Breast Cancer Cell Cultures on Electrospun Poly(ε-Caprolactone) as a Potential Tool for Preclinical Studies on Anticancer Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Scaffolds
2.3. Tensile Tests
2.4. Cell Cultures
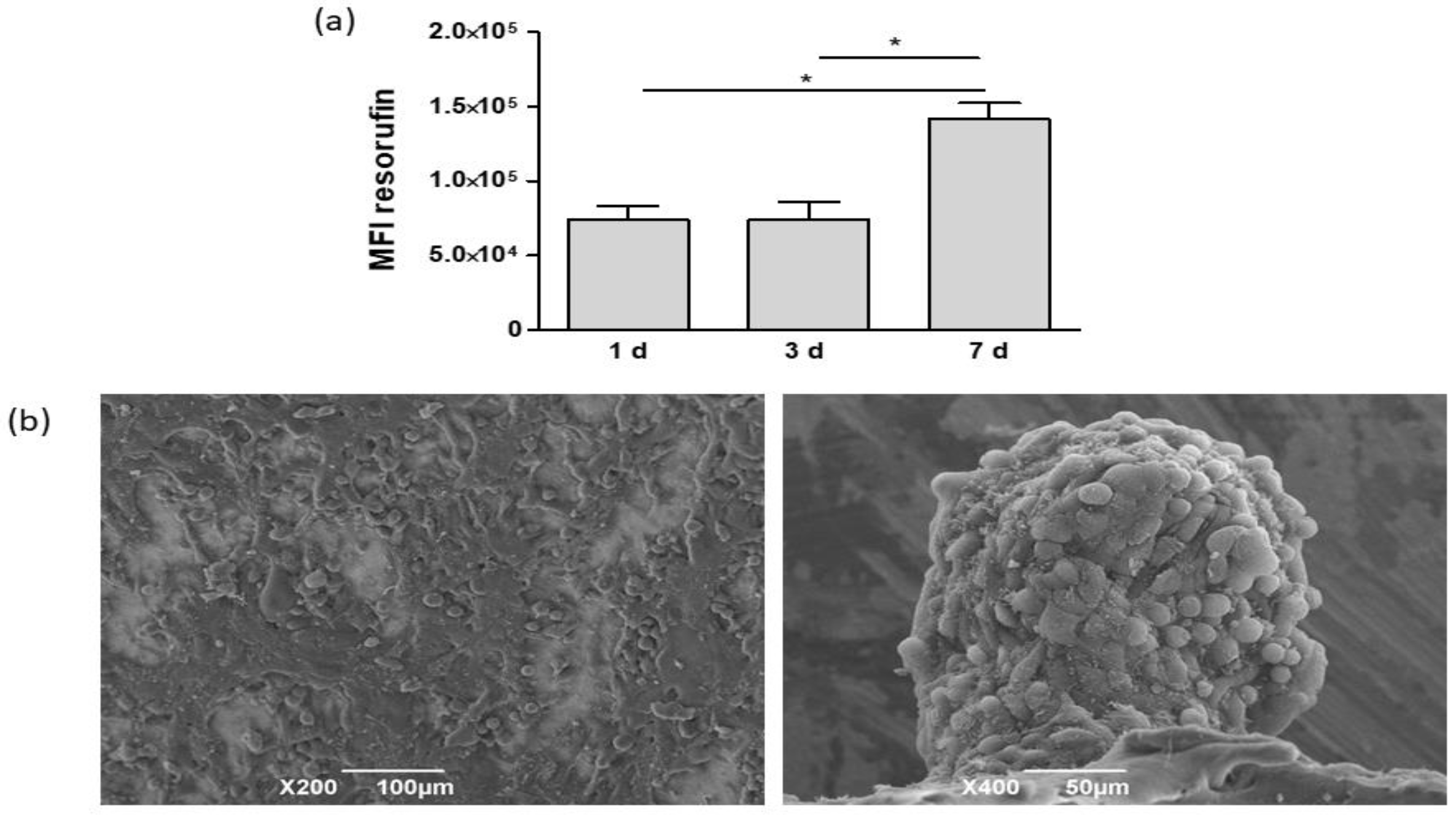
2.5. Scanning Electron Microscopy
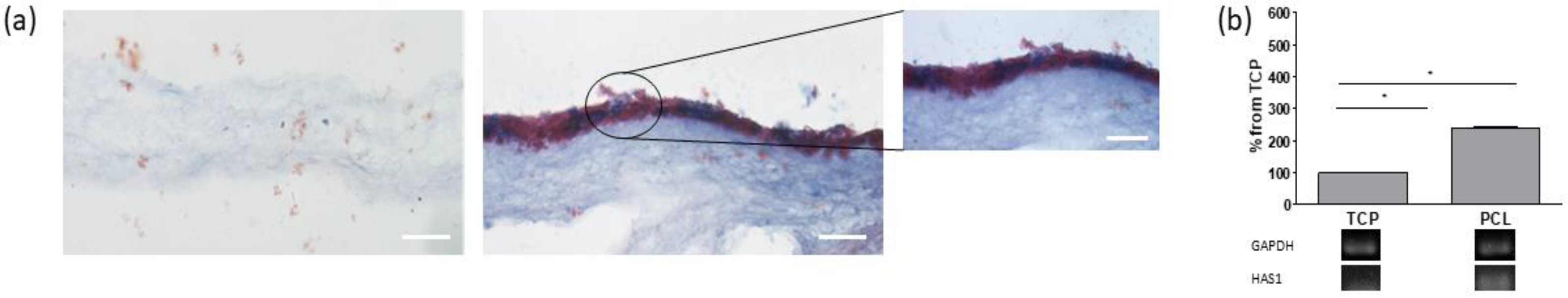
2.6. Histological Analysis
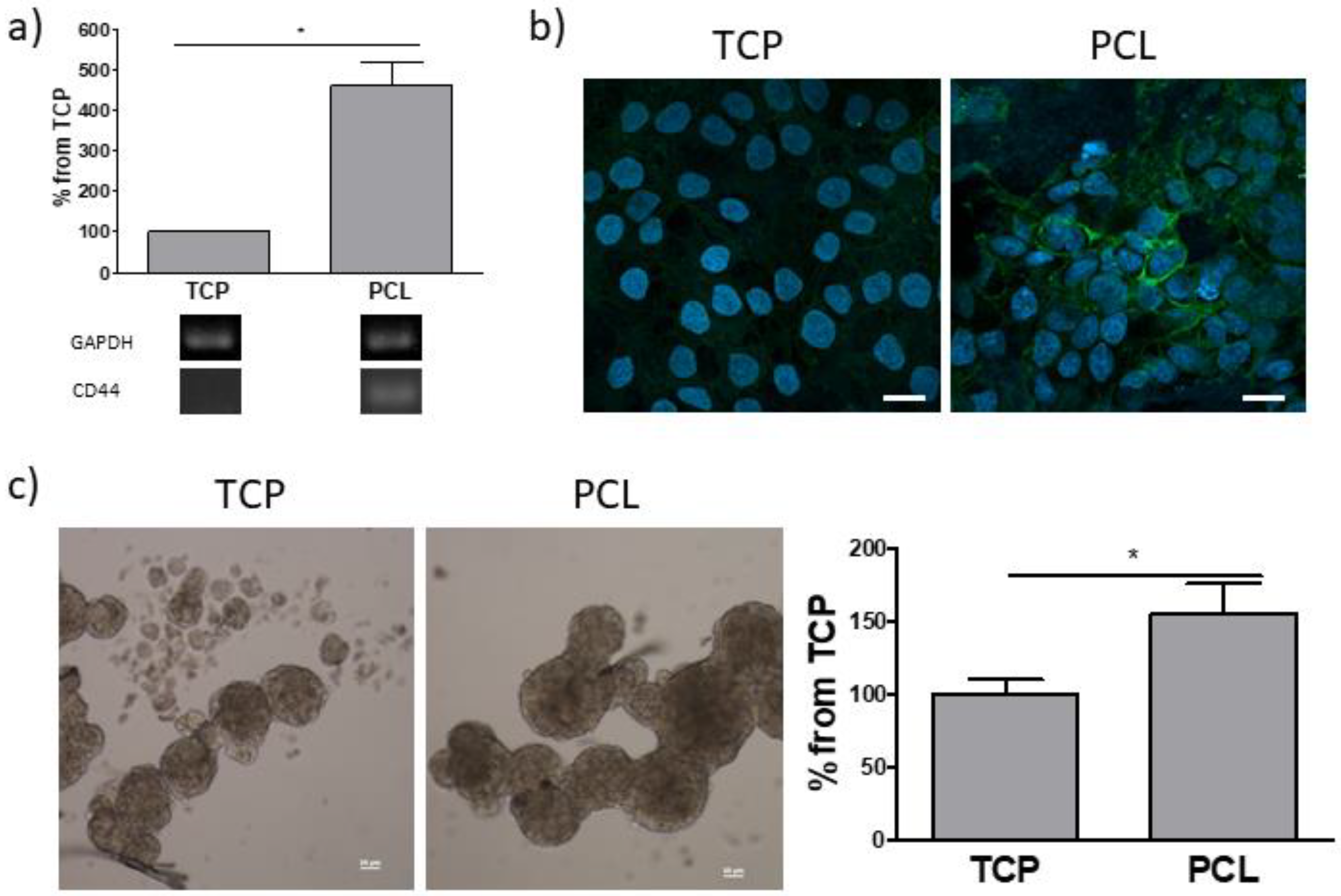
2.7. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.8. Immunofluorescence
2.9. Sphere-Forming Assay
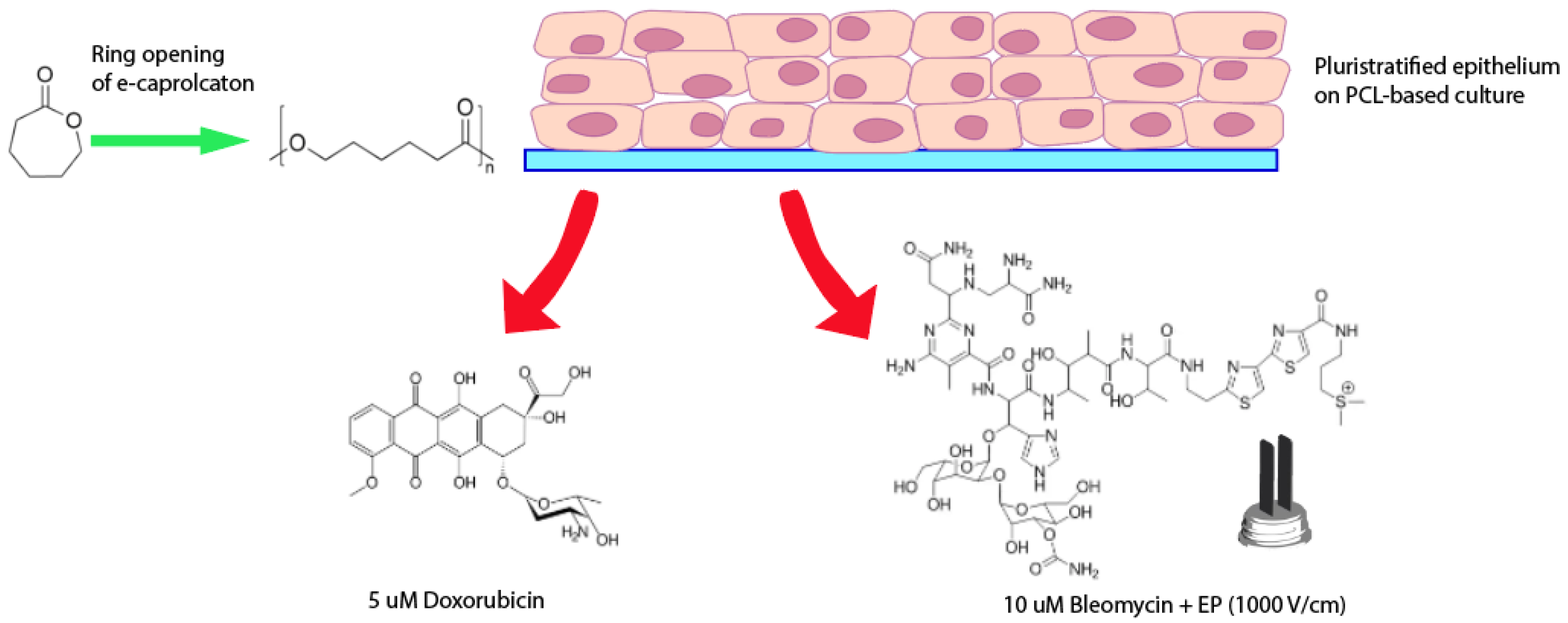
2.10. Cellular Response to Doxorubicin
2.11. Cellular Response to Bleomycin and Electroporation
2.12. Statistical Analysis
3. Results
3.1. Morphological and Mechanical Features of Electrospun PCL
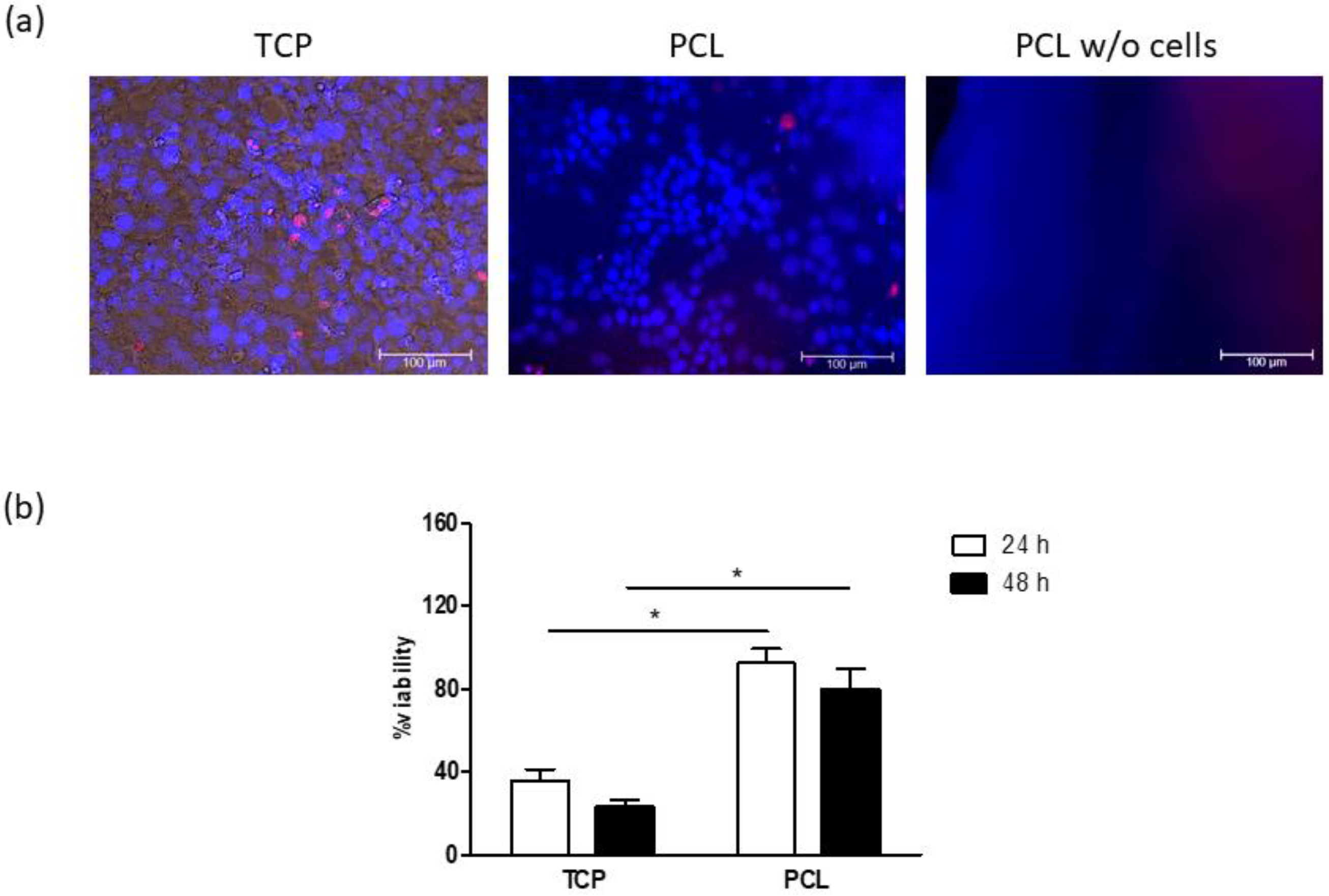
3.2. Cell Cultures on Electrospun PCL
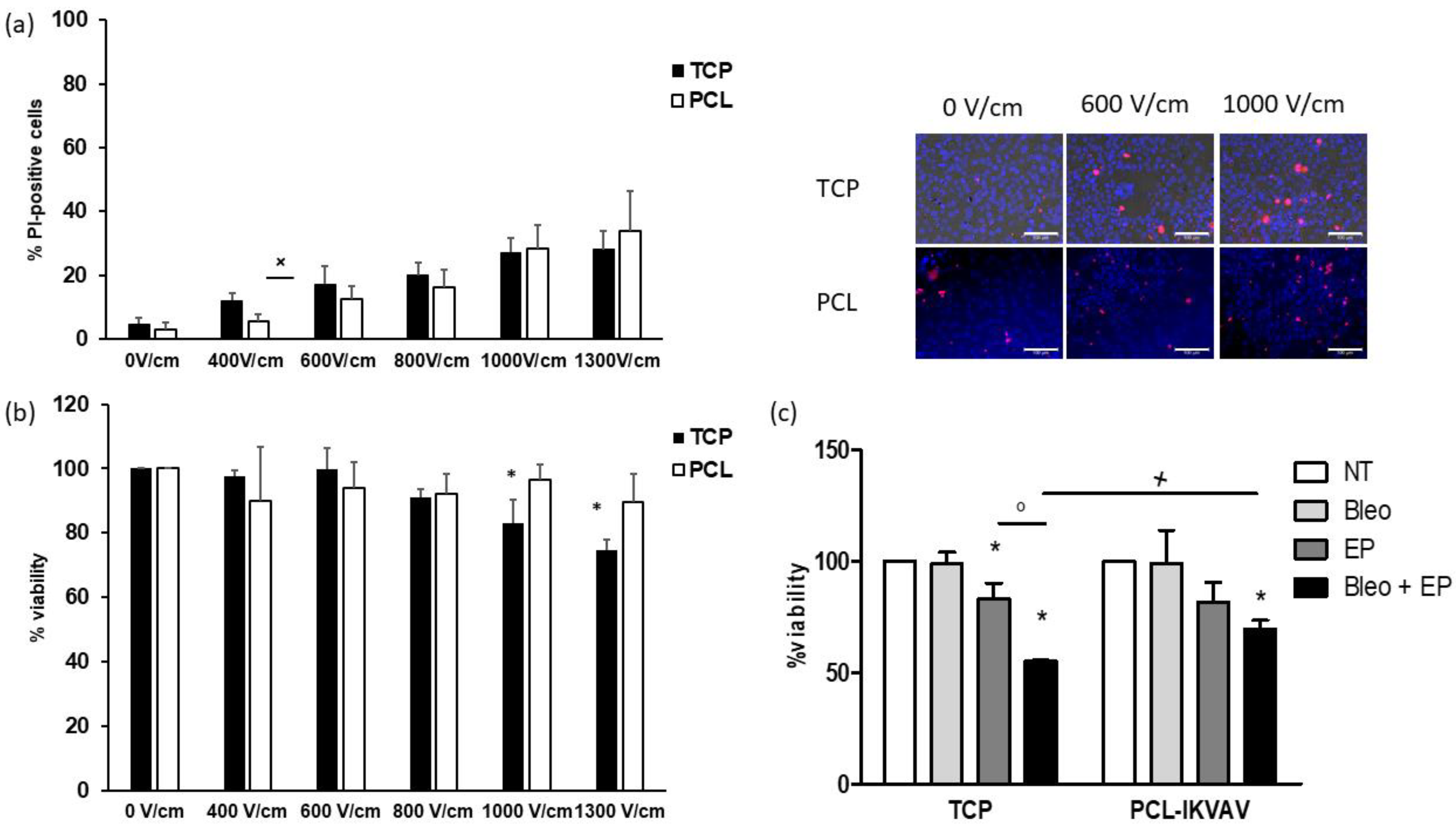
3.3. Responses of PCL-Based Cultures to Anticancer Drugs and EP
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| BSA | Bovine serum albumin |
| Col1a1 | Collagen 1a1 |
| ECM | Extracellular matrix |
| EP | Electroporation |
| FBS | Fetal bovine serum |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| HA | Hyaluronic acid |
| HAS1 | Hyaluronic acid synthase 1 |
| HFIP | Hexafluoroisopropanol |
| HOE | Hoechst 33,342 |
| MFI | Mean fluorescence intensity |
| MMP2 | Matrix Metalloproteinase 2 |
| PBS | Phosphate buffer |
| PCL | Poly (є-caprolactone) |
| PI | Propidium iodide |
| SEM | Scanning electron microscopy |
| TCP | Tissue culture polystyrene |
| β-cat | β-Catenin |
Appendix A

References
- Saji Joseph, J.; Tebogo Malindisa, S.; Ntwasa, M. Two-Dimensional (2D) and Three-Dimensional (3D) Cell Culturing in Drug Discovery. In Cell Culture; Ali Mehanna, R., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78984-866-3. [Google Scholar]
- Anisimov, V.N.; Ukraintseva, S.V.; Yashin, A.I. Cancer in rodents: Does it tell us about cancer in humans? Nat. Rev. Cancer 2005, 5, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Rijal, G.; Li, W. 3D scaffolds in breast cancer research. Biomaterials 2016, 81, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Farach-Carson, M.C.; Jia, X. Three-dimensional in vitro tumor models for cancer research and drug evaluation. Biotechnol. Adv. 2014, 32, 1256–1268. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, S.; Hassani, I.; Clary, J.M.; Lipke, E.A. Polymeric Biomaterials for In Vitro Cancer Tissue Engineering and Drug Testing Applications. Tissue Eng. Part B Rev. 2016, 22, 470–484. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharm. 2018, 9, 6. [Google Scholar] [CrossRef]
- Rabionet, M.; Yeste, M.; Puig, T.; Ciurana, J. Electrospinning PCL Scaffolds Manufacture for Three-Dimensional Breast Cancer Cell Culture. Polymers 2017, 9, 328. [Google Scholar] [CrossRef]
- Elamparithi, A.; Punnoose, A.M.; Kuruvilla, S.; Ravi, M.; Rao, S.; Paul, S.F.D. Electrospun polycaprolactone matrices with tensile properties suitable for soft tissue engineering. Artif. Cells Nanomed. Biotechnol. 2015, 44, 878–884. [Google Scholar] [CrossRef]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Espinoza, S.M.; Patil, H.I.; San Martin Martinez, E.; Casañas Pimentel, R.; Ige, P.P. Poly-ε-caprolactone (PCL), a promising polymer for pharmaceutical and biomedical applications: Focus on nanomedicine in cancer. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 85–126. [Google Scholar] [CrossRef]
- Malakpour Permlid, A.; Roci, P.; Fredlund, E.; Fält, F.; Önell, E.; Johansson, F.; Oredsson, S. Unique animal friendly 3D culturing of human cancer and normal cells. Toxicol. Vitr. 2019, 60, 51–60. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun Poly(ε-caprolactone) Microfiber and Multilayer Nanofiber/Microfiber Scaffolds: Characterization of Scaffolds and Measurement of Cellular Infiltration. Biomacromolecules 2006, 7, 2796–2805. [Google Scholar] [CrossRef] [PubMed]
- Palomeras, S.; Rabionet, M.; Ferrer, I.; Sarrats, A.; Garcia-Romeu, M.; Puig, T.; Ciurana, J. Breast Cancer Stem Cell Culture and Enrichment Using Poly(ε-Caprolactone) Scaffolds. Molecules 2016, 21, 537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sims-Mourtada, J.; Niamat, R.; Samuel, S.; Eskridge, C.; Kmiec, E. Enrichment of breast cancer stem-like cells by growth on electrospun polycaprolactone-chitosan nanofiber scaffolds. IJN 2014, 9, 995–1003. [Google Scholar] [CrossRef] [Green Version]
- Prieto, E.; Mojares, E.B.; Cortez, J.J.; Vasquez, M.R., Jr. Electrospun nanofiber scaffolds for the propagation and analysis of breast cancer stem cells in vitro. Biomed. Mater. 2020. [Google Scholar] [CrossRef]
- Feng, S.; Duan, X.; Lo, P.-K.; Liu, S.; Liu, X.; Chen, H.; Wang, Q. Expansion of breast cancer stem cells with fibrous scaffolds. Integr. Biol. 2013, 5, 768. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Ma, Y.; Zhu, J.; Chen, Y.; Sun, Y.; Yao, Y.; Yang, Z.; Xie, J. A Review on Electroporation-Based Intracellular Delivery. Molecules 2018, 23, 3044. [Google Scholar] [CrossRef] [Green Version]
- Tsong, T.Y. Electroporation of cell membranes. Biophys. J. 1991, 60, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Weaver, J.C.; Chizmadzhev, Y.A. Theory of electroporation: A review. Bioelectrochem. Bioenerg. 1996, 41, 135–160. [Google Scholar] [CrossRef]
- Rosazza, C.; Haberl Meglic, S.; Zumbusch, A.; Rols, M.-P.; Miklavcic, D. Gene Electrotransfer: A Mechanistic Perspective. Curr. Gene Ther. 2016, 16, 98–129. [Google Scholar] [CrossRef] [Green Version]
- Mir, L.M. Bases and rationale of the electrochemotherapy. Eur. J. Cancer Suppl. 2006, 4, 38–44. [Google Scholar] [CrossRef]
- Sersa, G.; Miklavcic, D.; Cemazar, M.; Rudolf, Z.; Pucihar, G.; Snoj, M. Electrochemotherapy in treatment of tumours. Eur. J. Surg. Oncol. (Ejso) 2008, 34, 232–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wichtowski, M.; Murawa, D.; Kulcenty, K.; Zaleska, K. Electrochemotherapy in Breast Cancer - Discussion of the Method and Literature Review. Breast Care 2017, 12, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Marty, M.; Sersa, G.; Garbay, J.R.; Gehl, J.; Collins, C.G.; Snoj, M.; Billard, V.; Geertsen, P.F.; Larkin, J.O.; Miklavcic, D.; et al. Electrochemotherapy—An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur. J. Cancer Suppl. 2006, 4, 3–13. [Google Scholar] [CrossRef]
- Hoejholt, K.L.; Mužić, T.; Jensen, S.D.; Dalgaard, L.T.; Bilgin, M.; Nylandsted, J.; Heimburg, T.; Frandsen, S.K.; Gehl, J. Calcium electroporation and electrochemotherapy for cancer treatment: Importance of cell membrane composition investigated by lipidomics, calorimetry and in vitro efficacy. Sci. Rep. 2019, 9, 4758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campana, L.; Mocellin, S.; Basso, M.; Puccetti, O.; De Salvo, G.; Chiarion-Sileni, V.; Vecchiato, A.; Corti, L.; Rossi, C.; Nitti, D. Bleomycin-Based Electrochemotherapy: Clinical Outcome from a Single Institution’s Experience with 52 Patients. Ann. Surg. Oncol. 2009, 16, 191–199. [Google Scholar] [CrossRef] [PubMed]
- De Virgilio, A.; Ralli, M.; Longo, L.; Mancini, P.; Attanasio, G.; Atturo, F.; De Vincentiis, M.; Greco, A. Electrochemotherapy in head and neck cancer: A review of an emerging cancer treatment (Review). Oncol. Lett. 2018. [Google Scholar] [CrossRef] [Green Version]
- Miklavčič, D.; Serša, G.; Brecelj, E.; Gehl, J.; Soden, D.; Bianchi, G.; Ruggieri, P.; Rossi, C.R.; Campana, L.G.; Jarm, T. Electrochemotherapy: Technological advancements for efficient electroporation-based treatment of internal tumors. Med. Biol. Eng. Comput. 2012, 50, 1213–1225. [Google Scholar] [CrossRef] [Green Version]
- Edhemovic, I.; Gadzijev, E.M.; Brecelj, E.; Miklavcic, D.; Kos, B.; Zupanic, A.; Mali, B.; Jarm, T.; Pavliha, D.; Marcan, M.; et al. Electrochemotherapy: A new technological approach in treatment of metastases in the liver. Technol. Cancer Res. Treat. 2011, 10, 475–485. [Google Scholar] [CrossRef] [Green Version]
- Edhemovic, I.; Brecelj, E.; Gasljevic, G.; Marolt Music, M.; Gorjup, V.; Mali, B.; Jarm, T.; Kos, B.; Pavliha, D.; Grcar Kuzmanov, B.; et al. Intraoperative electrochemotherapy of colorectal liver metastases. J. Surg. Oncol. 2014, 110, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, G.; Campanacci, L.; Ronchetti, M.; Donati, D. Electrochemotherapy in the Treatment of Bone Metastases: A Phase II Trial. World J. Surg. 2016, 40, 3088–3094. [Google Scholar] [CrossRef] [Green Version]
- Pavlin, M.; Pavselj, N.; Miklavcic, D. Dependence of induced transmembrane potential on cell density, arrangement, and cell position inside a cell system. IEEE Trans. Biomed. Eng. 2002, 49, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Bernardis, A.; Bullo, M.; Campana, L.G.; Di Barba, P.; Dughiero, F.; Forzan, M.; Mognaschi, M.E.; Sgarbossa, P.; Sieni, E. Electric field computation and measurements in the electroporation of inhomogeneous samples. Open Phys. 2017, 15. [Google Scholar] [CrossRef]
- Kranjc, M.; Bajd, F.; Sersa, I.; Miklavcic, D. Magnetic Resonance Electrical Impedance Tomography for Monitoring Electric Field Distribution during Tissue Electroporation. IEEE Trans. Med. Imaging 2011, 30, 1771–1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campana, L.G.; Barba, P.D.; Mognaschi, M.E.; Bullo, M.; Dughiero, F.; Forzan, M.; Sgarbossa, P.; Spessot, E.; Sieni, E. Electrical resistance in inhomogeneous samples during electroporation. In Proceedings of the 14th International Conference on Synthesis, Modeling, Analysis and Simulation Methods and Applications to Circuit Design (SMACD), Giardini Naxos, Italy, 12–15 June 2017. [Google Scholar]
- Dettin, M.; Zamuner, A.; Roso, M.; Gloria, A.; Iucci, G.; Messina, G.M.L.; D’Amora, U.; Marletta, G.; Modesti, M.; Castagliuolo, I.; et al. Electrospun Scaffolds for Osteoblast Cells: Peptide-Induced Concentration-Dependent Improvements of Polycaprolactone. PLoS ONE 2015, 10, e0137505. [Google Scholar] [CrossRef] [Green Version]
- ImageJ. Available online: http://imagej.nih.gov/ij/ (accessed on 3 October 2014).
- Mir, L.M.; Gehl, J.; Sersa, G.; Collins, C.G.; Garbay, J.-R.; Billard, V.; Geertsen, P.F.; Rudolf, Z.; O’Sullivan, G.C.; Marty, M. Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the CliniporatorTM by means of invasive or non-invasive electrodes. EJC Suppl. 2006, 4, 14–25. [Google Scholar] [CrossRef]
- Napotnik, T.B. Fluorescent Indicators of Membrane Permeabilization Due to Electroporation. In Handbook of Electroporation; Miklavcic, D., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–19. ISBN 978-3-319-26779-1. [Google Scholar]
- Batista Napotnik, T.; Miklavčič, D. In vitro electroporation detection methods—An overview. Bioelectrochemistry 2018, 120, 166–182. [Google Scholar] [CrossRef]
- Ongaro, A.; Campana, L.G.; De Mattei, M.; Dughiero, F.; Forzan, M.; Pellati, A.; Rossi, C.R.; Sieni, E. Evaluation of the Electroporation Efficiency of a Grid Electrode for Electrochemotherapy: From Numerical Model to In Vitro Tests. Technol. Cancer Res. Treat. 2016, 15, 296–307. [Google Scholar] [CrossRef]
- Steinkamp, J.A.; Lehnert, B.E.; Lehnert, N.M. Discrimination of damaged/dead cells by propidium iodide uptake in immunofluorescently labeled populations analyzed by phase-sensitive flow cytometry. J. Immunol. Methods 1999, 226, 59–70. [Google Scholar] [CrossRef]
- Yan, Y.; Zuo, X.; Wei, D. Concise Review: Emerging Role of CD44 in Cancer Stem Cells: A Promising Biomarker and Therapeutic Target: CD44 in Cancer Stem Cells. Stem Cells Transl. Med. 2015, 4, 1033–1043. [Google Scholar] [CrossRef]
- Garantziotis, S.; Savani, R.C. Hyaluronan biology: A complex balancing act of structure, function, location and context. Matrix Biol. 2019, 78–79, 1–10. [Google Scholar] [CrossRef]
- Wu, W.; Chen, L.; Wang, Y.; Jin, J.; Xie, X.; Zhang, J. Hyaluronic acid predicts poor prognosis in breast cancer patients: A protocol for systematic review and meta analysis. Medicine 2020, 99, e20438. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, F.M.; Vitale, D.L.; Sevic, I.; Alaniz, L. Hyaluronan in the Tumor Microenvironment. In Tumor Microenvironment; Birbrair, A., Ed.; Springer International Publishing: Cham, Switzerland, 2020; Volume 1245, pp. 67–83. ISBN 978-3-030-40145-0. [Google Scholar]
- Belgodere, J.A.; King, C.T.; Bursavich, J.B.; Burow, M.E.; Martin, E.C.; Jung, J.P. Engineering Breast Cancer Microenvironments and 3D Bioprinting. Front. Bioeng. Biotechnol. 2018, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Lao-On, U.; Rojvirat, P.; Chansongkrow, P.; Phannasil, P.; Siritutsoontorn, S.; Charoensawan, V.; Jitrapakdee, S. c-Myc directly targets an over-expression of pyruvate carboxylase in highly invasive breast cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165656. [Google Scholar] [CrossRef]
- Meng, Y.; Xu, Q.; Chen, L.; Wang, L.; Hu, X. The function of SOX2 in breast cancer and relevant signaling pathway. Pathol. Res. Pract. 2020, 216, 153023. [Google Scholar] [CrossRef] [PubMed]
- Emadian Saravi, O.; Naghshvar, F.; Torabizadeh, Z.; Sheidaei, S. Immunohistochemical Expression of Nanog and Its Relation with Clinicopathologic Characteristics in Breast Ductal Carcinoma. Iran. Biomed. J. 2019, 23, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Zhang, J.; Yang, H. OCT4, SOX2, and NAN OG positive expression correlates with poor differentiation, advanced disease stages, and worse overall survival in HER2+ breast cancer patients. OTT 2018, 11, 7873–7881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maksimenko, A.; Dosio, F.; Mougin, J.; Ferrero, A.; Wack, S.; Reddy, L.H.; Weyn, A.-A.; Lepeltier, E.; Bourgaux, C.; Stella, B.; et al. A unique squalenoylated and nonpegylated doxorubicin nanomedicine with systemic long-circulating properties and anticancer activity. Proc. Natl. Acad. Sci. USA 2014, 111, E217–E226. [Google Scholar] [CrossRef] [Green Version]
- Kotnik, T.; Kramar, P.; Pucihar, G.; Miklavcic, D.; Tarek, M. Cell membrane electroporation—Part 1: The phenomenon. IEEE Electr. Insul. Mag. 2012, 28, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Gibot, L. 3D Tissue Models to Bridge the Gap between Cell Culture and Tissue in Assessing Electroporation. In Handbook of Electroporation; Miklavcic, D., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–15. ISBN 978-3-319-26779-1. [Google Scholar]
- Campana, L.G.; Valpione, S.; Falci, C.; Mocellin, S.; Basso, M.; Corti, L.; Balestrieri, N.; Marchet, A.; Rossi, C.R. The activity and safety of electrochemotherapy in persistent chest wall recurrence from breast cancer after mastectomy: A phase-II study. Breast Cancer Res. Treat. 2012, 134, 1169–1178. [Google Scholar] [CrossRef]
- Probst, U.; Fuhrmann, I.; Beyer, L.; Wiggermann, P. Electrochemotherapy as a New Modality in Interventional Oncology: A Review. Technol Cancer Res. Treat. 2018, 17, 153303381878532. [Google Scholar] [CrossRef] [Green Version]
- Hale, J.S.; Li, M.; Lathia, J.D. The malignant social network: Cell-cell adhesion and communication in cancer stem cells. Cell Adhes. Migr. 2012, 6, 346–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Gene | Primer Sequence |
|---|---|
| GAPDH | F- TCTTCCAGGAGCGAGATC R- CAGAGATGATGACCCTTTTG |
| Hyaluronic Acid synthase 1 (HAS1) | F- TGCGATACTGGGTAGCCTTC R- GGTTGTACCAGGCCTCAAGA |
| Collagen 1a1 (Col1a1) | F- GACTGGTGAGACCTGCGTGT R- TTGTCCTTGGGGTTCTTGCT |
| Laminin B1 (LamB1) | F- GCGAGAATCCCAGTTCAAGG R- GGGGTGTTCCACAGGTCATT |
| Matrix Metalloproteinase 2 (MMP2) | F- CGACCGCGACAAGAAGTATG R- TGTTGCCCAGGAAAGTGAAG |
| c-myc | F- CTCCACACATCAGCACAACTA R- TGTCCAACTTGACCCTCTTG |
| β-Catenin (β-cat) | F- CTTCACCTGACAGATCCAAGTC R- CCTTCCATCCCTTCCTGTTTAG |
| NANOG | F- ACAGGTGAAGACCTGGTTCC R- TTGCTATTCTTCGGCCAGTT |
| SOX2 | F- ATGGGTTCGGTGGTCAAGT R- CTGATCATGTCCCGGAGGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazzolo, B.; Sieni, E.; Zamuner, A.; Roso, M.; Russo, T.; Gloria, A.; Dettin, M.; Conconi, M.T. Breast Cancer Cell Cultures on Electrospun Poly(ε-Caprolactone) as a Potential Tool for Preclinical Studies on Anticancer Treatments. Bioengineering 2021, 8, 1. https://doi.org/10.3390/bioengineering8010001
Bazzolo B, Sieni E, Zamuner A, Roso M, Russo T, Gloria A, Dettin M, Conconi MT. Breast Cancer Cell Cultures on Electrospun Poly(ε-Caprolactone) as a Potential Tool for Preclinical Studies on Anticancer Treatments. Bioengineering. 2021; 8(1):1. https://doi.org/10.3390/bioengineering8010001
Chicago/Turabian StyleBazzolo, Bianca, Elisabetta Sieni, Annj Zamuner, Martina Roso, Teresa Russo, Antonio Gloria, Monica Dettin, and Maria Teresa Conconi. 2021. "Breast Cancer Cell Cultures on Electrospun Poly(ε-Caprolactone) as a Potential Tool for Preclinical Studies on Anticancer Treatments" Bioengineering 8, no. 1: 1. https://doi.org/10.3390/bioengineering8010001
APA StyleBazzolo, B., Sieni, E., Zamuner, A., Roso, M., Russo, T., Gloria, A., Dettin, M., & Conconi, M. T. (2021). Breast Cancer Cell Cultures on Electrospun Poly(ε-Caprolactone) as a Potential Tool for Preclinical Studies on Anticancer Treatments. Bioengineering, 8(1), 1. https://doi.org/10.3390/bioengineering8010001










