Development of Cellular and Enzymatic Bioluminescent Assay Systems to Study Low-Dose Effects of Thorium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparations and Reagents
2.2. Bioluminescence Assay System Composition
2.2.1. Bacterial Assay
2.2.2. Enzymatic Assay
- 6 μL solution of the enzyme preparation;
- 25 μL 5.4 × 10−4 M FMN;
- 25 μL 0.0025% RCHO;
- 50 μL 0.05 M potassium phosphate buffer (pH 6.8);
- 100 μL 4 × 10−4 M NADH.
- 6 μL solution of the enzyme preparation in a Th-232 (3.5 × 10−2 M–3.5 × 10−8 M) solution;
- 25 μL 5.4 × 10−4 M FMN;
- 25 μL 0.0025% RCHO;
- 50 μL 0.05 M potassium phosphate buffer (pH 6.8);
- 100 μL 4 × 10−4 M NADH.
2.2.3. Effect of Th-232 on NADH Oxidation Rates
- NADH: 200 μL 4 × 10−4 M NADH; 255 μL 0.05 M potassium phosphate buffer (pH 6.8); 55 μL distilled H2O;
- NADH + enzyme preparation: 200 μL 4 × 10−4 M NADH; 5 μL enzyme preparation; 250 μL 0.05 M potassium phosphate buffer (pH 6.8); 55 μL distilled H2O;
- NADH + FMN: 200 μL 4 × 10−4 M NADH; 50 μL 5 × 10−4 M FMN; 255 μL 0.05 M potassium phosphate buffer (pH 6.8); 5 μL distilled H2O;
- NADH + FMN + enzyme preparation: 200 μL 4 × 10−4 M NADH; 5 μL enzyme preparation; 250 μL 0.05 M potassium phosphate buffer (pH 6.8); 50 μL 5 × 10−4 M FMN; 5 μL distilled H2O;
- NADH + Th-232: 200 μL 4 × 10−4 M NADH; 50 μL 10−6 M Th-232; 205 μL 0.05 M potassium phosphate buffer (pH 6.8); 55 μL distilled H2O;
- NADH + enzyme preparation + Th-232: 200 μL 4 × 10−4 M NADH; 5 μL enzyme preparation; 50 μL 10−6 M Th-232; 200 μL 0.05 M potassium phosphate buffer (pH 6.8); 55 μL distilled H2O;
- NADH + FMN + Th-232: 200 μL 4 × 10−4 M NADH; 50 μL 10−6 M Th-232; 50 μL 5 × 10−4 M FMN; 205 μL 0.05 M potassium phosphate buffer (pH 6.8); 5 μL distilled H2O;
- NADH + FMN + enzyme preparation + Th-232: 200 μL 4 × 10−4 M NADH; 5 μL enzyme preparation; 50 μL 10−6 M Th-232; 200 μL 0.05 M potassium phosphate buffer (pH 6.8); 50 μL 5 × 10−4 M FMN; 5 μL distilled H2O.
2.3. Bioluminescence Registration
2.4. Chemiluminescence Measurements
2.5. Acidity Measurements
2.6. Equipment
2.7. Statistical Processing
3. Results
3.1. Effect of Thorium on Bioluminescence of Bacteria
3.2. Effect of Th-232 on Bioluminescence of Enzymatic Assay
3.3. Effect of Th-232 on NADH Oxidation Rates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bulich, A.A.; Isenberg, D.L. Use of the Luminescent Bacterial System for the Rapid Assessment of Aquatic Toxicity. ISA Trans. 1981, 20, 29–33. [Google Scholar] [PubMed]
- Girotti, S.; Ferri, E.N.; Fumo, M.G.; Maiolini, E. Monitoring of Environmental Pollutants by Bioluminescent Bacteria. Anal. Chim. Acta 2008, 608, 2–29. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Guardigli, M.; Michelini, E.; Mirasoli, M. Bioluminescence in Analytical Chemistry and in Vivo Imaging. TrAC Trends Anal. Chem. 2009, 28, 307–322. [Google Scholar] [CrossRef]
- Ismailov, A.D.; Aleskerova, L.E. Photobiosensors Containing Luminescent Bacteria. Biochemistry 2015, 80, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Adil, M.; Ehtisham-ul-Haque, S.; Munir, B.; Yameen, M.; Ghaffar, A.; Shar, G.A.; Asif Tahir, M.; Iqbal, M. Vibrio Fischeri Bioluminescence Inhibition Assay for Ecotoxicity Assessment: A Review. Sci. Total Environ. 2018, 626, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Rozhko, T.V.; Nogovitsyna, E.I.; Badun, G.A.; Lukyanchuk, A.N.; Kudryasheva, N.S. Reactive Oxygen Species and Low-Dose Effects of Tritium on Bacterial Cells. J. Environ. Radioact. 2019, 208–209, 106035. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, M.; Sakuma, S. Development of Bioassay System for Evaluation of Materials for Personal Protective Equipment (PPE) against Toxic Effects of Ionizing Radiations. Ind. Health 2017, 55, 580–583. [Google Scholar] [CrossRef] [Green Version]
- Kratasyuk, V.A. Principle of luciferase biotesting. In Biological Luminescence; Jeżowska-Trzebiatowska, B., Kochel, B., Sławiński, J., Stręk, W., Eds.; WSPC: Singapore, 1990; p. 660. ISBN 978-9810204051. [Google Scholar]
- Kudryasheva, N.; Vetrova, E.; Kuznetsov, A.; Kratasyuk, V.; Stom, D. Bioluminescence Assays: Effects of Quinones and Phenols. Ecotoxicol. Environ. Saf. 2002, 53, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Esimbekova, E.N.; Kratasyuk, V.A.; Torgashina, I.G. Disk-Shaped Immobilized Multicomponent Reagent for Bioluminescent Analyses: Correlation between Activity and Composition. Enzyme Microb. Technol. 2007, 40, 343–346. [Google Scholar] [CrossRef]
- Nemtseva, E.V.; Kudryasheva, N.S. The Mechanism of Electronic Excitation in the Bacterial Bioluminescent Reaction. Russ. Chem. Rev. 2007, 76, 91–100. [Google Scholar] [CrossRef]
- Kudryasheva, N.S.; Kovel, E.S. Monitoring of Low-Intensity Exposures via Luminescent Bioassays of Different Complexity: Cells, Enzyme Reactions, and Fluorescent Proteins. Int. J. Mol. Sci. 2019, 20, 4451. [Google Scholar] [CrossRef] [Green Version]
- Kudryasheva, N.S. Bioluminescence and Exogenous Compounds: Physico-Chemical Basis for Bioluminescent Assay. J. Photochem. Photobiol. B Biol. 2006, 83, 77–86. [Google Scholar] [CrossRef]
- Rozhko, T.V.; Kudryasheva, N.S.; Kuznetsov, A.M.; Vydryakova, G.A.; Bondareva, L.G.; Bolsunovsky, A.Y. Effect of Low-Level α-Radiation on Bioluminescent Assay Systems of Various Complexity. Photochem. Photobiol. Sci. 2007, 6, 67–70. [Google Scholar] [CrossRef]
- Rozhko, T.V.; Badun, G.A.; Razzhivina, I.A.; Guseynov, O.A.; Guseynova, V.E.; Kudryasheva, N.S. On the Mechanism of Biological Activation by Tritium. J. Environ. Radioact. 2016, 157, 131–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozhko, T.V.; Nemtseva, E.V.; Gardt, M.V.; Raikov, A.V.; Lisitsa, A.E.; Badun, G.A.; Kudryasheva, N.S. Enzymatic Responses to Low-Intensity Radiation of Tritium. Int. J. Mol. Sci. 2020, 21, 8464. [Google Scholar] [CrossRef] [PubMed]
- Rozhko, T.V.; Kolesnik, O.V.; Badun, G.A.; Stom, D.I.; Kudryasheva, N.S. Humic Substances Mitigate the Impact of Tritium on Luminous Marine Bacteria. Involvement of Reactive Oxygen Species. Int. J. Mol. Sci. 2020, 21, 6783. [Google Scholar] [CrossRef]
- Alexandrova, M.; Rozhko, T.; Vydryakova, G.; Kudryasheva, N. Effect of Americium-241 on Luminous Bacteria. Role of Peroxides. J. Environ. Radioact. 2011, 102, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Selivanova, M.A.; Mogilnaya, O.A.; Badun, G.A.; Vydryakova, G.A.; Kuznetsov, A.M.; Kudryasheva, N.S. Effect of Tritium on Luminous Marine Bacteria and Enzyme Reactions. J. Environ. Radioact. 2013, 120, 19–25. [Google Scholar] [CrossRef]
- Selivanova, M.; Rozhko, T.; Devyatlovskaya, A.; Kudryasheva, N. Comparison of Chronic Low-Dose Effects of Alpha- and Beta-Emitting Radionuclides on Marine Bacteria. Cent. Eur. J. Biol. 2014, 9, 951–959. [Google Scholar] [CrossRef]
- Kudryasheva, N.S.; Rozhko, T.V. Effect of Low-Dose Ionizing Radiation on Luminous Marine Bacteria: Radiation Hormesis and Toxicity. J. Environ. Radioact. 2015, 142, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Kudryasheva, N.S.; Petrova, A.S.; Dementyev, D.V.; Bondar, A.A. Exposure of Luminous Marine Bacteria to Low-Dose Gamma-Radiation. J. Environ. Radioact. 2017, 169, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Min, J.; Lee, C.W.; Gu, M.B. Gamma-Radiation Dose-Rate Effects on DNA Damage and Toxicity in Bacterial Cells. Radiat. Environ. Biophys. 2003, 42, 189–192. [Google Scholar] [CrossRef]
- The International Commission on Radiological Protection. ICRP Publication 99. Low-Dose Extrapolation of Radiation-Related Cancer Risk. Annals of the ICPR; Valentin, J., Ed.; Elsevier: New York, NY, USA, 2005. [Google Scholar]
- Kudryashov, Y.B. Radiation Biophysics (Ionizing Radiation); Fizmatlit: Moscow, Russia, 2004; ISBN 5-9221-0388-1. (In Russian) [Google Scholar]
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of Ionizing Radiation on Biological Molecules—Mechanisms of Damage and Emerging Methods of Detection. Antioxid. Redox Signal. 2014, 21, 260–292. [Google Scholar] [CrossRef] [PubMed]
- Rozhko, T.; Bondareva, L.; Mogilnaya, O.; Vydryakova, G.; Bolsunovsky, A.; Stom, D.; Kudryasheva, N. Detoxification of AM-241 Solutions by Humic Substances: Bioluminescent Monitoring. Anal. Bioanal. Chem. 2011, 400, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Rozhko, T.V.; Guseynov, O.A.; Guseynova, V.E.; Bondar, A.A.; Devyatlovskaya, A.N.; Kudryasheva, N.S. Is Bacterial Luminescence Response to Low-Dose Radiation Associated with Mutagenicity? J. Environ. Radioact. 2017, 177, 261–265. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, E. Hormesis: Path and Progression to Significance. Int. J. Mol. Sci. 2018, 19, 2871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jargin, S.V. Hormesis and Radiation Safety Norms: Comments for an Update. Hum. Exp. Toxicol. 2018, 37, 1233–1243. [Google Scholar] [CrossRef]
- Shibamoto, Y.; Nakamura, H. Overview of Biological, Epidemiological, and Clinical Evidence of Radiation Hormesis. Int. J. Mol. Sci. 2018, 19, 2387. [Google Scholar] [CrossRef] [Green Version]
- Ge, H.; Zhou, M.; Lv, D.; Wang, M.; Xie, D.; Yang, X.; Dong, C.; Li, S.; Lin, P. Novel Segmented Concentration Addition Method to Predict Mixture Hormesis of Chlortetracycline Hydrochloride and Oxytetracycline Hydrochloride to Aliivibrio Fischeri. Int. J. Mol. Sci. 2020, 21, 481. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, H.; HamadaA, N.; Takahashi, A.; Kobayashi, Y.; Ohinishi, T. Vanguards of Paradigm Shift in Radiation Biology: Radiation-Induced Adaptive and Bystander Responses. J. Radiat. Res. 2007, 48, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Azzam, E.I.; Jay-Gerin, J.-P.; Pain, D. Ionizing Radiation-Induced Metabolic Oxidative Stress and Prolonged Cell Injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.W.; Wang, J.; Schültke, E.; Seymour, C.B.; Bräuer-Krisch, E.; Laissue, J.A.; Blattmann, H.; Mothersill, C.E. Proteomic Changes in the Rat Brain Induced by Homogenous Irradiation and by the Bystander Effect Resulting from High Energy Synchrotron X-Ray Microbeams. Int. J. Radiat. Biol. 2013, 89, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Herb, M.; Schramm, M. Functions of Ros in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Paiva, C.N.; Bozza, M.T. Are Reactive Oxygen Species Always Detrimental to Pathogens? Antioxid. Redox Signal. 2014, 20, 1000–1034. [Google Scholar] [CrossRef] [Green Version]
- Brynildsen, M.P.; Winkler, J.A.; Spina, C.S.; MacDonald, I.C.; Collins, J.J. Potentiating Antibacterial Activity by Predictably Enhancing Endogenous Microbial ROS Production. Nat. Biotechnol. 2013, 31, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Ezraty, B.; Gennaris, A.; Barras, F.; Collet, J.-F. Oxidative Stress, Protein Damage and Repair in Bacteria. Nat. Rev. Microbiol. 2017, 15, 385–396. [Google Scholar] [CrossRef]
- Dayem, A.A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.M.; Choi, H.Y.; Cho, S.G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, B.C.; Chang, C.J. Chemistry and Biology of Reactive Oxygen Species in Signaling or Stress Responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakhvataev, V.E. Stress-Induced Bystander Signaling as a Possible Factor Contributing to Neuronal Excitability and Seizure Generation/epileptogenesis. Med. Hypotheses 2016, 90, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Touyz, R.M.; Zweier, J.L.; Dikalov, S.; Chilian, W.; Chen, Y.-R.; Harrison, D.G.; Bhatnagar, A. Measurement of Reactive Oxygen Species, Reactive Nitrogen Species, and Redox-Dependent Signaling in the Cardiovascular System. Circ. Res. 2016, 119, 39–75. [Google Scholar] [CrossRef]
- Suzen, S.; Gurer-Orhan, H.; Saso, L. Detection of Reactive Oxygen and Nitrogen Species by Electron Paramagnetic Resonance (EPR) Technique. Molecules 2017, 22, 181. [Google Scholar] [CrossRef]
- Proctor, P. Electron-Transfer Factors in Psychosis and Dyskinesia. Physiol. Chem. Phys. 1972, 4, 349–360. [Google Scholar] [PubMed]
- Nadeev, A.D.; Goncharov, N.V. Reactive Oxygen Species in the Cells of Cardiovascular System. Complex Issues Cardiovasc. Dis. 2015, 80–94. [Google Scholar] [CrossRef]
- Aprioku, J.S. Pharmacology of Free Radicals and the Impact of Reactive Oxygen Species on the Testis. J. Reprod. Infertil. 2013, 14, 158–172. [Google Scholar] [PubMed]
- Imlay, J.A. The Molecular Mechanisms and Physiological Consequences of Oxidative Stress: Lessons from a Model Bacterium. Nat. Rev. Microbiol. 2013, 11, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Moloney, J.N.; Cotter, T.G. ROS Signalling in the Biology of Cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Fetoni, A.R.; Paciello, F.; Rolesi, R.; Paludetti, G.; Troiani, D. Targeting Dysregulation of Redox Homeostasis in Noise-Induced Hearing Loss: Oxidative Stress and ROS Signaling. Free Radic. Biol. Med. 2019, 135, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.T.; Desikan, R.; Neill, S.J. Role of Reactive Oxygen Species in Cell Signalling Pathways. Biochem. Soc. Trans. 2001, 29, 345. [Google Scholar] [CrossRef]
- Kashmiri, Z.N.; Mankar, S.A. Free Radicals and Oxidative Stress in Bacteria. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 34–40. [Google Scholar]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA Damage Response in Cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Luzina, E.L.; Popov, A. V Synthesis and Anticancer Activity of N-Bis(trifluoromethyl)alkyl-N′-Thiazolyl and N-Bis(trifluoromethyl)alkyl-N′-Benzothiazolyl Ureas. Eur. J. Med. Chem. 2009, 44, 4944–4953. [Google Scholar] [CrossRef] [PubMed]
- Jella, K.K.; Moriarty, R.; McClean, B.; Byrne, H.J.; Lyng, F.M. Reactive Oxygen Species and Nitric Oxide Signaling in Bystander Cells. PLoS ONE 2018, 13, e0195371. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, M.; Neumann, R. Changes in Gene Expression as One of the Key Mechanisms Involved in Radiation-induced Bystander Effect (Review). Biomed. Rep. 2018, 9, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Casartelli, E.A.; Miekeley, N. Determination of Thorium and Light Rare-Earth Elements in Soil Water and Its High Molecular Mass Organic Fractions by Inductively Coupled Plasma Mass Spectrometry and on-Line-Coupled Size-Exclusion Chromatography. Anal. Bioanal. Chem. 2003, 377, 58–64. [Google Scholar] [CrossRef]
- Zelikman, A.N. Metallurgy of Rare Earth Metals, Thorium and Uranium; Kamaeva, O.M., Attopovich, M.K., Eds.; Metallurgizdat: Moscow, Russia, 1960. (In Russian) [Google Scholar]
- Otansev, P.; Taşkin, H.; Başsari, A.; Varinlioğlu, A. Distribution and Environmental Impacts of Heavy Metals and Radioactivity in Sediment and Seawater Samples of the Marmara Sea. Chemosphere 2016, 154, 266–275. [Google Scholar] [CrossRef]
- Robinson, L.F.; Noble, T.L.; McManus, J.F. Measurement of Adsorbed and Total 232Th/230Th Ratios from Marine Sediments. Chem. Geol. 2008, 252, 169–179. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Duarte, I.M.; Paiva, A.V.; Yunes, S.N.; Almeida, C.E.; Mattos, R.C.; Sarcinelli, P.N. The Role of Chemical Interactions between Thorium, Cerium, and Lanthanum in Lymphocyte Toxicity. Arch. Environ. Occup. Health 2014, 69, 40–45. [Google Scholar] [CrossRef]
- Zhu, Z. Effects of Thorium on Paddy Soil Enzymes and Microbial Diversity. Radioprotection 2019, 54, 219–224. [Google Scholar] [CrossRef]
- Doose, C.; Morin, S.; Malbezin, L.; Vedrenne, J.; Fortin, C. Effects of Thorium on Bacterial, Microalgal and Micromeiofaunal Community Structures in a Periphytic Biofilm. Ecotoxicol. Environ. Saf. 2021, 218, 112276. [Google Scholar] [CrossRef]
- Kuznetsov, A.M.; Rodicheva, E.K.; Shilova, E. V Bioassay Based on Lyophilized Bacteria. Biotekhnologiya 1996, 9, 57–61. (In Russian) [Google Scholar]
- Khan, P.; Idrees, D.; Moxley, M.A.; Corbett, J.A.; Ahmad, F.; von Figura, G.; Sly, W.S.; Waheed, A.; Hassan, M.I. Luminol-Based Chemiluminescent Signals: Clinical and Non-Clinical Application and Future Uses. Appl. Biochem. Biotechnol. 2014, 173, 333–355. [Google Scholar] [CrossRef] [Green Version]
- Vasil’ev, R.F.; Veprintsev, T.L.; Dolmatova, L.S.; Naumov, V.V.; Trofimov, A.V.; Tsaplev, Y.B. Kinetics of Ethylbenzene Oxy-Chemiluminescence in the Presence of Antioxidants from Tissues of the Marine Invertebrate Eupentacta Fraudatrix: Estimating the Concentration and Reactivity of the Natural Antioxidants. Kinet. Catal. 2014, 55, 148–153. [Google Scholar] [CrossRef]
- Gmurman, V.E. Fundamentals of Probability Theory and Mathematical Statistics; Berenblut, I.I., Ed.; American Elsevier Publishing, Co.: New York, NY, USA, 1968; ISBN 0592039315. [Google Scholar]
- Krieger, N.; Hastings, J.W. Bioluminescence: pH Activity Profies of Related Luciferase Fractions. Science 1968, 161, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Kydryasheva, N.S.; Zuzikova, E.V.; Gutnik, T.V. Mechanism of Action of Metal Salts on Bacterial Bioluminescent System in Vitro. Biofizika 1999, 44, 249–250. (In Russian) [Google Scholar]
- Thakre, N.A.; Shanware, A.S. Promising Biological Indicator of Heavy Metal Pollution: Bioluminescent Bacterial Strains Isolated and Characterized from Marine Niches of Goa, India. Indian J. Microbiol. 2015, 55, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Parmar, P.; Shukla, A.; Goswami, D.; Gaur, S.; Patel, B.; Saraf, M. Comprehensive Depiction of Novel Heavy Metal Tolerant and EPS Producing Bioluminescent Vibrio Alginolyticus PBR1 and V. Rotiferianus PBL1 Confined from Marine Organisms. Microbiol. Res. 2020, 238, 126526. [Google Scholar] [CrossRef] [PubMed]
- Rozhko, T.V.; Kudryasheva, N.S.; Alexandrova, M.A.; Bondareva, L.G.; Bolsunovsky, A.Y.; Vydryakova, G.V. Comparison of Effects of Uranium and Americium on Bioluminescent Bacteria. J. Sib. Fed. Univ. Biol. 2008, 1, 60–65. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Chernikov, A.V.; Bruskov, V.I. Chemical and Radiological Toxicity of Uranium Compounds. Russ. J. Gen. Chem. 2016, 86, 1531–1538. [Google Scholar] [CrossRef]
- Akiska, E.; Karakas, Z.; Öztürk, C. Uranium, Thorium and Rare Earth Element Deposits of Turkey. In Modern Approaches in Solid Earth Sciences; Pirajno, F., Ünlü, T., Dönmez, C., Şahin, M.B., Eds.; Springer: Cham, Switzerland, 2019; Volume 16, pp. 655–679. ISBN 978-3-030-02948-7. [Google Scholar]
- Wilson, T.; Hastings, J.W. Bioluminescence. Annu. Rev. Cell Dev. Biol. 1998, 14, 197–230. [Google Scholar] [CrossRef]
- Lee, J.; Müller, F.; Visser, A.J.W.G. The Sensitized Bioluminescence Mechanism of Bacterial Luciferase. Photochem. Photobiol. 2019, 95, 679–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
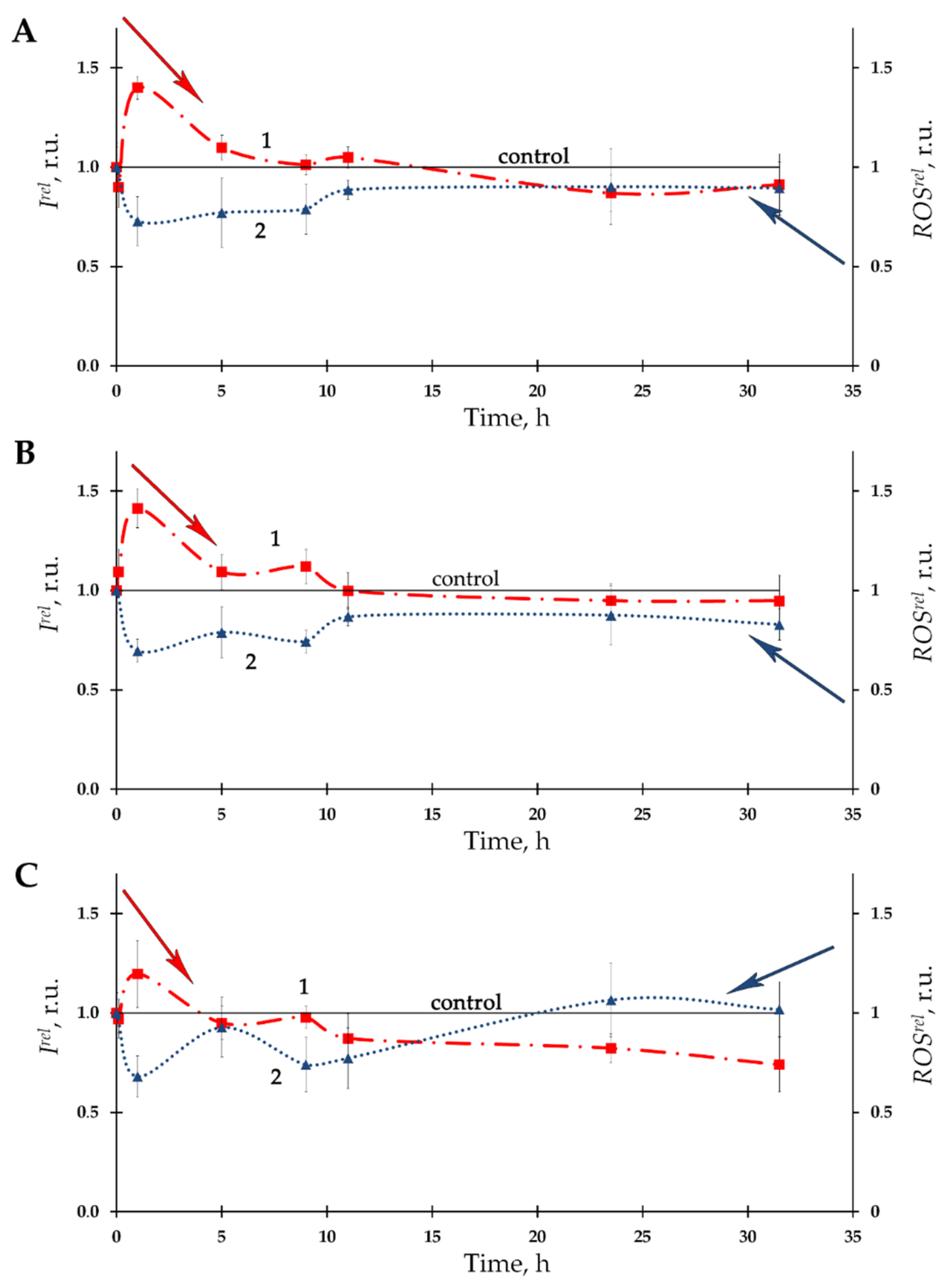
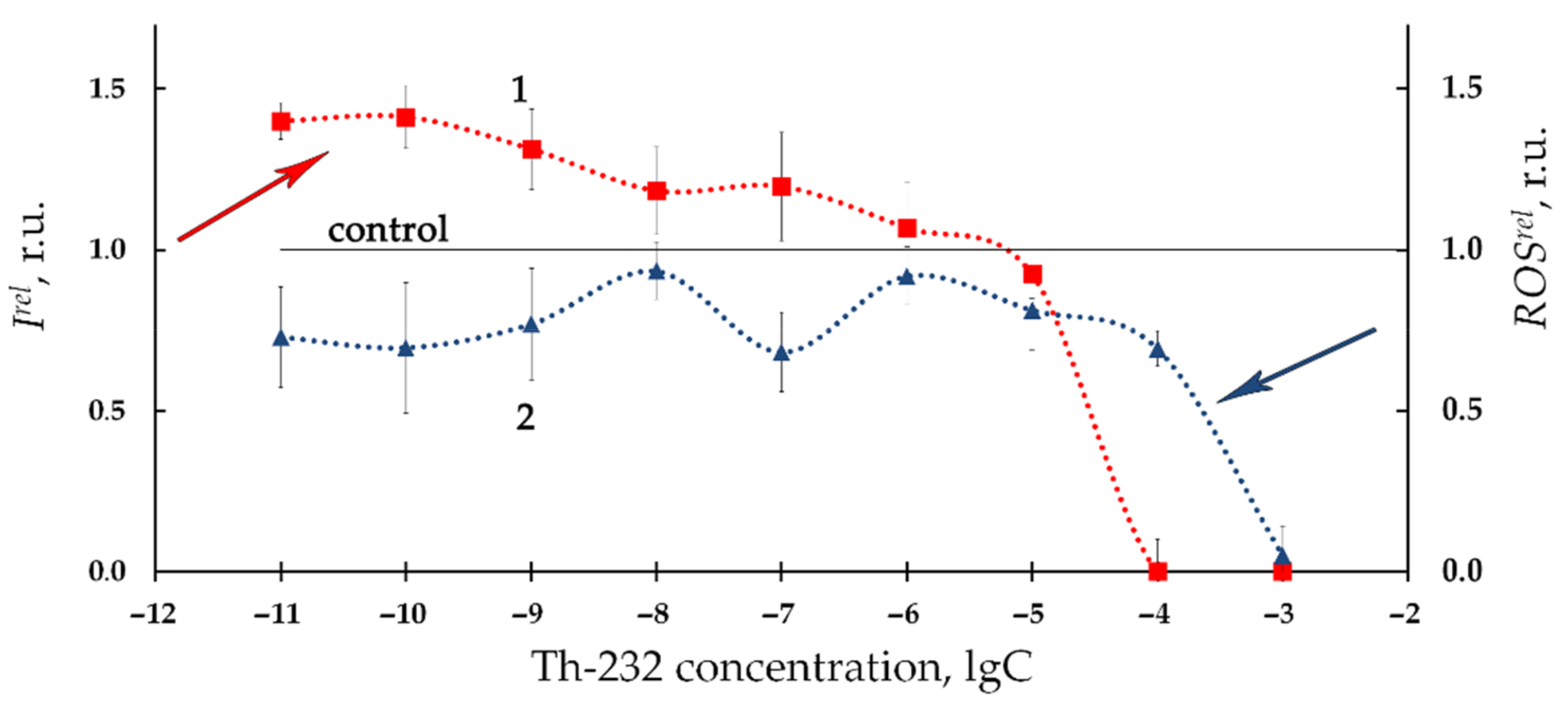
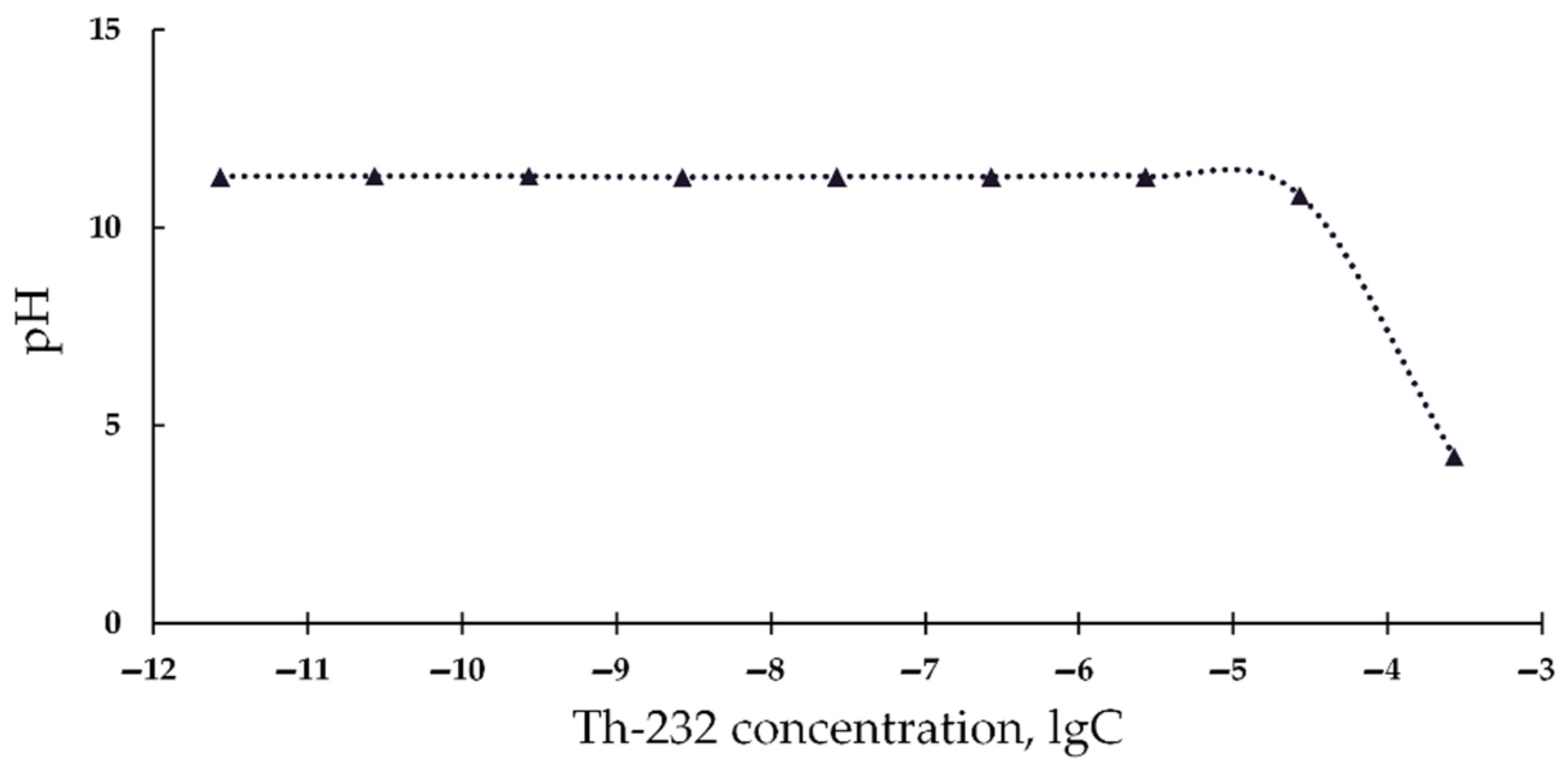
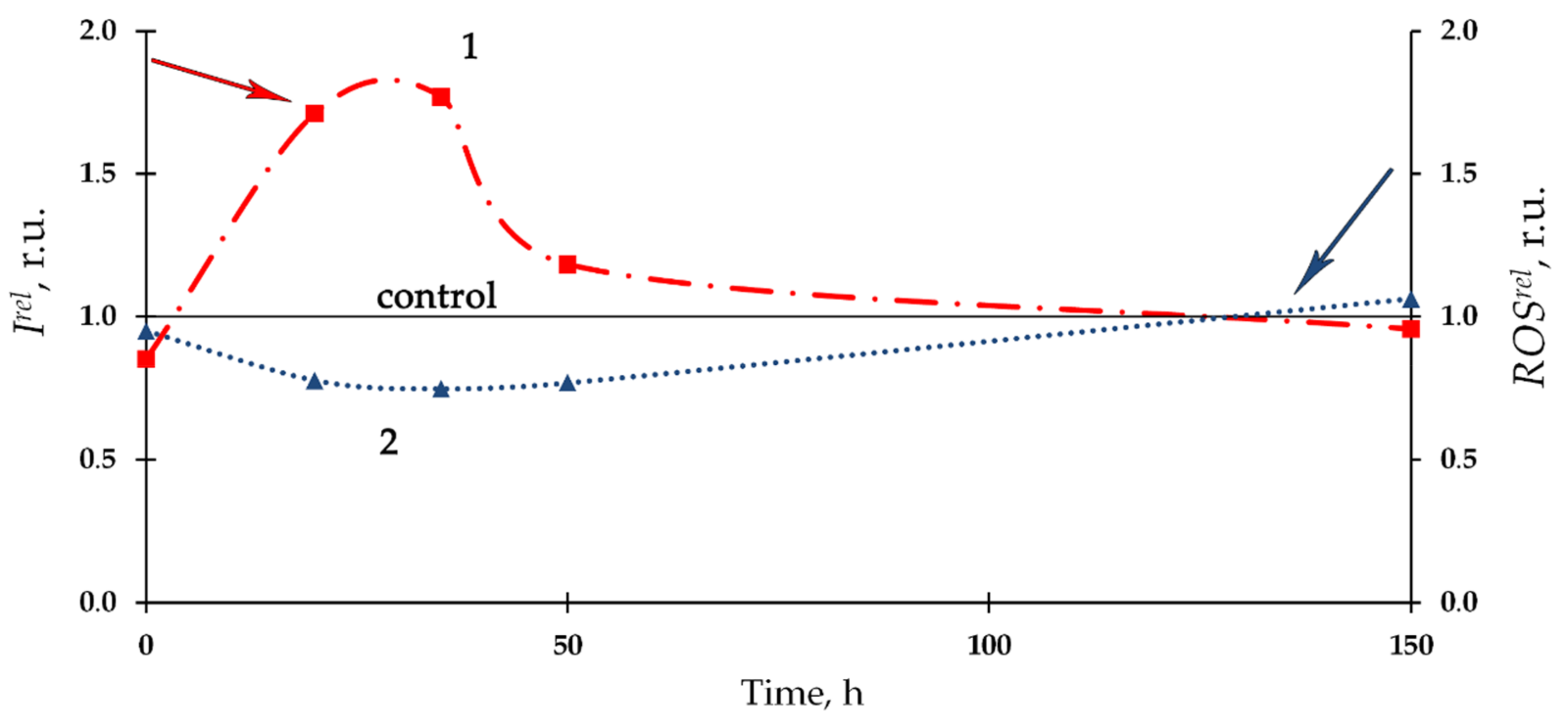

| Number of Solutions | Components of Solutions | V × 108, M | |
|---|---|---|---|
| without Th | with Th | ||
| 1 | NADH | 2.43 | 4.05 |
| 2 | NADH + enzyme preparation | 4.05 | 6.07 |
| 3 | NADH + FMN | 14.20 | 20.60 |
| 4 | NADH + FMN + enzyme preparation | 16.20 | 26.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolesnik, O.V.; Rozhko, T.V.; Lapina, M.A.; Solovyev, V.S.; Sachkova, A.S.; Kudryasheva, N.S. Development of Cellular and Enzymatic Bioluminescent Assay Systems to Study Low-Dose Effects of Thorium. Bioengineering 2021, 8, 194. https://doi.org/10.3390/bioengineering8120194
Kolesnik OV, Rozhko TV, Lapina MA, Solovyev VS, Sachkova AS, Kudryasheva NS. Development of Cellular and Enzymatic Bioluminescent Assay Systems to Study Low-Dose Effects of Thorium. Bioengineering. 2021; 8(12):194. https://doi.org/10.3390/bioengineering8120194
Chicago/Turabian StyleKolesnik, Olga V., Tatiana V. Rozhko, Maria A. Lapina, Vladislav S. Solovyev, Anna S. Sachkova, and Nadezhda S. Kudryasheva. 2021. "Development of Cellular and Enzymatic Bioluminescent Assay Systems to Study Low-Dose Effects of Thorium" Bioengineering 8, no. 12: 194. https://doi.org/10.3390/bioengineering8120194







