Versatile Covalent Postsynthetic Modification of Metal Organic Frameworks via Thermal Condensation for Fluoride Sensing in Waters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization
3. Results
3.1. Post-Synthetic Modification of Al-BDC-NH MOF
3.2. Structural and Textural Characterization of PSM-MOFs
3.3. Chemical Interaction of Modified MOFs with Fluoride
4. Discussion
4.1. Post Synthetic Modification of MOFs
4.2. Interpretation of the MOF-Fluoride Interaction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Demelash, H.; Beyene, A.; Abebe, Z.; Melese, A. Fluoride concentration in ground water and prevalence of dental fluorosis in Ethiopian Rift Valley. systematic review and meta-analysis. (BMC) Public Health 2019, 19, 1298. [Google Scholar] [CrossRef] [Green Version]
- Fawell, J.; Bailey, K.; Chilton, J.; Dahi, E.; Magara, Y. Fluoride in Drinking-Water. In WHO Water Series; IWA Publishing: London, UK, 2013; pp. 29–36. [Google Scholar]
- Envision2030: 17 Goals to Transform the World for Persons with Disabilities|United Nations. Available online: https://www.un.org/development/desa/disabilities/envision2030.html (accessed on 27 October 2021).
- Otal, E.H.; Kim, M.L.; Dietrich, S.; Takada, R.; Nakaya, S.; Kimura, M. Open-Source Portable Device for the Determination of Fluoride in Drinking Water. ACS Sens. 2021, 6, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Otal, E.H.; Tanaka, H.; Kim, M.L.; Hinestroza, J.P.; Kimura, M. The Long and Bright Path of a Lanthanide MOF: From Basics towards the Application. Chem.-A Eur. J. 2021, 27, 7376–7382. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.L.; Hurley, A. Collected Fictions; Viking: New York, NY, USA, 1998. [Google Scholar]
- Ibarra, I.A.; Bayliss, P.A.; Pérez, E.; Yang, S.; Blake, A.J.; Nowell, H.; Allan, D.R.; Poliakoff, M.; Schröder, M. Near-critical water, a cleaner solvent for the synthesis of a metal–organic framework. Green Chem. 2012, 14, 117–122. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.; Getachew, N.; Díaz, K.; Díaz-García, M.; Chebude, Y.; Díaz, I. Synthesis of metal–organic frameworks in water at room temperature: Salts as linker sources. Green Chem. 2015, 17, 1500–1509. [Google Scholar] [CrossRef]
- Bayliss, P.A.; Ibarra, I.A.; Pérez, E.; Yang, S.; Tang, C.C.; Poliakoff, M.; Schröder, M. Synthesis of metal–organic frameworks by continuous flow. Green Chem. 2014, 16, 3796–3802. [Google Scholar] [CrossRef] [Green Version]
- Julien, P.A.; Mottillo, C.; Friščić, T. Metal–organic frameworks meet scalable and sustainable synthesis. Green Chem. 2017, 19, 2729–2747. [Google Scholar] [CrossRef]
- Kiang, Y.H.; Gardner, G.B.; Lee, S.; Xu, Z.; Lobkovsky, E.B. Variable Pore Size, Variable Chemical Functionality, and an Example of Reactivity within Porous Phenylacetylene Silver Salts. J. Am. Chem. Soc. 1999, 121, 8204–8215. [Google Scholar] [CrossRef]
- Wang, Z.; Cohen, S.M. Postsynthetic Covalent Modification of a Neutral Metal-Organic Framework. J. Am. Chem. Soc. 2007, 129, 12368–12369. [Google Scholar] [CrossRef] [PubMed]
- Haneda, T.; Kawano, M.; Kawamichi, T.; Fujita, M. Direct Observation of the Labile Imine Formation through Single-Crystal-to-Single-Crystal Reactions in the Pores of a Porous Coordination Network. J. Am. Chem. Soc. 2008, 130, 1578–1579. [Google Scholar] [CrossRef]
- Cohen, S.M. Postsynthetic Methods for the Functionalization of Metal–Organic Frameworks. Chem. Rev. 2012, 112, 970–1000. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M. The Postsynthetic Renaissance in Porous Solids. J. Am. Chem. Soc. 2017, 139, 2855–2863. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, Y.; Yaghi, O.M. Covalent Chemistry beyond Molecules. J. Am. Chem. Soc. 2016, 138, 3255–3265. [Google Scholar] [CrossRef]
- Kalaj, M.; Cohen, S.M. Postsynthetic Modification: An Enabling Technology for the Advancement of Metal–Organic Frameworks. ACS Cent. Sci. 2020, 6, 1046–1057. [Google Scholar] [CrossRef]
- Evans, J.D.; Sumby, C.J.; Doonan, C.J. Post-synthetic metalation of metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 5933–5951. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Song, I.; Jung, G.Y.; Choi, W.; Ohtsu, H.; Lee, J.H.; Koo, J.Y.; Liu, B.; Ahn, J.; Kawano, M.; et al. Chiral self-sorted multifunctional supramolecular biocoordination polymers and their applications in sensors. Nat. Commun. 2018, 9, 3933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Yang, S.; Chen, X.; Liu, Q.; Li, H.; Wang, H.; Wei, B.; Jiang, D. Energy-storage covalent organic frameworks: Improving performance via engineering polysulfide chains on walls. Chem. Sci. 2019, 10, 6001–6006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, F.G.; Liu, H.; Yang, N.N.; Gao, E.Q. Aldehyde-Tagged Zirconium Metal–Organic Frameworks: A Versatile Platform for Postsynthetic Modification. Inorg. Chem. 2016, 55, 4701–4703. [Google Scholar] [CrossRef] [PubMed]
- Otal, E.H.; Kim, M.L.; Calvo, M.E.; Karvonen, L.; Fabregas, I.O.; Sierra, C.A.; Hinestroza, J.P. A panchromatic modification of the light absorption spectra of metal–organic frameworks. Chem. Commun. 2016, 52, 6665–6668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hindelang, K.; Kronast, A.; Vagin, S.I.; Rieger, B. Functionalization of Metal–Organic Frameworks through the Postsynthetic Transformation of Olefin Side Groups. Chem. A Eur. J. 2013, 19, 8244–8252. [Google Scholar] [CrossRef] [PubMed]
- Ahnfeldt, T.; Gunzelmann, D.; Loiseau, T.; Hirsemann, D.; Senker, J.; Férey, G.; Stock, N. Synthesis and Modification of a Functionalized 3D Open-Framework Structure with MIL-53 Topology. Inorg. Chem. 2009, 48, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Hinterholzinger, F.M.; Rühle, B.; Wuttke, S.; Karaghiosoff, K.; Bein, T. Highly sensitive and selective fluoride detection in water through fluorophore release from a metal-organic framework. Sci. Rep. 2013, 3, 2562. [Google Scholar] [CrossRef] [PubMed]
- Modrow, A.; Zargarani, D.; Herges, R.; Stock, N. Introducing a photo-switchable azo-functionality inside Cr-MIL-101-NH2 by covalent post-synthetic modification. Dalton Trans. 2012, 41, 8690–8696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, X.D.; Hoang, V.T.; Kaliaguine, S. MIL-53(Al) mesostructured metal-organic frameworks. Microporous Mesoporous Mater. 2011, 141, 135–139. [Google Scholar] [CrossRef]
- Bourrelly, S.; Llewellyn, P.L.; Serre, C.; Millange, F.; Loiseau, T.; Férey, G. Different Adsorption Behaviors of Methane and Carbon Dioxide in the Isotypic Nanoporous Metal Terephthalates MIL-53 and MIL-47. J. Am. Chem. Soc. 2005, 127, 13519–13521. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zakharova, N.; Adeyilola, A.; Zeng, L. Experimental Study on the Pore Shape Damage of Shale Samples during the Crushing Process. Energy Fuels 2021, 35, 2183–2191. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S. Adsorption by Powders and Porous Solids; Academic Press: London, UK, 1999. [Google Scholar]
- Adams, M.C.; Davis, J. Kinetics of fluorescein decay and its application as a geothermal tracer. Geothermics 1991, 20, 53–66. [Google Scholar] [CrossRef]
- Barbano, D.M.; Dellavalle, M.E. Thermal Degradation of FD & C Red No. 3 and Release of Free Iodide. J. Food Prot. 1984, 47, 668–669. [Google Scholar] [CrossRef]
- Qiu, S.; Chu, H.; Zou, Y.; Xiang, C.; Zhang, H.; Sun, L.; Xu, F. Thermochemical studies of Rhodamine B and Rhodamine 6G by modulated differential scanning calorimetry and thermogravimetric analysis. J. Therm. Anal. Calorim. 2016, 123, 1611–1618. [Google Scholar] [CrossRef]
- Alarcon, E.I.; Poblete, H.; Roh, H.; Couture, J.F.; Comer, J.; Kochevar, I.E. Rose Bengal Binding to Collagen and Tissue Photobonding. ACS Omega 2017, 2, 6646–6657. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Du, L.; Zhu, P.; Chen, Q.; Tan, K. An erythrosin B-based “turn on” fluorescent sensor for detecting perfluorooctane sulfonate and perfluorooctanoic acid in environmental water samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 201, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, B.B.; Ganguly, P. Photophysics of xanthene dyes in surfactant solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 1997–2003. [Google Scholar] [CrossRef] [PubMed]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Boston, MA, USA, 1990. [Google Scholar]
- Zeng, X.; Hu, J.; Zhang, M.; Wang, F.; Wu, L.; Hou, X. Visual Detection of Fluoride Anions Using Mixed Lanthanide Metal–Organic Frameworks with a Smartphone. Anal. Chem. 2020, 92, 2097–2102. [Google Scholar] [CrossRef] [PubMed]


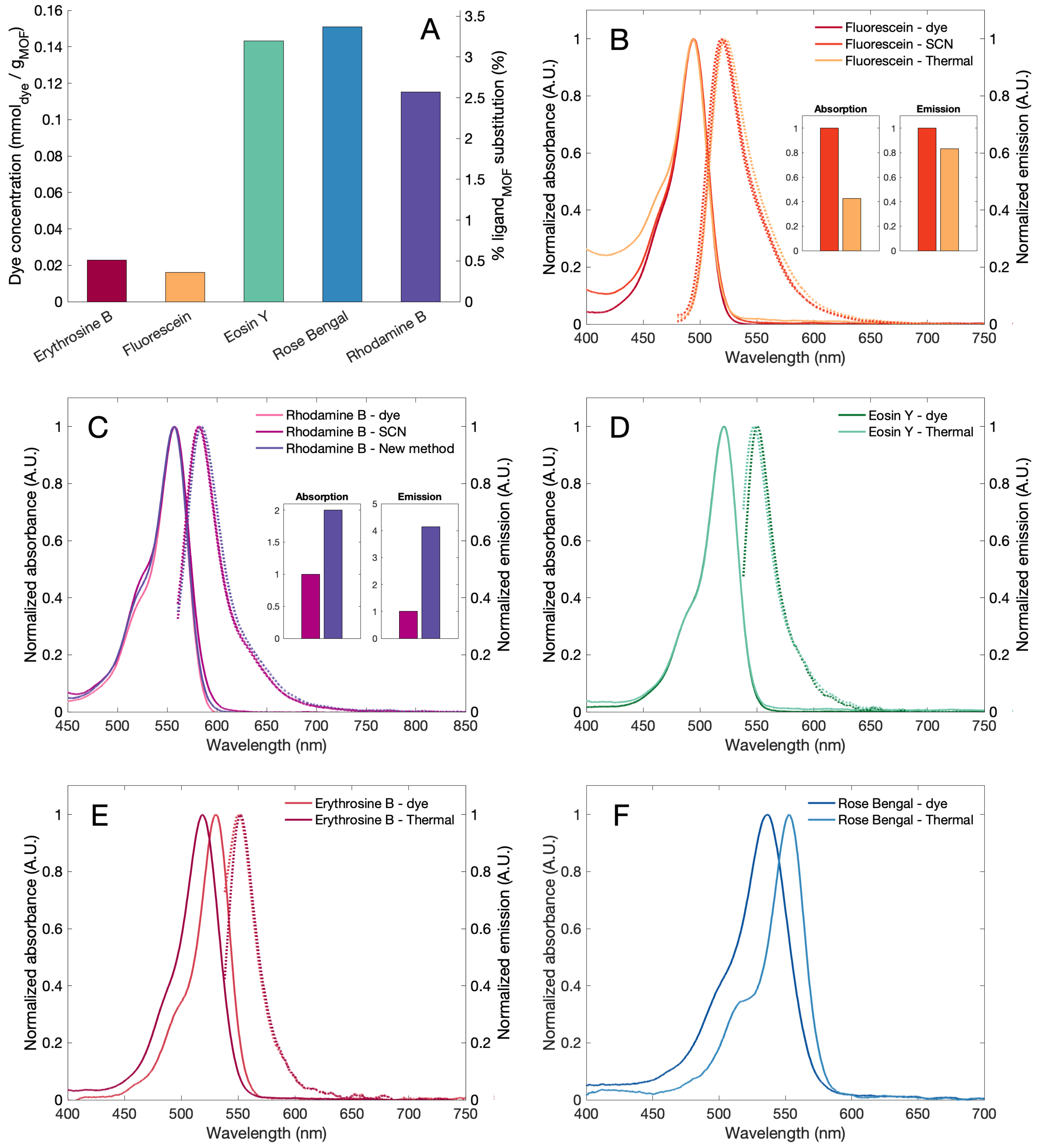
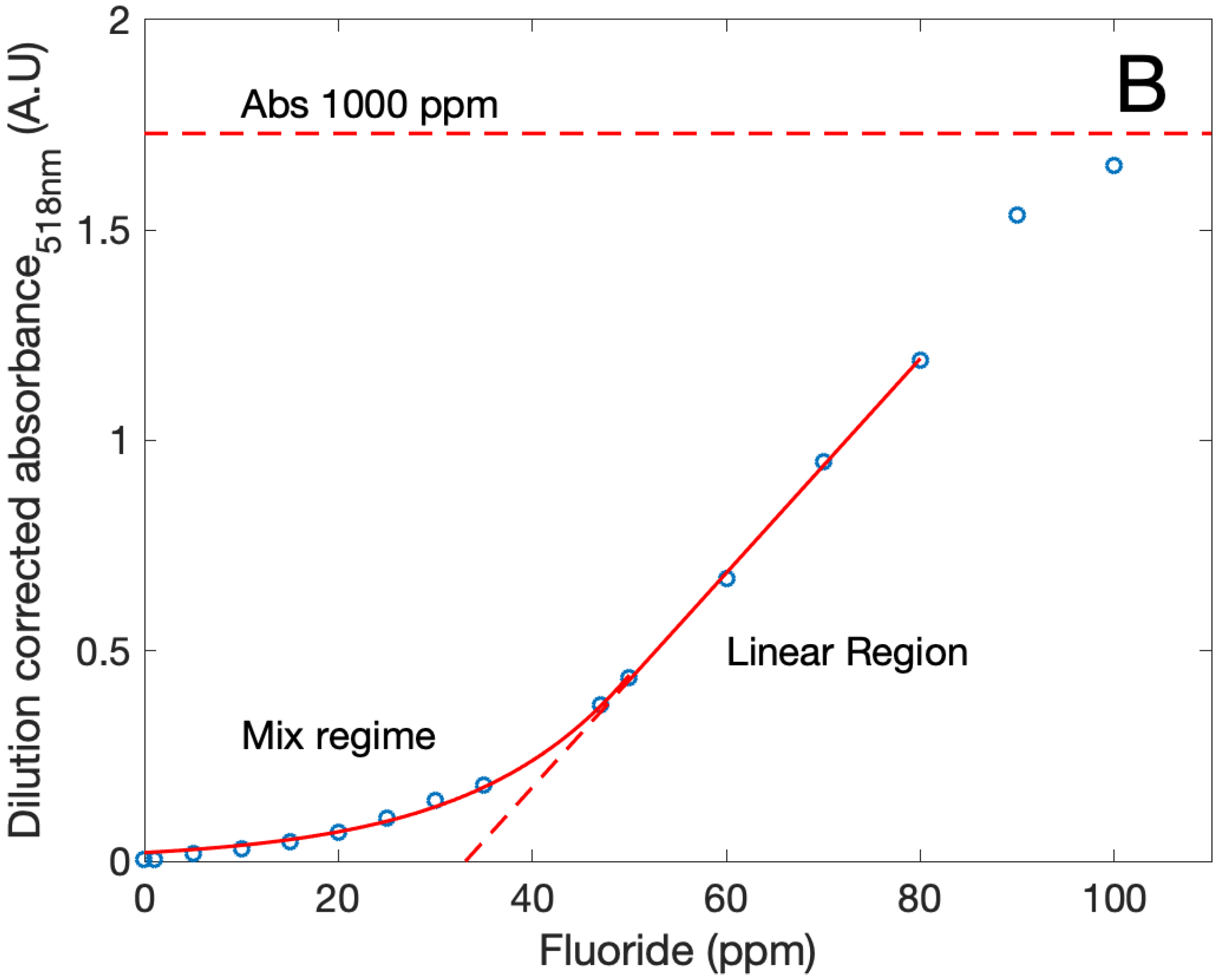
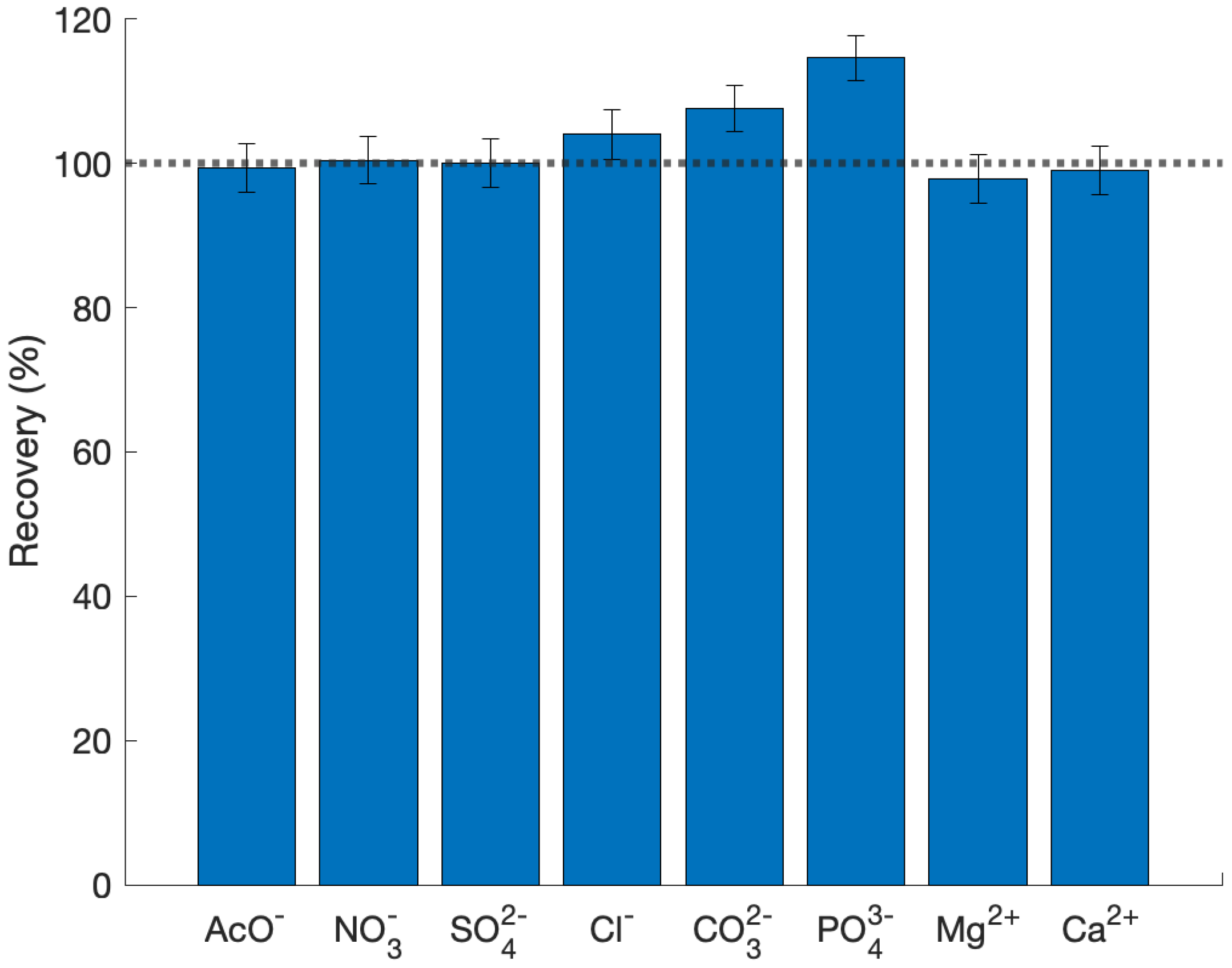
| Sample | S (m/g) | VP (cm/g) | V (cm/g) |
|---|---|---|---|
| Pristine MOF | 1386 | 0.599 | 0.784 |
| Fluorescein | 559 | 0.221 | 0.409 |
| Rhodamine B | 222 | 0.111 | 0.277 |
| Rose bengal | 322 | 0.161 | 0.295 |
| Eosin Y | 521 | 0.225 | 0.331 |
| Erythrosine B | 864 | 0.394 | 0.558 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otal, E.H.; Kim, M.L.; Hattori, Y.; Kitazawa, Y.; Hinestroza, J.P.; Kimura, M. Versatile Covalent Postsynthetic Modification of Metal Organic Frameworks via Thermal Condensation for Fluoride Sensing in Waters. Bioengineering 2021, 8, 196. https://doi.org/10.3390/bioengineering8120196
Otal EH, Kim ML, Hattori Y, Kitazawa Y, Hinestroza JP, Kimura M. Versatile Covalent Postsynthetic Modification of Metal Organic Frameworks via Thermal Condensation for Fluoride Sensing in Waters. Bioengineering. 2021; 8(12):196. https://doi.org/10.3390/bioengineering8120196
Chicago/Turabian StyleOtal, Eugenio Hernan, Manuela Leticia Kim, Yoshiyuki Hattori, Yu Kitazawa, Juan Paulo Hinestroza, and Mutsumi Kimura. 2021. "Versatile Covalent Postsynthetic Modification of Metal Organic Frameworks via Thermal Condensation for Fluoride Sensing in Waters" Bioengineering 8, no. 12: 196. https://doi.org/10.3390/bioengineering8120196








