Implantable Drug Delivery Systems and Foreign Body Reaction: Traversing the Current Clinical Landscape
Abstract
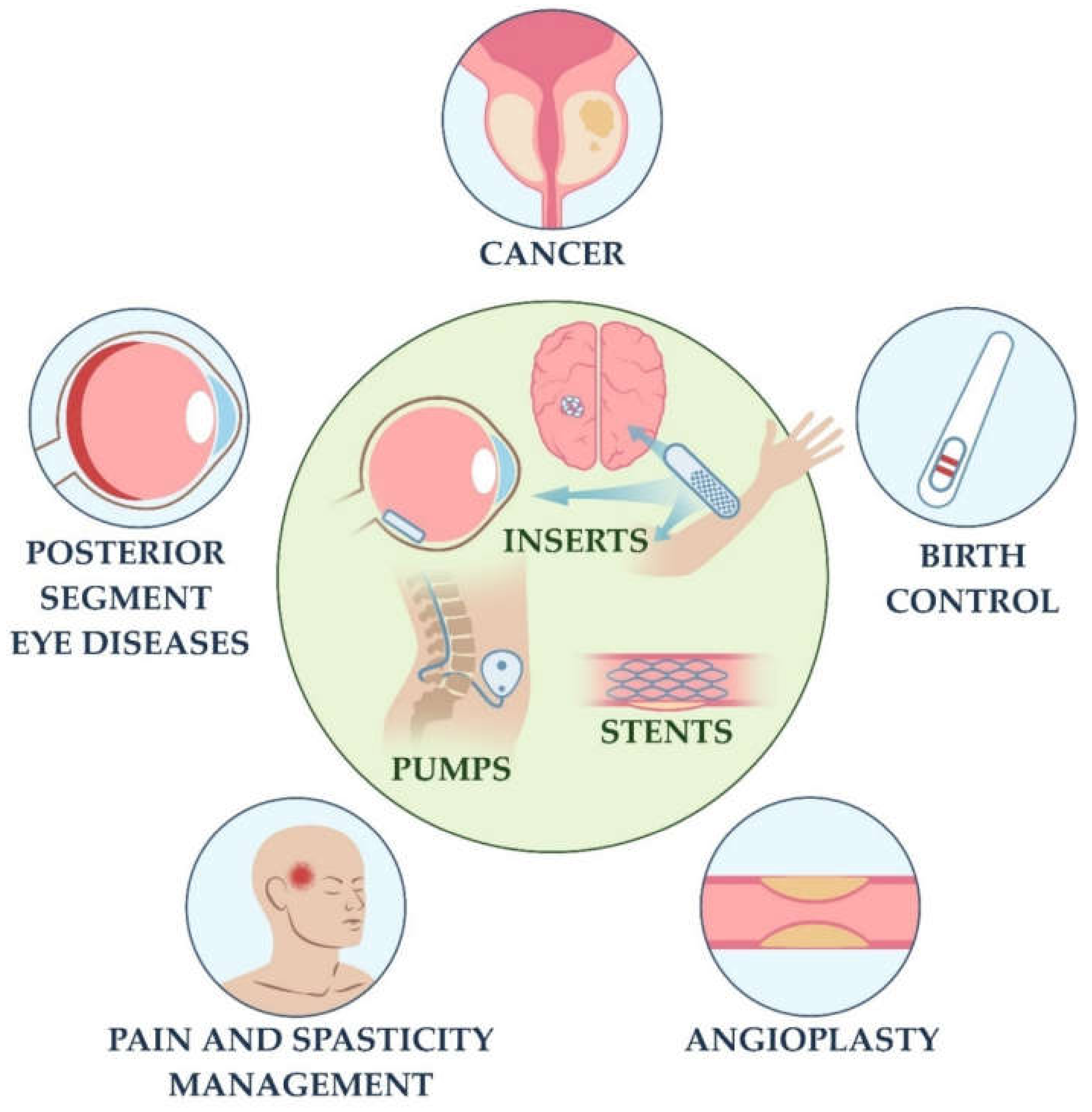
1. Introduction
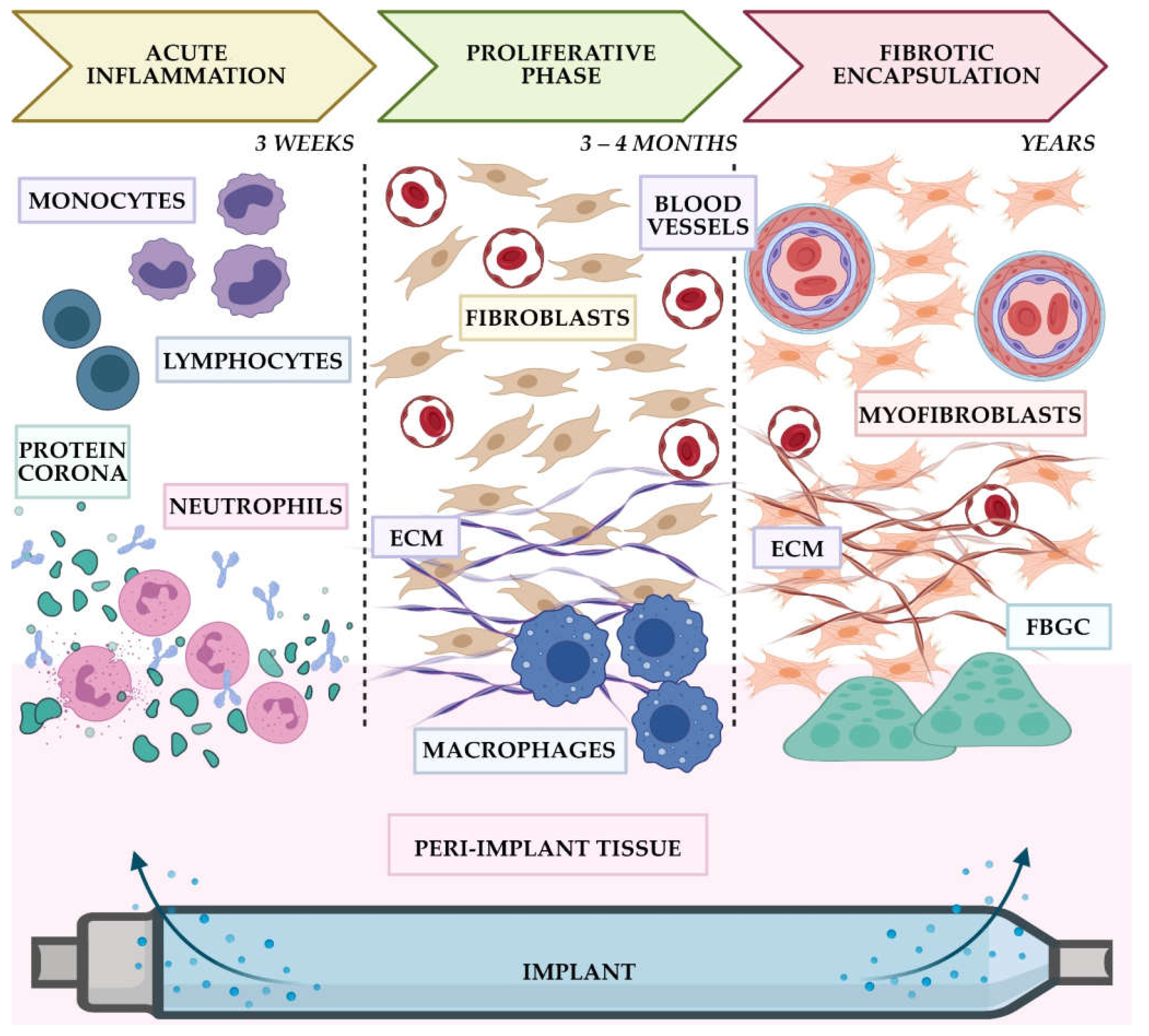
2. IDDSs and FBR: Current Clinical Landscape
2.1. Subcutaneous IDDSs
2.2. Pump IDDSs
2.3. Ocular IDDSs
2.4. Neurological IDDS
2.5. Cardiovascular IDDSs
3. General Discussion and Future Prospects
3.1. Main Findings
- (1)
- Implantation site (the immune reactivity of the host tissue);
- (2)
- The volume of the implant (defining the volume of surgical trauma and the local tissue extension);
- (3)
- The drug load of IDDS (anti-inflammatory, anti-proliferative, and immunosuppressing drugs contribute to less prominent FBR).
3.2. Analysis of the Application-Specific Trends
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pond, S.M.; Tozer, T.N. First-pass elimination. Basic concepts and clinical consequences. Clin. Pharmacokinet. 1984, 9, 1–25. [Google Scholar] [CrossRef]
- Massot Mesquida, M.; de la Fuente, J.A.; Andres Lorca, A.M.; Arteaga Pillasagua, I.; Balboa Blanco, E.; Gracia Vidal, S.; Pablo Reyes, S.; Gomez Iparraguirre, P.; Seda Gombau, G.; Toran-Monserrat, P. Primary Care Records of Chronic-Disease Patient Adherence to Treatment. Int. J. Environ. Res. Public Health 2021, 18, 3710. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, E.; Hearnshaw, H.; Van Royen, P.; Denekens, J. Patient adherence to treatment: Three decades of research. A comprehensive review. J. Clin. Pharm. Ther. 2001, 26, 331–342. [Google Scholar] [CrossRef]
- Jain, K.K. Drug delivery systems—An overview. Methods Mol. Biol. 2008, 437, 1–50. [Google Scholar] [CrossRef]
- Choi, S.H.; Wang, Y.; Conti, D.S.; Raney, S.G.; Delvadia, R.; Leboeuf, A.A.; Witzmann, K. Generic drug device combination products: Regulatory and scientific considerations. Int. J. Pharm. 2018, 544, 443–454. [Google Scholar] [CrossRef]
- Al-Jawadi, S.; Capasso, P.; Sharma, M. The road to market implantable drug delivery systems: A review on US FDA’s regulatory framework and quality control requirements. Pharm. Dev. Technol. 2018, 23, 953–963. [Google Scholar] [CrossRef]
- Pons-Faudoa, F.P.; Ballerini, A.; Sakamoto, J.; Grattoni, A. Advanced implantable drug delivery technologies: Transforming the clinical landscape of therapeutics for chronic diseases. Biomed. Microdevices 2019, 21, 47. [Google Scholar] [CrossRef]
- Kleiner, L.W.; Wright, J.C. Implants and Inserts. In Biomaterials Science; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 1062–1071. [Google Scholar]
- Kleiner, L.W.; Wright, J.C.; Wang, Y. Evolution of implantable and insertable drug delivery systems. J. Control. Release Off. J. Control. Release Soc. 2014, 181, 1–10. [Google Scholar] [CrossRef]
- Salam, M.T.; Mirzaei, M.; Ly, M.S.; Nguyen, D.K.; Sawan, M. An implantable closedloop asynchronous drug delivery system for the treatment of refractory epilepsy. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, M.P.; Klaff, L.J.; Brazg, R.; Chang, A.R.; Levy, C.J.; Lam, D.; Denham, D.S.; Atiee, G.; Bode, B.W.; Walters, S.J.; et al. A Prospective Multicenter Evaluation of the Accuracy of a Novel Implanted Continuous Glucose Sensor: PRECISE II. Diabetes Technol. Ther. 2018, 20, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Danckwerts, M.; Fassihi, A. Implantable Controlled Release Drug Delivery Systems—A Review. Drug Dev. Ind. Pharm. 1991, 17, 1465–1502. [Google Scholar] [CrossRef]
- Santos, A.; Sinn Aw, M.; Bariana, M.; Kumeria, T.; Wang, Y.; Losic, D. Drug-releasing implants: Current progress, challenges and perspectives. J. Mater. Chem. B 2014, 2, 6157–6182. [Google Scholar] [CrossRef]
- Kumar, A.; Pillai, J. Implantable drug delivery systems. In Nanostructures for the Engineering of Cells, Tissues and Organs; Grumezescu, A.M., Ed.; William Andrew Publishing: Cambridge, MA, USA, 2018; pp. 473–511. [Google Scholar]
- Quarterman, J.C.; Geary, S.M.; Salem, A.K. Evolution of drug-eluting biomedical implants for sustained drug delivery. Eur. J. Pharm. Biopharm. 2021, 159, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J.; Long, D.M. The use of silicone rubber as a carrier for prolonged drug therapy. J. Surg. Res. 1964, 4, 139–142. [Google Scholar] [CrossRef]
- FDA. IDE Definitions and Acronyms. Available online: https://www.fda.gov/medical-devices/investigational-device-exemption-ide/ide-definitions-and-acronyms (accessed on 28 October 2021).
- Major, I.; Lastakchi, S.; Dalton, M.; McConville, C. Implantable drug delivery systems. In Engineering Drug Delivery Systems; Seyfoddin, A., Dezfooli, S.M., Greene, C.A., Eds.; Woodhead Publishing: Kidnlington, UK, 2020; pp. 111–146. [Google Scholar]
- Stewart, S.A.; Dominguez-Robles, J.; Donnelly, R.F.; Larraneta, E. Implantable Polymeric Drug Delivery Devices: Classification, Manufacture, Materials, and Clinical Applications. Polymers 2018, 10, 1379. [Google Scholar] [CrossRef]
- Mohtashami, Z.; Esmaili, Z.; Vakilinezhad, M.A.; Seyedjafari, E.; Akbari Javar, H. Pharmaceutical implants: Classification, limitations and therapeutic applications. Pharm. Dev. Technol. 2020, 25, 116–132. [Google Scholar] [CrossRef] [PubMed]
- FDA Center for Devices; Radiological Health Center for Biologics Evaluation and Research; U.S. Department of Health and Human Services Food and Drug Administration. Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process: Guidance for Industry and Food and Drug Administration Staff. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-international-standard-iso-10993-1-biological-evaluation-medical-devices-part-1-evaluation-and (accessed on 26 October 2021).
- Coleman, D.L.; King, R.N.; Andrade, J.D. The foreign body reaction: A chronic inflammatory response. J. Biomed. Mater. Res. 1974, 8, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Chandorkar, Y.; Ravikumar, K.; Basu, B. The Foreign Body Response Demystified. ACS Biomater. Sci. Eng. 2019, 5, 19–44. [Google Scholar] [CrossRef]
- Serpooshan, V.; Mahmoudi, M.; Zhao, M.; Wei, K.; Sivanesan, S.; Motamedchaboki, K.; Malkovskiy, A.V.; Gladstone, A.B.; Cohen, J.E.; Yang, P.C.; et al. Protein Corona Influences Cell-Biomaterial Interactions in Nanostructured Tissue Engineering Scaffolds. Adv. Funct. Mater. 2015, 25, 4379–4389. [Google Scholar] [CrossRef]
- Cunningham, J.J.; Nikolovski, J.; Linderman, J.J.; Mooney, D.J. Quantification of fibronectin adsorption to silicone-rubber cell culture substrates. Biotechniques 2002, 32, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of biomaterial-cell interactions by adsorbed proteins: A review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.I.; Wang, Y. Cell Responses to Surface and Architecture of Tissue Engineering Scaffolds. Regen. Med. Tissue Eng.—Cells Biomater. 2011, 569–588. [Google Scholar] [CrossRef]
- Rostam, H.M.; Singh, S.; Salazar, F.; Magennis, P.; Hook, A.; Singh, T.; Vrana, N.E.; Alexander, M.R.; Ghaemmaghami, A.M. The impact of surface chemistry modification on macrophage polarisation. Immunobiology 2016, 221, 1237–1246. [Google Scholar] [CrossRef]
- Matriano, J. CHAPTER 7. Addressing Immunogenicity for Implantable Drug-delivery Devices and Long-acting Injectables, Including Pharmacokinetic and Pharmacodynamic Correlations. In Implantable Technologies; Drug Development and Pharmaceutical Science; The Royal Society of Chemistry: Croydon, UK, 2021; pp. 131–159. [Google Scholar]
- McCullough, A.R.; Khera, M.; Goldstein, I.; Hellstrom, W.J.; Morgentaler, A.; Levine, L.A. A multi-institutional observational study of testosterone levels after testosterone pellet (Testopel((R))) insertion. J. Sex. Med. 2012, 9, 594–601. [Google Scholar] [CrossRef]
- McCullough, A. A Review of Testosterone Pellets in the Treatment of Hypogonadism. Curr. Sex. Health Rep. 2014, 6, 265–269. [Google Scholar] [CrossRef]
- Chaudhry, F. Adverse reaction to Nexplanon(R). J. Fam. Plan. Reprod. Health Care 2013, 39, 231–232. [Google Scholar] [CrossRef]
- Schuchard, M.; Lanning, R.; North, R.; Reig, E.; Krames, E. Neurologic sequelae of intraspinal drug delivery systems: Results of a survey of american implanters of implantable drug delivery systems. Neuromodulation 1998, 1, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Kleppner, S.R.; Patel, R.; McDonough, J.; Costantini, L.C. In-vitro and in-vivo characterization of a buprenorphine delivery system. J. Pharm. Pharmacol. 2006, 58, 295–302. [Google Scholar] [CrossRef]
- McDonald-Mosley, R.; Burke, A.E. Contraceptive implants. Semin. Reprod. Med 2010, 28, 110–117. [Google Scholar] [CrossRef]
- Ramdhan, R.C.; Simonds, E.; Wilson, C.; Loukas, M.; Oskouian, R.J.; Tubbs, R.S. Complications of Subcutaneous Contraception: A Review. Cureus 2018, 10, e2132. [Google Scholar] [CrossRef]
- Wysowski, D.K.; Green, L. Serious adverse events in Norplant users reported to the Food and Drug Administration’s MedWatch Spontaneous Reporting System. Obstet. Gynecol. 1995, 85, 538–542. [Google Scholar] [CrossRef]
- Chadha-Gupta, A.; Moss, A. Fat atrophy at the site of a subdermal contraceptive implant. J. Fam. Plan. Reprod. Health Care 2007, 33, 123–124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Funk, S.; Miller, M.M.; Mishell, D.R., Jr.; Archer, D.F.; Poindexter, A.; Schmidt, J.; Zampaglione, E.; Implanon, U.S.S.G. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel. Contraception 2005, 71, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Gwinnell, E. Expulsion of Implanon. J. Fam. Plan. Reprod. Health Care 2007, 33, 211. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.R. A foreign body reaction to a contraceptive implant. J. Womens Health Gynecol. 2014, 1, 1–3. [Google Scholar] [CrossRef]
- Park, J.U.; Bae, H.S.; Lee, S.M.; Bae, J.; Park, J.W. Removal of a subdermal contraceptive implant (Implanon NXT) that migrated to the axilla by C-arm guidance: A case report and review of the literature. Medicine 2017, 96, e8627. [Google Scholar] [CrossRef] [PubMed]
- Mansour, D. Comment on ‘Adverse reaction to Nexplanon®’. J. Fam. Plan. Reprod. Health Care 2013, 39, 232–233. [Google Scholar] [CrossRef]
- Serati, M.; Bogani, G.; Kumar, S.; Cromi, A.; Ghezzi, F. Delayed-type hypersensitivity reaction against Nexplanon(R). Contraception 2015, 91, 91–92. [Google Scholar] [CrossRef]
- Fowler, J.E. Patient-reported experience with the Viadur 12-month leuprolide implant for prostate cancer. Urology 2001, 58, 430–434. [Google Scholar] [CrossRef]
- Wright, J.C. Critical variables associated with nonbiodegradable osmotically controlled implants. AAPS J. 2010, 12, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Shore, N. Introducing Vantas: The First Once-Yearly Luteinising Hormone-Releasing Hormone Agonist. Eur. Urol. Suppl. 2010, 9, 701–705. [Google Scholar] [CrossRef]
- Lewis, K.A.; Goldyn, A.K.; West, K.W.; Eugster, E.A. A single histrelin implant is effective for 2 years for treatment of central precocious puberty. J. Pediatr. 2013, 163, 1214–1216. [Google Scholar] [CrossRef] [PubMed]
- Olson-Kennedy, J.; Streeter, L.H.; Garofalo, R.; Chan, Y.M.; Rosenthal, S.M. Histrelin Implants for Suppression of Puberty in Youth with Gender Dysphoria: A Comparison of 50 mcg/Day (Vantas) and 65 mcg/Day (SupprelinLA). Transgend. Health 2021, 6, 36–42. [Google Scholar] [CrossRef]
- Miller, B.S.; Shukla, A.R. Sterile abscess formation in response to two separate branded long-acting gonadotropin-releasing hormone agonists. Clin. Ther. 2010, 32, 1749–1751. [Google Scholar] [CrossRef] [PubMed]
- Barnwal, P.; Das, S.; Mondal, S.; Ramasamy, A.; Maiti, T.; Saha, A. Probuphine(R) (buprenorphine implant): A promising candidate in opioid dependence. Ther. Adv. Psychopharm. 2017, 7, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Frost, M.; Bobb, R. Buprenorphine Implant Removal 7 Years Postinsertion: A Case Report. J. Addict. Med. 2019, 13, 79–80. [Google Scholar] [CrossRef]
- Johnston, J.; Reich, S.; Bailey, A.; Sluetz, J. Shiley INFUSAID Pump technology. Ann. N. Y. Acad. Sci. 1988, 531, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Hohn, D.C.; Rayner, A.A.; Economou, J.S.; Ignoffo, R.J.; Lewis, B.J.; Stagg, R.J. Toxicities and complications of implanted pump hepatic arterial and intravenous floxuridine infusion. Cancer 1986, 57, 465–470. [Google Scholar] [CrossRef]
- Haq, M.M.; Valdes, L.G.; Peterson, D.F.; Gourley, W.K. Fibrosis of Extrahepatic Biliary System after Continuous Hepatic-Artery Infusion of Floxuridine through an Implantable Pump (Infusaid Pump). Cancer 1986, 57, 1281–1283. [Google Scholar] [CrossRef]
- Broussolle, C.; Jeandidier, N.; Hanaire-Broutin, H. French multicentre experience of implantable insulin pumps. The EVADIAC Study Group. Evaluation of Active Implants in Diabetes Society. Lancet 1994, 343, 514–515. [Google Scholar] [CrossRef]
- Teddy, P.; Jamous, A.; Gardner, B.; Wang, D.; Silver, J. Complications of intrathecal baclofen delivery. Br. J. Neurosurg. 1992, 6, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Farid, R.; Binz, K.; Emerson, J.A.; Murdock, F. Accuracy and Precision of the SynchroMed II Pump. Neuromodulation 2019, 22, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Bourge, R.C.; Waxman, A.B.; Gomberg-Maitland, M.; Shapiro, S.M.; Tarver, J.H., 3rd; Zwicke, D.L.; Feldman, J.P.; Chakinala, M.M.; Frantz, R.P.; Torres, F.; et al. Treprostinil Administered to Treat Pulmonary Arterial Hypertension Using a Fully Implantable Programmable Intravascular Delivery System: Results of the DelIVery for PAH Trial. Chest 2016, 150, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Taira, T.; Ueta, T.; Katayama, Y.; Kimizuka, M.; Nemoto, A.; Mizusawa, H.; Liu, M.; Koito, M.; Hiro, Y.; Tanabe, H. Rate of Complications Among the Recipients of Intrathecal Baclofen Pump in J apan: A Multicenter Study. Neuromodul. Technol. Neural Interface 2013, 16, 266–272. [Google Scholar] [CrossRef] [PubMed]
- North, R.B.; Cutchis, P.N.; Epstein, J.A.; Long, D.M. Spinal cord compression complicating subarachnoid infusion of morphine: Case report and laboratory experience. Neurosurgery 1991, 29, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Johansen, M.J.; Gradert, T.L.; Satterfield, W.C.; Baze, W.B.; Hildebrand, K.; Trissel, L.; Hassenbusch, S.J. Safety of continuous intrathecal midazolam infusion in the sheep model. Anesth. Analg. 2004, 98, 1528–1535. [Google Scholar] [CrossRef]
- Michael, A.; Buffen, E.; Rauck, R.; Anderson, W.; McGirt, M.; Mendenhall, H.V. An in vivo canine study to assess granulomatous responses in the MedStream Programmable Infusion System (TM) and the SynchroMed II Infusion System(R). Pain Med. 2012, 13, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Borrini, L.; Bensmail, D.; Thiebaut, J.B.; Hugeron, C.; Rech, C.; Jourdan, C. Occurrence of adverse events in long-term intrathecal baclofen infusion: A 1-year follow-up study of 158 adults. Arch. Phys. Med. Rehabil. 2014, 95, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; Rawlins, P.K. The diagnosis of intrathecal infusion pump system failure. Pain Physician 2005, 8, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Gofeld, M.; McQueen, C.K. Ultrasound-guided intrathecal pump access and prevention of the pocket fill. Pain Med. 2011, 12, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Jones, R. Spinal Cord Stimulation and Implanted Intrathecal Drug Infusion. In Pain Procedures in Clinical Practice; Elsevier: Philadelphia, PA, USA, 2011; pp. 483–506. [Google Scholar]
- Delhaas, E.M.; Harhangi, B.S.; Frankema, S.P.G.; Huygen, F.; van der Lugt, A. Plain radiography in patients treated with intrathecal drug delivery using an implantable pump device. Insights Imaging 2017, 8, 499–511. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Intera 3000. Available online: https://interaoncology.com/intera-3000 (accessed on 3 December 2021).
- Manickavasagam, D.; Oyewumi, M.O. Critical assessment of implantable drug delivery devices in glaucoma management. J. Drug Deliv. 2013, 2013, 895013. [Google Scholar] [CrossRef] [PubMed]
- Geroski, D.H.; Edelhauser, H.F. Transscleral drug delivery for posterior segment disease. Adv. Drug Deliv. Rev. 2001, 52, 37–48. [Google Scholar] [CrossRef]
- Abdelkader, H.; Fathalla, Z.; Seyfoddin, A.; Farahani, M.; Thrimawithana, T.; Allahham, A.; Alani, A.W.G.; Al-Kinani, A.A.; Alany, R.G. Polymeric long-acting drug delivery systems (LADDS) for treatment of chronic diseases: Inserts, patches, wafers, and implants. Adv. Drug Deliv. Rev. 2021, 177, 113957. [Google Scholar] [CrossRef] [PubMed]
- El-Ghrably, I.A.; Saad, A.; Dinah, C. A Novel Technique for Repositioning of a Migrated ILUVIEN (R) (Fluocinolone Acetonide) Implant into the Anterior Chamber. Ophthalmol. Ther. 2015, 4, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Rofagha, S.; Bhisitkul, R.B.; Boyer, D.S.; Sadda, S.R.; Zhang, K.; Group, S.-U.S. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: A multicenter cohort study (SEVEN-UP). Ophthalmology 2013, 120, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.W.; Feng, X.; Wabner, K.; Conston, S.R.; Sierra, D.H.; Folden, D.V.; Smith, M.E.; Cameron, J.D. Cannulation of the suprachoroidal space: A novel drug delivery methodology to the posterior segment. Am. J. Ophthalmol. 2006, 142, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, C.L.; Santini, J.T., Jr.; Langer, R. Reservoir-based drug delivery systems utilizing microtechnology. Adv. Drug Deliv. Rev. 2012, 64, 1590–1602. [Google Scholar] [CrossRef]
- Jervis, L. A summary of recent advances in ocular inserts and implants. J. Bioequiv. Bioavailab. 2017, 9, 320–323. [Google Scholar]
- Musch, D.C.; Martin, D.F.; Gordon, J.F.; Davis, M.D.; Kuppermann, B.D. Treatment of cytomegalovirus retinitis with a sustained-release ganciclovir implant. The Ganciclovir Implant Study Group. N. Engl. J. Med. 1997, 337, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.I.; Wolitz, R.A.; Dowling, A.H.; Bloom, H.R.; Irvine, A.R.; Schwartz, D.M. Visual and anatomic outcomes associated with posterior segment complications after ganciclovir implant procedures in patients with AIDS and cytomegalovirus retinitis. Am. J. Ophthalmol. 1999, 127, 288–293. [Google Scholar] [CrossRef]
- Lallemand, F.; Felt-Baeyens, O.; Besseghir, K.; Behar-Cohen, F.; Gurny, R. Cyclosporine A delivery to the eye: A pharmaceutical challenge. Eur. J. Pharm. Biopharm. 2003, 56, 307–318. [Google Scholar] [CrossRef]
- Nicholson, B.P.; Singh, R.P.; Sears, J.E.; Lowder, C.Y.; Kaiser, P.K. Evaluation of fluocinolone acetonide sustained release implant (Retisert) dissociation during implant removal and exchange surgery. Am. J. Ophthalmol. 2012, 154, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, G.J.; Martin, D.; Callanan, D.; Pearson, P.A.; Levy, B.; Comstock, T.; Fluocinolone Acetonide Uveitis Study Group. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: Thirty-four-week results of a multicenter randomized clinical study. Ophthalmology 2006, 113, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Berger, B.B. Fluocinolone Implant for Idiopathic Non-Infectious Posterior Uveitis. Retinal Physician, May 2012; pp. 2–6. [Google Scholar]
- Sims, J.L.; Chee, S.P. Cytomegalovirus endotheliitis following fluocinolone acetonide (Retisert) implant. Eye 2010, 24, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Park, U.C.; Kim, S.J.; Yu, H.G. Cytomegalovirus endotheliitis after fluocinolone acetonide (Retisert) implant in a patient with Behcet uveitis. Ocul. Immunol. Inflamm. 2011, 19, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Callanan, D.G.; Jaffe, G.J.; Martin, D.F.; Pearson, P.A.; Comstock, T.L. Treatment of posterior uveitis with a fluocinolone acetonide implant: Three-year clinical trial results. Arch. Ophthalmol. 2008, 126, 1191–1201. [Google Scholar] [CrossRef]
- Borkar, D.S.; Ung, C.; Sobrin, L. Sustained Release Corticosteroid Therapy for Noninfectious Uveitis. Int. Ophthalmol. Clin. 2017, 57, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Logan, S.A.; Weng, C.Y.; Carvounis, P.E. Intravitreal Steroid Implants in the Management of Retinal Disease and Uveitis. Int. Ophthalmol. Clin. 2016, 56, 127–149. [Google Scholar] [CrossRef] [PubMed]
- Yasin, M.N.; Svirskis, D.; Seyfoddin, A.; Rupenthal, I.D. Implants for drug delivery to the posterior segment of the eye: A focus on stimuli-responsive and tunable release systems. J. Control. Release 2014, 196, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Massa, H.; Nagar, A.M.; Vergados, A.; Dadoukis, P.; Patra, S.; Panos, G.D. Intravitreal fluocinolone acetonide implant (ILUVIEN®) for diabetic macular oedema: A literature review. J. Int. Med Res. 2019, 47, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Papastavrou, V.T.; Zambarakji, H.; Dooley, I.; Eleftheriadis, H.; Jackson, T.L. Observation: Fluocinolone acetonide (ILUVIEN) implant migration into the anterior chamber. Retin. Cases brief Rep. 2017, 11, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Testi, I.; Pavesio, C. Preliminary evaluation of YUTIQ (fluocinolone acetonide intravitreal implant 0.18 mg) in posterior uveitis. Ther. Deliv. 2019, 10, 621–625. [Google Scholar] [CrossRef]
- Williams, G.A.; Haller, J.A.; Kuppermann, B.D.; Blumenkranz, M.S.; Weinberg, D.V.; Chou, C.; Whitcup, S.M.; Dexamethasone DDS Phase II Study Group. Dexamethasone posterior-segment drug delivery system in the treatment of macular edema resulting from uveitis or Irvine-Gass syndrome. Am. J. Ophthalmol. 2009, 147, 1048–1054. [Google Scholar] [CrossRef]
- Chang-Lin, J.E.; Attar, M.; Acheampong, A.A.; Robinson, M.R.; Whitcup, S.M.; Kuppermann, B.D.; Welty, D. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Investig. Ophthalmol. Vis. Sci. 2011, 52, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Fassbender Adeniran, J.M.; Jusufbegovic, D.; Schaal, S. Common and Rare Ocular Side-effects of the Dexamethasone Implant. Ocul. Immunol. Inflamm. 2017, 25, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Malcles, A.; Dot, C.; Voirin, N.; Vie, A.L.; Agard, E.; Bellocq, D.; Denis, P.; Kodjikian, L. Safety of intravitreal dexamethasone implant (ozurdex): The SAFODEX study. Incidence and Risk Factors of Ocular Hypertension. Retina 2017, 37, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Dugel, P.; Eliott, D.; Cantrill, H.; Mahmoud, T.; Avery, R.; Erickson, S. I-VationTM TA: 24-month clinical results of the phase I safety and preliminary efficacy study. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4332. [Google Scholar]
- Bakri, S.J.; Alniemi, S.T. Fibrotic encapsulation of a dexamethasone intravitreal implant following vitrectomy and silicone oil for rhegmatogenous retinal detachment. Ophthalmic. Surg. Lasers Imaging Retin. 2014, 45, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Fernandez-Sanz, G.; Bala, S.; Addison, P.K. Desegmentation of Ozurdex implant in vitreous cavity: Report of two cases. Br. J. Ophthalmol. 2014, 98, 961–963. [Google Scholar] [CrossRef] [PubMed]
- Eadie, J.A.; Lesser, R.; Capone, A., Jr. Migration of Ozurdex implant into the anterior chamber. Retin. Cases Brief Rep. 2012, 6, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Lopez, D.; Frances-Munoz, E.; Gallego-Pinazo, R.; Diaz-Llopis, M. Anterior chamber migration of dexametasone intravitreal implant (Ozurdex(R)). Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 1703–1704. [Google Scholar] [CrossRef]
- Suner, I.J.; Peden, M.C. Dexamethasone Sustained-Release Intracanalicular Insert for Control of Postoperative Inflammation After Pars Plana Vitrectomy. Clin. Ophthalmol. 2021, 15, 3859–3864. [Google Scholar] [CrossRef] [PubMed]
- Walters, T.; Bafna, S. Efficacy and Safety of Sustained Release Dexamethasone for the Treatment of Ocular Pain and Inflammation after Cataract Surgery: Results from Two Phase 3 Studies. J. Clin. Exp. Ophthalmol. 2016, 7, 1000572. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, A.; Joshi, M.; Christoforidis, J. Drug delivery implants in the treatment of vitreous inflammation. Mediat. Inflamm. 2013, 2013, 780634. [Google Scholar] [CrossRef]
- Holekamp, N.M.; Campochiaro, P.A.; Chang, M.A.; Miller, D.; Pieramici, D.; Adamis, A.P.; Brittain, C.; Evans, E.; Kaufman, D.; Maass, K.F.; et al. Archway Randomized Phase 3 Trial of the Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2021. [Google Scholar] [CrossRef] [PubMed]
- Nayak, K.; Misra, M. A review on recent drug delivery systems for posterior segment of eye. Biomed. Pharmacother. 2018, 107, 1564–1582. [Google Scholar] [CrossRef]
- Thrimawithana, T.R.; Young, S.; Bunt, C.R.; Green, C.; Alany, R.G. Drug delivery to the posterior segment of the eye. Drug Discov. Today 2011, 16, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Donnenfeld, E.; Holland, E. Dexamethasone Intracameral Drug-Delivery Suspension for Inflammation Associated with Cataract Surgery: A Randomized, Placebo-Controlled, Phase III Trial. Ophthalmology 2018, 125, 799–806. [Google Scholar] [CrossRef]
- Hu, M.; Huang, G.; Karasina, F.; Wong, V.G. VerisomeTM, a Novel injectable, Sustained Release, Biodegradable, Intraocular Drug Delivery System and Triamcinolone Acetonide. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5627. [Google Scholar]
- Haghjou, N.; Soheilian, M.; Abdekhodaie, M.J. Sustained release intraocular drug delivery devices for treatment of uveitis. J. Ophthalmic. Vis. Res. 2011, 6, 317–329. [Google Scholar]
- Zhang, K.; Hopkins, J.J.; Heier, J.S.; Birch, D.G.; Halperin, L.S.; Albini, T.A.; Brown, D.M.; Jaffe, G.J.; Tao, W.; Williams, G.A. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 6241–6245. [Google Scholar] [CrossRef] [PubMed]
- Ciolino, J.B.; Stefanescu, C.F.; Ross, A.E.; Salvador-Culla, B.; Cortez, P.; Ford, E.M.; Wymbs, K.A.; Sprague, S.L.; Mascoop, D.R.; Rudina, S.S.; et al. In vivo performance of a drug-eluting contact lens to treat glaucoma for a month. Biomaterials 2014, 35, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.P.; Adamson, D.C. Current FDA-Approved Therapies for High-Grade Malignant Gliomas. Biomedicines 2021, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Champeaux, C.; Weller, J. Implantation of carmustine wafers (Gliadel((R))) for high-grade glioma treatment. A 9-year nationwide retrospective study. J. Neurooncol. 2020, 147, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Attenello, F.J.; Mukherjee, D.; Datoo, G.; McGirt, M.J.; Bohan, E.; Weingart, J.D.; Olivi, A.; Quinones-Hinojosa, A.; Brem, H. Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: A 10-year institutional experience. Ann. Surg. Oncol. 2008, 15, 2887–2893. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers. J. Control. Release 2012, 159, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Muntasser, H.; Liaquat, I.; Barlow, A.; Whittle, I. Gliadel wafers acting as a lattice for bacterial growth: A case illustration. Acta Neurochir. 2011, 153, 2099–2100. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, I.; Hanihara, M.; Watanabe, T.; Dan, M.; Sato, S.; Kuroda, H.; Inamura, A.; Inukai, M.; Hara, A.; Yasui, Y.; et al. Tumor microenvironment after biodegradable BCNU wafer implantation: Special consideration of immune system. J. Neurooncol. 2018, 137, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, I.; Miyasaka, K.; Sekiguchi, A.; Ishiyama, H.; Inukai, M.; Yasui, Y.; Watanabe, T.; Sato, S.; Hide, T.; Kumabe, T. Long-term follow-up after BCNU wafer implantation in patients with newly diagnosed glioblastoma. J. Clin. Neurosci. 2021, 86, 202–210. [Google Scholar] [CrossRef]
- Sato, K.; Dan, M.; Yamamoto, D.; Miyajima, Y.; Hara, A.; Kumabe, T. Chronic Phase Intracranial Hemorrhage Caused by Ruptured Pseudoaneurysm Induced by Carmustine Wafer Implantation for Insulo-opercular Anaplastic Astrocytoma: A Case Report. Neurol. Med. Chir. 2015, 55, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Manoukian, O.S.; Baker, J.T.; Rudraiah, S.; Arul, M.R.; Vella, A.T.; Domb, A.J.; Kumbar, S.G. Functional polymeric nerve guidance conduits and drug delivery strategies for peripheral nerve repair and regeneration. J. Control. Release 2020, 317, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Ziemba, A.M.; Gilbert, R.J. Biomaterials for Local, Controlled Drug Delivery to the Injured Spinal Cord. Front. Pharmacol. 2017, 8, 245. [Google Scholar] [CrossRef]
- Wessely, R. New drug-eluting stent concepts. Nat. Rev. Cardiol. 2010, 7, 194–203. [Google Scholar] [CrossRef]
- Shlofmitz, E.; Iantorno, M.; Waksman, R. Restenosis of Drug-Eluting Stents: A New Classification System Based on Disease Mechanism to Guide Treatment and State-of-the-Art Review. Circ. Cardiovasc. Interv. 2019, 12, e007023. [Google Scholar] [CrossRef]
- Mei, X.; Cheng, K. Recent Development in Therapeutic Cardiac Patches. Front. Cardiovasc. Med. 2020, 7, 610364. [Google Scholar] [CrossRef] [PubMed]
- Torii, S.; Jinnouchi, H.; Sakamoto, A.; Kutyna, M.; Cornelissen, A.; Kuntz, S.; Guo, L.; Mori, H.; Harari, E.; Paek, K.H.; et al. Drug-eluting coronary stents: Insights from preclinical and pathology studies. Nat. Rev. Cardiol. 2020, 17, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, D.; Raczynska, D.; Raczynska, K. Five-month observation of persistent diabetic macular edema after intravitreal injection of Ozurdex implant. Mediat. Inflamm. 2014, 2014, 364143. [Google Scholar] [CrossRef] [PubMed]
- Fayzullin, A.; Churbanov, S.; Ignatieva, N.; Zakharkina, O.; Tokarev, M.; Mudryak, D.; Khristidis, Y.; Balyasin, M.; Kurkov, A.; Golubeva, E.N.; et al. Local Delivery of Pirfenidone by PLA Implants Modifies Foreign Body Reaction and Prevents Fibrosis. Biomedicines 2021, 9, 853. [Google Scholar] [CrossRef] [PubMed]





| IDDS | FDA Approval Date | Drug 1 | Drug Class 2 | Drug Release Duration | Material 3 | Indication |
|---|---|---|---|---|---|---|
| Testopel | 1972 | TS | Hormone | 4–6 months | PVP | Testosterone deficiency syndrome |
| Norplant (Jadelle) | 1990 | LG | PG | 5 years | Silicone, PDMS | Pregnancy control |
| Implanon (Nexplanon) | 2006 | EG | PG | 3–5 years | EVA | Pregnancy control |
| Viadur | 200 | LP | GTRHA | 1 year | Titanium | Prostate cancer |
| Vantas/ Supprelin LA | 2004/2007 | HS | GTRHA | 1 year | EVA | Advanced prostate cancer/central precocious puberty |
| Probuphine | 2016 | BPH | Opioid | 6 months | EVA | Opioid use disorder |
| Implant | FDA Approval Date | Drug | Indication |
|---|---|---|---|
| Infusaid pump | 1982 | Heparin, floxuridine, fluorouracil, amikacin | Recurrent thromboembolic disease, hepatic arterial infusions for tumor site, osteomyelitis |
| SynchroMed (SynchroMed II) | 1991 | Baclofen, Morphine, Ziconotide, Treprostinil | Severe spasticity, pain management, pulmonary arterial hypertension |
| Intera 3000 (Codman 3000) | 2011 (1996) | Morphine, baclofen, floxuridine | Pain management, hepatic arterial infusions for tumor site |
| Prometra II | 2012 | Morphine, baclofen | Severe spasticity, pain management |
| Implant | FDA Approval Date | Drug | Drug Class 1 | Release Duration | Material 2 | Indication |
|---|---|---|---|---|---|---|
| Vitrasert | 1996 | Ganciclovir | NSA | 5–8 months | PVA, EVA | Cytomegalovirus retinitis |
| Retisert | 2005 | Fluocinolone acetonide | GC | 2.5 years | PVA, Silicone | Noninfectious posterior uveitis, diabetic macular edema, central retinal vein occlusion |
| Ozurdex | 2009 | Dexamethasone | GC | 6 months | PLGA | Retinal vein occlusion, uveitis, diabetic macular edema |
| Iluvien | 2014 | Fluocinolone acetonide | GC | 3 years | PI | Diabetic macular edema, retinal vein occlusion |
| YUTIQ | 2018 | Fluocinolone acetonide | GC | 3 years | PI | Posterior segment uveitis |
| Dextenza | 2018 | Dexamethasone | GC | 1 month | PEG | Postoperative ocular inflammation, conjunctivitis, allergy |
| BIM Ring (Durysta) | 2020 | Bimatoprost | SAPG | 6 months | Silicone, PP | Glaucoma |
| Susvimo | 2021 | Ranibizumab | a-VEGF MAB | 6 months | PSu, Silicone | Neovascular age-related macular degeneration |
| Implant | FDA Approval Date | Drug 1 | Release Duration | Material 2 | Biodegradability |
|---|---|---|---|---|---|
| Cypher | 2003 | SM | 3 months | Stainless steel, PEVA/PBMA | Permanent |
| Taxus Express | 2004 | PTX | 6 months | Stainless steel, SIBS | Permanent |
| Xience Alpine | 2014 | ELM | 4 months | CoCr, PVDF-HFP | Permanent |
| Resolute Integrity | 2012 | ZLM | 6 months | CoNi, BioLinx | Permanent |
| Orsiro | 2019 | SM | 4 months | CoCr, PLLA | Coating biodegrades after 15 months |
| Synergy | 2015 | ELM | 3 months | PtCr, PLGA | Coating biodegrades after 4 months |
| Absorb GT1 Bioresorbable Vascular Scaffold System | 2016 | ELM | 3 months | PLLA, Pt markers | Stent degrades after >24 months |
| IDDS | Biodegradability/ Bio-Erodibility | Requires Removal? | Type of Implant |
|---|---|---|---|
| Norplant | No | Yes | Reservoir implant in PDMS tubing |
| Jadelle | No | Yes | Reservoir in PDMS core with silicone sheath |
| Implanon/ Nexplanon | No | Yes | Reservoir in EVA core and sheath |
| Testopel | Yes | No | Matrix in PVP |
| Viadur | No | Yes | Titanium cylinder with osmotically driven drug release (osmotic pump) |
| Vantas/ Supprelin LA | No | Yes | Reservoir in EVA |
| Probuphine | No | Yes | Matrix in EVA |
| Infusaid pump | No | Yes | Infusion pump with gas-mediated constant release |
| SynchroMed (SynchroMed II) | No | Yes | Peristaltic pumps |
| Codman 3000 (Intera 3000) | No | Yes | Infusion pumps without battery |
| Prometra II | No | Yes | Infusion pumps with battery |
| Vitrasert/ Retisert | No | Yes | Reservoir in drug pellet in PVOH/ EVA coating |
| Ozurdex | Yes | No | Matrix in PLGA |
| Iluvien/ YUTIQ | No | No | Reservoir in PVOH core in polyimide sheath |
| Dextenza | Yes | No | PEG hydrogel matrix |
| Susvimo | No | No | Refillable permanent reservoir system |
| BIM Ring (Durysta)–to complete description | Yes | No | Injectable polymer matrix |
| Gliadel | Yes (slow rate) | No | Drug eluting polymer matrix |
| Cypher | No | No | Stent with permanent DEPC |
| Taxus Express | No | No | Stent with permanent DEPC |
| Xience Alpine | No | No | Stent with permanent DEPC |
| Resolute Integrity | No | No | Stent with permanent DEPC |
| Orsiro | Coating biodegrades after 15 months | No | Stent with biodegradable DEPC |
| Synergy | Coating biodegrades after 4 months | No | Stent with biodegradable DEPC |
| Absorb GT1 Bioresorbable Vascular Scaffold System | Stent degrades after >24 months | No | Stent with biodegradable DEPC |
| Type of IDDSs | Reported Biointegration Issues that May Be Linked to FBR | References |
|---|---|---|
| Subcutaneous | Implant migration | [43] |
| Difficulty with implant removal due to peri-implant scarring | [36] | |
| Implantation site aseptic inflammation or implant rejection | [51] | |
| Infusion pumps as IDDSs | Inflammatory and dystrophic changes in spinal cord | [34] |
| Fibrotic transformation of the skin pocket around metallic reservoir of the pump can affect the integrity of the reservoir–catheter connection and result in skin pocket fill with high doses of the drug | [67] | |
| Ocular IDDSs | Poststeroid cataracts | [128] |
| Intracerebral IDDS (Gliadel) | Peri-implant inflammation | [116] |
| Drug-eluting Stents | Proliferation of neointima and thrombogenic complications (successfully prevented in modern IDDSs) | [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fayzullin, A.; Bakulina, A.; Mikaelyan, K.; Shekhter, A.; Guller, A. Implantable Drug Delivery Systems and Foreign Body Reaction: Traversing the Current Clinical Landscape. Bioengineering 2021, 8, 205. https://doi.org/10.3390/bioengineering8120205
Fayzullin A, Bakulina A, Mikaelyan K, Shekhter A, Guller A. Implantable Drug Delivery Systems and Foreign Body Reaction: Traversing the Current Clinical Landscape. Bioengineering. 2021; 8(12):205. https://doi.org/10.3390/bioengineering8120205
Chicago/Turabian StyleFayzullin, Alexey, Alesia Bakulina, Karen Mikaelyan, Anatoly Shekhter, and Anna Guller. 2021. "Implantable Drug Delivery Systems and Foreign Body Reaction: Traversing the Current Clinical Landscape" Bioengineering 8, no. 12: 205. https://doi.org/10.3390/bioengineering8120205
APA StyleFayzullin, A., Bakulina, A., Mikaelyan, K., Shekhter, A., & Guller, A. (2021). Implantable Drug Delivery Systems and Foreign Body Reaction: Traversing the Current Clinical Landscape. Bioengineering, 8(12), 205. https://doi.org/10.3390/bioengineering8120205








