grafts, Good patency rate after 28 days of implantation, Successful endothelialization | Hytοnen et al. [67] |
| ePTFE | In vitro | Isolation of porcine ECs from jungular vein Successful endothelialization of ePTFE grafts Development of a bio-hybrid scaffold for vascular applications | Mall et al. [68] |
| ePTFE | Implantation in distal infrarenal aorta of rabbits | Development of ammonia plasma modified grafts Improved endothelialization of graft’s inner surface. | Sipehia et al. [69] |
| ePTFE | In vitro and in vivo evaluation | Development of polyurethane/polyurethane film Improved antiplatelet properties Lower hemolysis and no cytotoxicity (in vitro) Better biocompatibility, no occlusion, and successful endothelialization | Zhang et al. [70] |
| Dacron and ePTFE | In vitro | Immobilization of heparin, collagen, laminin, prostaglandin E1 (PGE1) Reduction of fibrinogen adsorption, and platelets deposition. Improved biocompatibility properties of both grafts | Chandy et al. [71] |
| Dacron and ePTFE | Implantation in mongrel dogs | Thrombus formation was reported 3 and 4 weeks postoperatively in ePTFE grafts. Patency rate of ePTFE grafts drop from 66% (3 weeks) to 33% (4 weeks) Patency rate of Dacron grafts changed from 55% (3 weeks) to 44% (4 weeks) ECs seeded grafts presented better patency rates and no graft occlusion due to thrombus formation. All animals received antiplatelet treatment | Hikro et al. [72] |
| Material Composition | Application | Comments | Research Team |
|---|---|---|---|
| PCL | In vitro | Production of electrospun PCL SDVGs Modified surface with polyethyleneimine and heparin Prolonged anticoagulant action of the modified SDVGs Mild inflammation reaction (when implanted subcutaneously) May be characterized by great long-term patency. Future plan, implantation to animal models | Wang et al. [77] |
| PCL | Implantation in sheep | Thrombosis formation in the control group Good patency rate of PCL SDVGs (50% after 1st year of implantation) | Antonova et al. [80] |
| PCL | Implantation in mice | Acellular electrospun PCL-derived vascular grafts implanted as a carotid interposition graft Successful recellularization by host’s cells Complete endothelium formation within 28 days | Chan et al. [81] |
| PCL and PU | In vitro | Production of endothelialized SDVGs Good Biomechanical properties No significant differences in hemocompatibility between non-endothelialized and endonthelialized SDVGs | Mervado—Pagan et al. [82] |
| PGS | In vitro | Minimal platelet adhesion in the produced vascular graft No cytotoxicity to erythrocytes | Liu et al. [83] Motlagh et al. [84] |
| PLA | Implantation into rats | Antithrombogenic properties of MSCs Successful in vivo remodeling process Improved patency rate and no graft occlusion in BM-MSCs seeded vascular grafts | Hashi et al. [85] |
| PGA | In vitro | PGA derived vascular graft, seeded with VSMCs Maturation in a pulsatile flow bioreactor for 8 weeks Improved biomechanical properties (burst pressure 2150 mmHg) | Niklason et al. [86] |
| PGA | Implantation in baboons, canine | Implantation in baboons as arteriovenous conduits Implantation in canines as coronary artery interposition graft. Recellularization of PGA vascular graft with ECs. No aneurysm formation was reported Good patency rate in the majority of the vascular grafts after 1, 3, and 6 months in both animal models. Recellularization with host’s VSMCs and ECs | Dahl et al. [87] |
| PGA | In vitro and in vivo | Recellularization of PGA vascular graft with ECs and maturation in a pulsatile flow bioreactor ECs and induced pluripotent stem cells (iPSCs) in vascular tissue engineering | Gui and Niklason. [88] |
| PGA | Human Use | Recellularization of PGA vascular grafts with human ECs obtained from cadaveric donors Implanted in 59 patients as arteriovenous graft Improved patency rate compared to ePTFE grafts. | Lawson et al. [79] |
| Material Composition | Application | Comments | Research Team |
|---|---|---|---|
| Fibrin | In vitro | Combination of human dermal fibroblasts with vascular graft derived from fibrin gel Successful cell migration and collagen deposition Low biomechanical properties (burst pressure 543 mmHg) | Huyhn et al. [131] |
| Fibrin | In vivo | Fabrication of fibrin-based vascular graft Maturation of the graft in a pulsatile flow-stretch bioreactor Significant biomechanical properties (burst pressure 3164 ± 342 mmHg) corresponded to 99.8% of the reported value of human internal mammary artery Implantation as arteriovenous graft in olive male baboons The majority of the grafts remained patent for 6 months. Successful repopulation by host’s cells | Syedain et al. [132] |
| Fibrin | In vivo | Production of fibrin-based vascular grafts, seeded with ovine dermal fibroblasts. Implantation of the grafts as pulmonary artery replacements in Dorset lamps Implanted grafts were characterized by physiological strength and stiffness, complete lumen endothelialization, and repopulation by SMCs The lamps exhibited somatic growth and normal physiological function for nearly one year. | Syedain et al. [133] |
| Fibrin, collagen, collagen-fibrin | In vitro | Collagen and collagen fibrin vascular grafts share common biomechanical properties Fibrin-based vascular grafts are characterized by lower biomechanical properties than the above grafts SMCs proliferated equally in all vascular scaffolds | Cummings et al. [134] |
| Hyaluronan | In vitro | Addition of sodium ascorbate to hyaluronan-based vascular grafts Improvement in SMC proliferation and cell viability. Well organized ECM and good biomechanical properties | Arrigoni et al. [135] |
| Silk | In vivo (Implantation into Sprague-Dawley rats as abdominal aorta graft) | Better patency rate after 1 year of implantation, compared to ePTFE graft ECs and SMCs proliferation into the grafts within a short time after the implantation Good ECM organization and in vivo remodeling properties (inner and media layer) Observation of vasa vasorum | Enomoto et al. [113] |
| Silk | In vivo | Silk-based vascular grafts have equal mechanical properties as the rat abdominal aorta. Low platelet adhesion High proliferation potential of silk-based vascular grafts seeded with HUVECs and SMCs Vascular remodeling after implantation experiments in rats | Lovett et al. [136] |
| Collagen | In vivo | Development of collagen-based vascular grafts with burst pressure 1313 mmHg Endothelialization of collagen tubes after implantation in femoral artery of rats | Li et al. [137] |
| Chitosan | In vitro | Development of chitosan (2% w/v) vascular graft Burst pressure over 4000 mmHg Successful seeding with VSMCs obtained from rabbit aorta | Zhang et al. [138] |
| Material Composition | Application | Comments | Research Team |
|---|---|---|---|
| PCL/collagen | In vivo | Development of hybrid scaffold with electrospinning method. Applied in aortoiliac bypass in rabbits, the graft remained for 1 month. Minimal cellular infiltration in the implanted vascular graft. Patency rate was 87.5% after 1 month of implantation | Tillman et al. [143] |
| PET/PU/PCL | In vitro and In vivo | Development of an electrospun triad-hybrid graft with an inner diameter of 5 mm. Burst pressure over 1689 mmHg Successful cell seeding and proliferation as it was indicated by the MTT assay Moderate immune reaction was observed after subcutaneous implantation in rats | Jirofti et al. [150] |
| PU/PET | In vitro | Development of PU/PET SDVGs with the electrospinning method Comparable biomechanical properties with native veins and arteries | Khodadoust et al. [151] |
| PU/PCL | In vitro | No cytotoxic PU/PCL vascular graft Successful seeded and proliferation of fibroblasts and ECs, as it was indicated by the MTT assay Confirmation of cell adhesion by SEM analysis | Nguyen et al. [152] |
| Gelatin/vinyl acetate | In vitro | Development of electrospun gelatin/vinyl acetate vascular grafts/ SMCs are used for seeding applications. Well organized ECM, accompanied by good biomechanical properties | Thomas and Nair et al. [148] |
| PCL and PU/collagen | In vivo | Electrospun PCL and PU/collagen vascular grafts were implanted as femoral artery interposition grafts in canines The grafts remained patent for 8 weeks Infiltration by ECs resulted in endothelium development | Lu et al. [153] |
| PCL/elastin | In vivo | Electrospun PCL/elastin vascular grafts were implanted as carotid arteries bypass grafts in rabbits The hybrid vascular graft was characterized by good biomechanical properties (tensile strength and Young’s Elastic Modulus) Low platelet attachment Preservation of biomechanical properties after implantation | Wise et al. [144] |
| Material Composition | Application | Comments | Research Team |
|---|---|---|---|
| Bovine carotid artery | In vitro | Decellularization of bovine carotid arteries with 1% w/v SD, 1% w/v CHAPS, 1% v/v Triton X-100 or 0.1% SDS Successful decellularization of carotid arteries Preservation of ECM structure Good biomechanical properties | Daugs et al. [186] |
| Ovine carotid artery | In vitro | Decellularization of carotid arteries with 1% w/v SDS, 0.05% v/v Trypsin, 0.02% EDTA Histological analysis with H&E, Masson’s Trichrome, and Verhoeff van Gieson revealed the preservation of ECM structure. Successful seeding and recellularization with MSCs | Mancuso et al. [187] |
| SIS | In vivo | Development of a vascular graft utilizing porcine SIS Implantation as a carotid artery interposition graft Functional comparison with autogenous saphenous vein No aneurism formation was found in both grafts. Equal patency rates between the two grafts | Sandusky et al. [188] |
| Bovine ureter | In vivo | Decellularized based on a patented process Comparison between ePTFE and decellularized bovine ureter. Applied as arteriovenous conduits Enrolled 60 patients No significant advantage of decellularized bovine ureter compared to ePTFE as AVF | Chemla and Morsy [189] |
| Bovine mesenteric vein | In vivo | Bovine mesenteric vein (MVB) evaluated as a vascular graft in hemodialysis Compared with ePTFE vascular graft Better patency rates of MVB than ePTFE graft (12 months was 35.6% for MVB versus 28.4% synthetic grafts. At 24 months, secondary patency was 60.3% MVB, 42.9% synthetic) Superior vascular graft compared to ePTFE grafts | Katzman et al. [190] |
| Canine carotid artery | In vivo | Decellularization of canine carotid arteries with 0.5% v/v Triton X-100, 0.05% v/v ammonium hydroxide Seeded with bone marrow MSCs derived from canine animal models Seeded grafts were implanted as carotid arteries interposition grafts Comparable suture retention strength between native and decellularized carotid arteries Successful in vivo remodeling after implantation, collagen and elastin production | Cho et al. [191] |
| Material Composition | Application | Comments | Research Team |
|---|---|---|---|
| Cadaveric femoral vein | In vivo (large-scale clinical trial) | Commercially available decellularized human femoral vein (Synergraft®) Applied as allograft for Hemodialysis Comparison between Synergraft®, Cryovein and ePTFE grafts Impaired patency rate of human allografts compared to ePTFE grafts Aneurism formation observed in human allografts Human allografts cost 5 times more than ePTFE grafts Ethical concerns | Madden et al. [213] |
| Iliac vein | In vivo (Proof of concept study) | Decellularization of iliac vein with 1% v/v Triton X-100, 1% v/v tri-n-butyl phosphate, and 4 mg/L deoxyribonuclease Evaluation of presence of HLA class I and I antigens Recellularization with patient’s ECs and SMCs Vessel implantation After 1st year of implantation, the graft was occluded and a new surgical operation was performed. The second recellularized vascular graft remained patent. No need for immunosuppressive agents | Olausson et al. [195] |
| HUV | In vivo (large-scale clinical trial) | Stabilized hUV applied in femoropopliteal bypass grafting in 171 patients 6% of the patients died within the 1st year The patency rate was 65% and 50% within the first and fifth year, respectively. | Jarrett and Mahood [214] |
| HUV | In vitro | HUV denudation either with 0.1% w/v collagenase, hypotonic media, or with gentle gas stream for ECs dehydration Better denudation using stream of gas, according to histological, SEM and biomechanical results | Hoenika et al. [215] |
| HUA | In vitro and in vivo | Trypsin de-endothelialization of hUVs Development multilayer of PSS/PAH films Implantation as a carotid interposition graft in rabbits. Good patency over 12 weeks. Successful cell infiltration by PECAM+ ECs and α-SMA+ SMCs | Kerdjoudj et al. [206] |
| HUA | In vitro and in vivo | Decellularized hUAs with CHAPS, SDS, EDTA, and EGM-2 buffers Preservation of ECM structure while no cells were evident. Implantation as acellular abdominal interposition grafts. Thrombus formation, but the vessel lumen did not rupture | Gui et al. [208] |
| HUA | In vitro and in vivo | Decellularization of hUAs with CHAPS, SDS, and α-ΜΕΜ with 40% FBS. Good preservation of ECM structure, no cellular or nuclear material, good biomechanical properties. Implantation as common carotid interposition graft. Thrombus formation within 30 days after the implantation. In vivo remodeling of hUAs, elastic fibers production | Mallis et al. [172] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallis, P.; Kostakis, A.; Stavropoulos-Giokas, C.; Michalopoulos, E. Future Perspectives in Small-Diameter Vascular Graft Engineering. Bioengineering 2020, 7, 160. https://doi.org/10.3390/bioengineering7040160
Mallis P, Kostakis A, Stavropoulos-Giokas C, Michalopoulos E. Future Perspectives in Small-Diameter Vascular Graft Engineering. Bioengineering. 2020; 7(4):160. https://doi.org/10.3390/bioengineering7040160
Chicago/Turabian StyleMallis, Panagiotis, Alkiviadis Kostakis, Catherine Stavropoulos-Giokas, and Efstathios Michalopoulos. 2020. "Future Perspectives in Small-Diameter Vascular Graft Engineering" Bioengineering 7, no. 4: 160. https://doi.org/10.3390/bioengineering7040160
APA StyleMallis, P., Kostakis, A., Stavropoulos-Giokas, C., & Michalopoulos, E. (2020). Future Perspectives in Small-Diameter Vascular Graft Engineering. Bioengineering, 7(4), 160. https://doi.org/10.3390/bioengineering7040160








