Lower Limb Kinematics in Individuals with Hip Osteoarthritis during Gait: A Focus on Adaptative Strategies and Interlimb Symmetry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
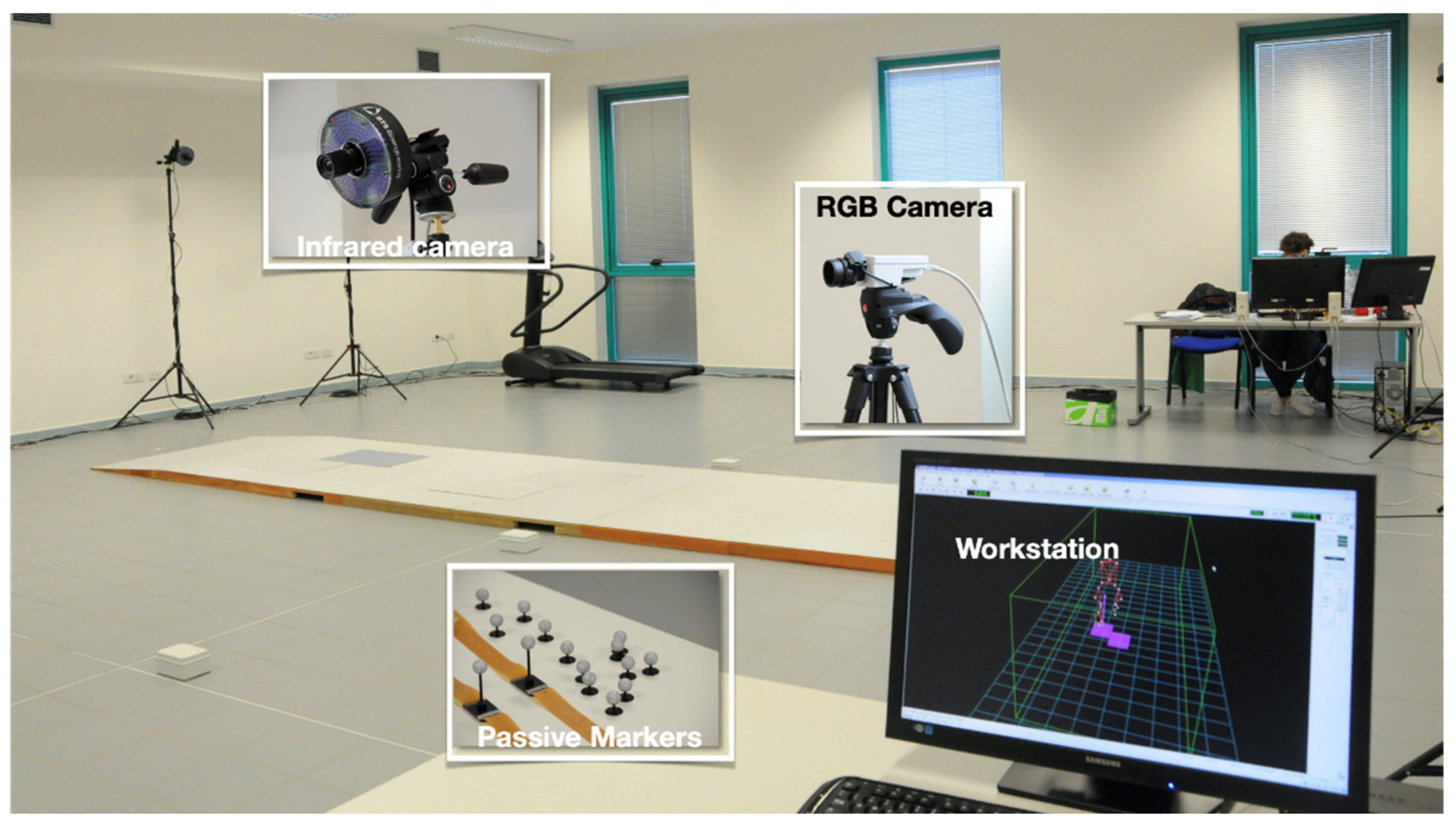
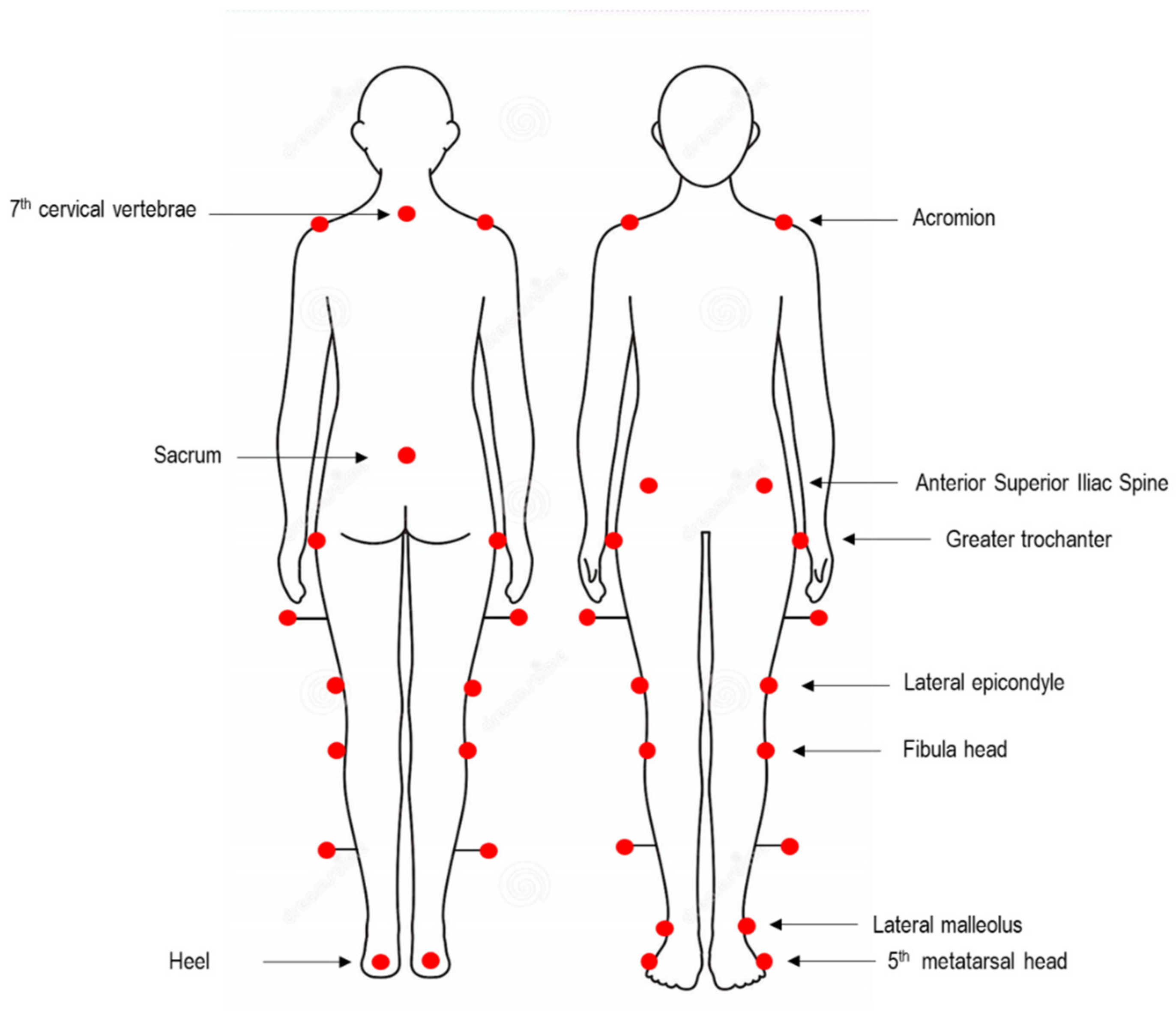
2.2. Spatio-Temporal and Kinematic Data Collection and Processing
- Five spatiotemporal gait parameters, namely gait speed, cadence, step length, step width, stance, swing, and double support phase duration;
- Nine kinematic parameters: pelvic tilt, rotation, and obliquity; hip flexion-extension, adduction-abduction, and rotation; knee flexion-extension, ankle dorsi-plantarflexion, and foot progression. From these, summary indexes of gait deviation from normality (Gait Variable Score GVS and Gait Profile Score, GPS, [18]) were obtained. GPS and GVS were previously employed to characterize gait quality in individuals who underwent THR surgery [19];
- The dynamic range of motion (ROM) for hip and knee flexion-extension and ankle dorsi-plantarflexion. They were calculated as the difference between the maximum and minimum angle value recorded during the gait cycle;
- The kinematics of hip, knee, and ankle on the sagittal plane, i.e., hip and knee flexion-extension and ankle dorsi-plantarflexion angle variations during the gait cycle. Such data were also employed to calculate the interlimb symmetry parameters as described later in detail.
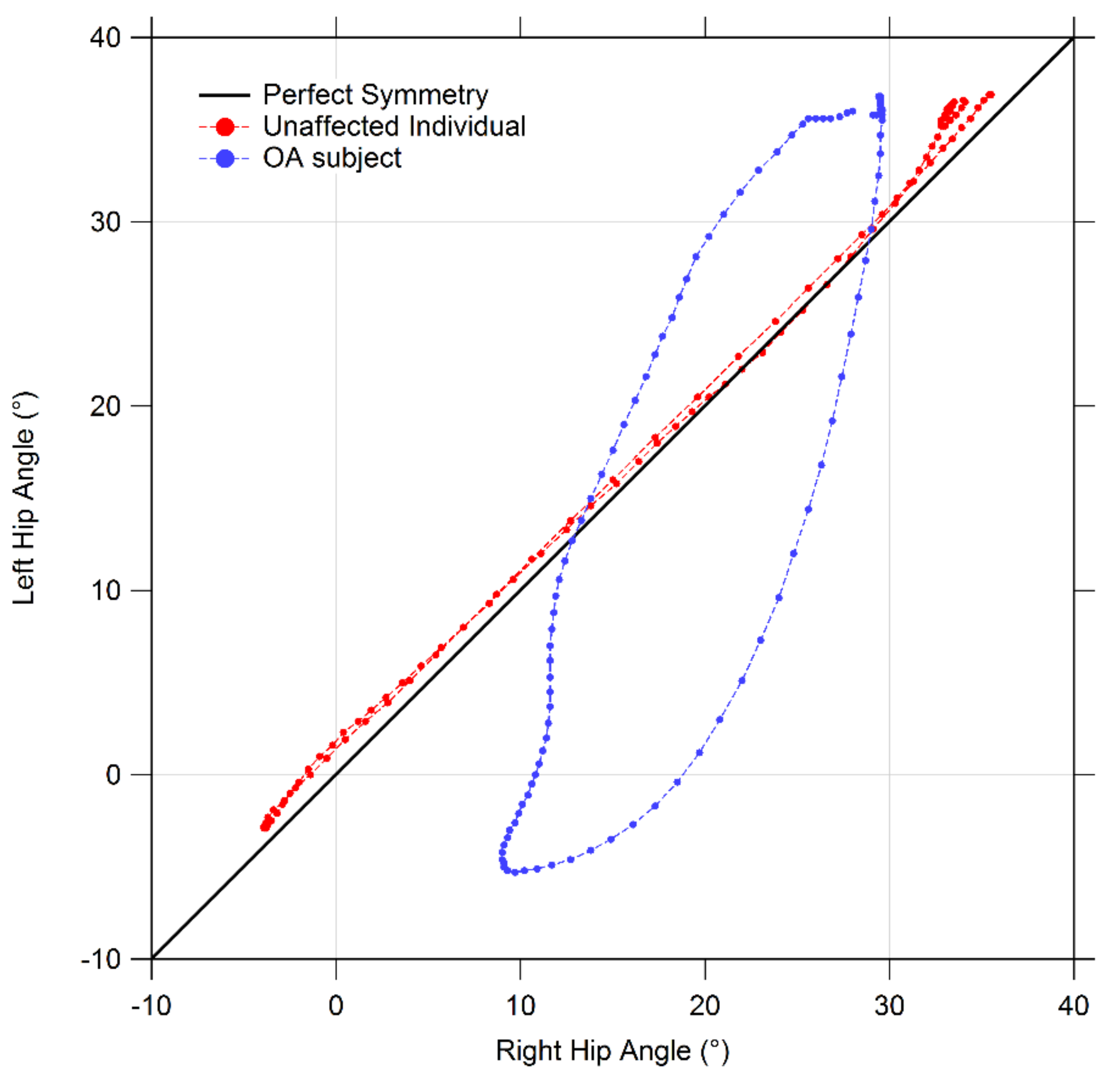
2.3. Gait Symmetry Quantification by Means of Cyclograms
- Cyclogram area (degrees2): the area enclosed by the curve obtained from each angle-angle diagram [21]. In the case of perfectly symmetrical gait, left and right joints assume the same angular position at a certain time, thus all cyclogram points lie on a 45° line in the diagram, making the area null. As asymmetry increases, so does the area;
- Cyclogram orientation (degrees): this parameter is expressed by the absolute value of angle ϕ formed by the 45° line (which corresponds to perfect interlimb symmetry) and the orientation of the principal axis of inertia, which corresponds to the minimum moment of inertia of the cyclogram [20,22]. The latter was calculated as the direction of the eigenvector of the matrix of inertia of the cyclogram points distribution in the x-y (i.e., left joint angle-right joint angle) reference system. Smaller values of this angle indicate higher interlimb symmetry. Higher interlimb symmetries are associated with smaller values of this angle;
- Trend Symmetry: this dimensionless parameter is calculated to assess the similarity between two waveforms (in our case time-normalized right leg and left leg angular trend across the gait cycles for each joint of interest) using an eigenvector analysis (see [23] for details of the mathematical procedure as proposed by Crenshaw et al.). In particular, it is obtained by dividing the variability about the eigenvector to the variability along the eigenvector. Trend Symmetry is not affected by the presence of a shift or by magnitude differences in the two waveforms. A null value indicates perfect symmetry, while increasing interlimb asymmetry corresponds to higher Trend Symmetry values.
2.4. Statistical Analysis
- Spatio-temporal parameters were analyzed after separating them into two groups: (i) gait speed, cadence, and step width (for which both limbs are involved) and (ii) stance, swing, double support phases, and step length, which can be analyzed distinguishing between limbs. The possible differences introduced by the presence of OA in spatiotemporal parameters were explored using a one-way multivariate analysis of variance (MANOVA) considering group (OA/CG) as the independent variable and the 3 parameters previously listed as dependent variables. In contrast, we ran a one-way MANOVA for the phase duration parameters considering the limb (affected, unaffected and CG) as independent variables and the 4 parameters previously mentioned as dependent variables.
- For dynamic ROM, GVS, and GPS analysis, two separate MANOVAs were carried out considering the limb (affected, unaffected, and CG) as the independent variable and the three ROM or the 9 GVS and GPS as dependent variables.
- The possible differences in joint kinematics were assessed by comparing the “angle vs. time” curves of the affected limb, the unaffected limb, and CG on a point-by-point basis using a one-way ANOVA for each of the 3 joints of interest, similarly to what was proposed by Bruening et al. [24]. It was thus possible to define the periods of the gait cycle in which significant differences associated with participants’ limb were present.
- Finally, possible differences introduced by OA in gait symmetry parameters were investigated using a MANOVA, considering group (OA/CG) as the independent variable and the 3 symmetry parameters previously listed as dependent variables.
3. Results
3.1. Spatio-Temporal Parameters of Gait
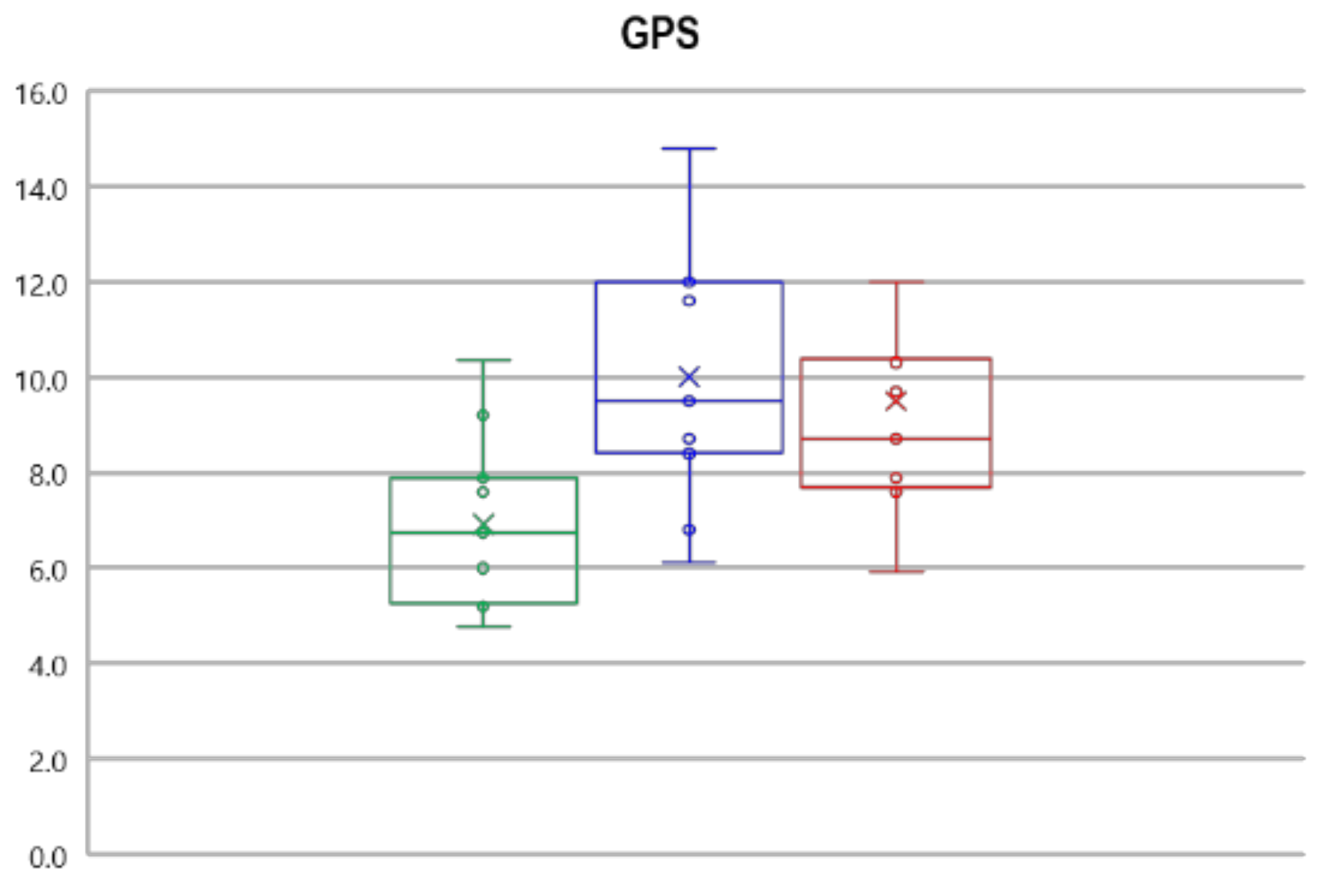
3.2. Gait Kinematics, GPS and GVS
3.3. Dynamic ROM
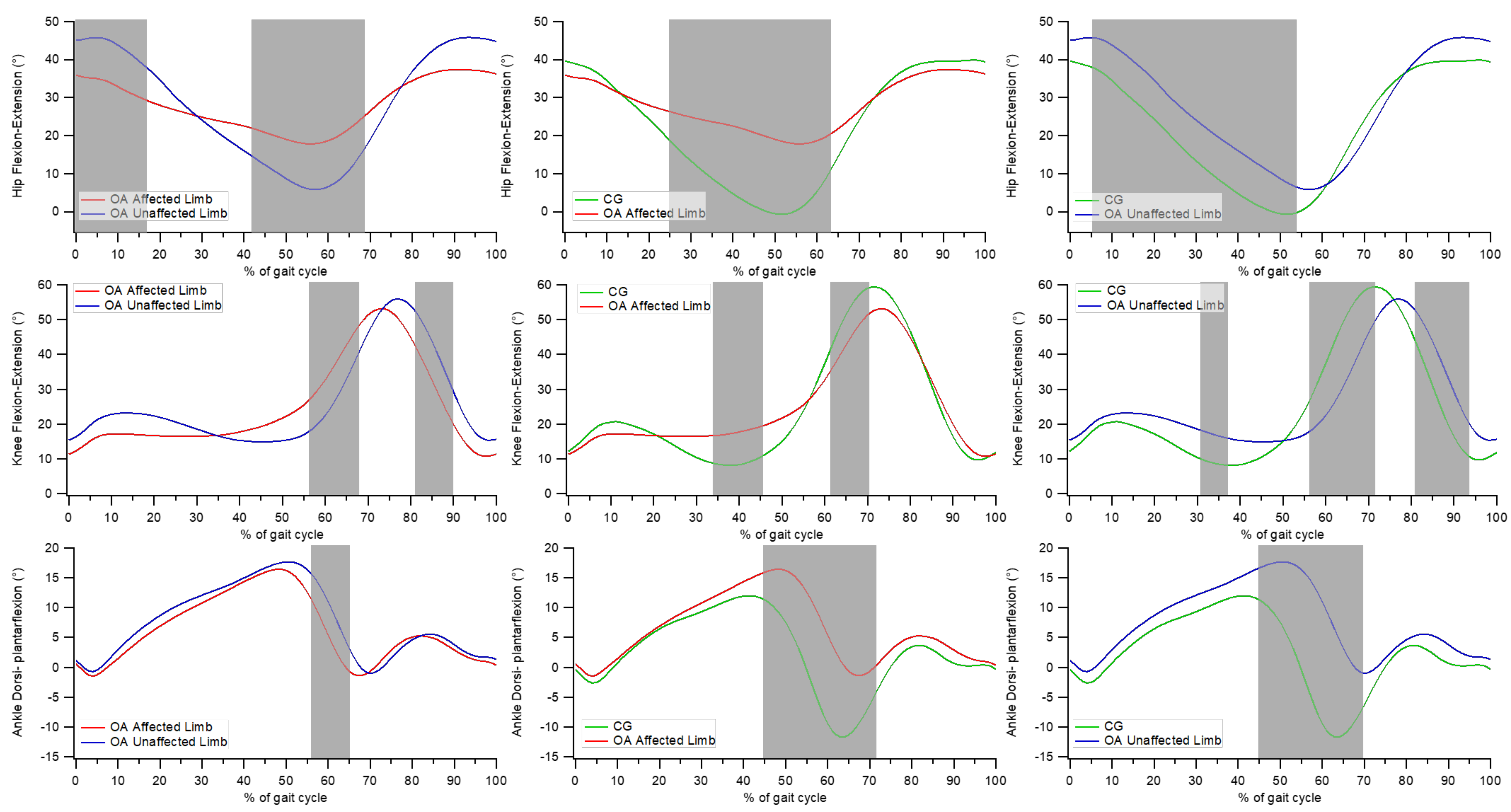
3.4. Point-by-Point Analysis of Kinematic Curves
- significant differences between the affected and unaffected limbs in individuals with OA at the hip joint from 0 to 17% and from 42 to 68% of the gait cycle, at the knee joint between 56 to 67% and between 81 and 90% of the gait cycle, and at the ankle joint from 56 to 65% of the gait cycle;
- significant differences between the affected limb of individuals with OA and CG at the hip joint from 25 to 64% of the gait cycle, at the knee joint between 34 and 46% and between 62 and 70% of the gait cycle, and at the ankle joint from 45 to 71% of the gait cycle;
- significant differences between the unaffected limb of individuals with OA and CG at the hip joint between 5 and 54% of the gait cycle; at the knee joint between 31–37%, 56–72%, and 81–94% of the gait cycle; and at the ankle joint between 45 and 70% of the gait cycle.
3.5. Symmetry Indexes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quintana, J.M.; Arostegui, I.; Escobar, A.; Azkarate, J.; Goenaga, J.I.; Lafuente, I. Prevalence of knee and hip osteoarthritis and the appropriateness of joint replacement in an older population. Arch. Intern. Med. 2008, 168, 1576–1584. [Google Scholar] [CrossRef]
- Zhang, Y.; Jordan, J.M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef] [Green Version]
- Heliövaara, M.; Mäkelä, M.; Impivaara, O.; Knekt, P.; Aromaa, A.; Sievers, K. Association of overweight; trauma and workload with coxarthrosis. A health survey of 7217 persons. Acta Orthop. Scand. 1993, 64, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T. Epidemiology of hip and knee osteoarthritis. Epidemiol. Rev. 1988, 10, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.D.; Chen, J.C.; Callahan, L.F.; Golightly, Y.M.; Helmick, C.G.; Renner, J.B.; Jordan, J.M. Associations of occupational tasks with knee and hip osteoarthritis: The Johnston County Osteoarthritis Project. J. Rheumatol. 2010, 37, 842–850. [Google Scholar] [CrossRef] [Green Version]
- Hochberg, M.C.; Lethbridge-Cejku, M. Epidemiologic considerations in the primary prevention of osteoarthritis. In Osteoarthritis: Public Health Implications for an Aging Population; Hamerman, D., Ed.; Johns Hopkins University Press: Baltimore, MD, USA, 1997; pp. 169–186. [Google Scholar]
- Vigdorchik, J.M.; Nepple, J.J.; Eftekhary, N.; Leunig, M.; Clohisy, J.C. What Is the Association of Elite Sporting Activities With the Development of Hip Osteoarthritis? Am. J. Sports Med. 2017, 45, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Hoaglund, F.T.; Steinbach, L.S. Primary osteoarthritis of the hip: Etiology and epidemiology. J. Am. Acad. Orthop. Surg. 2001, 9, 320–327. [Google Scholar] [CrossRef]
- Clynes, M.A.; Jameson, K.A.; Edwards, M.H.; Cooper, C.; Dennison, E.M. Impact of osteoarthritis on activities of daily living: Does joint site matter? Aging Clin. Exp. Res. 2019, 31, 1049–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahl, J.S.; Nelson, M.J.; Taylor, M.; Solomon, L.B.; Arnold, J.B.; Thewlis, D. Biomechanical changes and recovery of gait function after total hip arthroplasty for osteoarthritis: A systematic review and meta-analysis. Osteoarthr. Cartil. 2018, 26, 847–863. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.I.; Cha, Y.H.; Kim, K.J.; Kim, H.Y.; Choy, W.S.; Hwang, S.C. Gait analysis after total hip arthroplasty using direct anterior approach versus anterolateral approach: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2019, 20, 63. [Google Scholar] [CrossRef] [PubMed]
- Ornetti, P.; Laroche, D.; Morisset, C.; Beis, J.N.; Tavernier, C.; Maillefert, J.F. Three-dimensional kinematics of the lower limbs in hip osteoarthritis during walking. J. Back Musculoskelet. Rehabil. 2011, 24, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, D.; Buckingham, L.; Moreside, J.; Wong, I.; Richardson, G. Knee motion and muscle activation patterns are altered in hip osteoarthritis: The effect of severity on walking mechanics. Clin. Biomech. 2018, 59, 1–7. [Google Scholar] [CrossRef]
- Ismailidis, P.; Nüesch, C.; Kaufmann, M.; Clauss, M.; Pagenstert, G.; Eckardt, A.; Ilchmann, T.; Mündermann, A. Measuring gait kinematics in patients with severe hip osteoarthritis using wearable sensors. Gait Posture 2020, 81, 49–55. [Google Scholar] [CrossRef]
- Winiarski, S.; Rutkowska-Kucharska, A.; Pozowski, A.; Aleksandrowicz, K. A New Method of Evaluating the Symmetry of Movement Used to Assess the Gait of Patients after Unilateral Total Hip Replacement. Appl. Bionics Biomech. 2019, 2019, 7863674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, R.B.; Õunpuu, S.; Tyburski, D. A gait analysis data collection and reduction technique. Hum. Mov. Sci. 1991, 10, 575–587. [Google Scholar] [CrossRef]
- Baker, R.; McGinley, J.L.; Schwartz, M.H.; Beynon, S.; Rozumalski, A.; Graham, H.K.; Tirosh, O. The gait profile score and movement analysis profile. Gait Posture 2009, 30, 265–269. [Google Scholar] [CrossRef]
- Temporiti, F.; Zanotti, G.; Furone, R.; Molinari, S.; Zago, M.; Loppini, M.; Galli, M.; Grappiolo, G.; Gatti, R. Gait analysis in patients after bilateral versus unilateral total hip arthroplasty. Gait Posture 2019, 72, 46–50. [Google Scholar] [CrossRef]
- Goswami, A. Kinematic quantification of gait symmetry based on bilateral cyclograms. In Proceedings of the International Society of Biomechanics XIXth Congress, Dunedin, New Zealand, 6–11 July 2003. [Google Scholar]
- Hershler, C.; Milner, M. Angle-angle diagrams in the assessment of locomotion. Am. J. Phys. Med. 1980, 59, 109–125. [Google Scholar]
- Goswami, A. A new gait parameterization technique by means of cyclogram moments: Application to human slope walking. Gait Posture 1998, 8, 15–36. [Google Scholar] [CrossRef]
- Crenshaw, S.J.; Richards, J.G. A method for analyzing joint symmetry and normalcy, with an application to analyzing gait. Gait Posture 2006, 24, 515–521. [Google Scholar] [CrossRef]
- Bruening, D.A.; Frimenko, R.E.; Goodyear, C.D.; Bowden, D.R.; Fullenkamp, A.M. Sex differences in whole body gait kinematics at preferred speeds. Gait Posture 2015, 41, 540–545. [Google Scholar] [CrossRef] [Green Version]
- Ornetti, P.; Maillefert, J.F.; Laroche, D.; Morisset, C.; Dougados, M.; Gossec, L. Gait analysis as a quantifiable outcome measure in hip or knee osteoarthritis: A systematic review. Jt. Bone Spine 2010, 77, 421–425. [Google Scholar] [CrossRef]
- Constantinou, M.; Barrett, R.; Brown, M.; Mills, P. Spatial-temporal gait characteristics in individuals with hip osteoarthritis: A systematic literature review and meta-analysis. J. Orthop. Sports Phys. Ther. 2014, 44, 291-B7. [Google Scholar] [CrossRef] [Green Version]
- Hurwitz, D.E.; Hulet, C.H.; Andriacchi, T.P.; Rosenberg, A.G.; Galante, J.O. Gait compensations in patients with osteoarthritis of the hip and their relationship to pain and passive hip motion. J. Orthop. Res. 1997, 15, 629–635. [Google Scholar] [CrossRef]
- Zeni, J., Jr.; Pozzi, F.; Abujaber, S.; Miller, L. Relationship between physical impairments and movement patterns during gait in patients with end-stage hip osteoarthritis. J. Orthop. Res. 2015, 33, 382–389. [Google Scholar] [CrossRef]
- Foucher, K.C. Preoperative gait mechanics predict clinical response to total hip arthroplasty. J. Orthop. Res. 2017, 35, 366–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neto, V.E.P.; Rombaldi, A.J.; de Oliveira Saes, M.; Vinholes Siqueira, F. Physical activity and gait kinematics in the elderly. Revis. Braz. Ativ. Fís. Saúde 2015, 20, 243–251. [Google Scholar] [CrossRef]
- Wang, L.; Lee, M.; Zhang, Z.; Moodie, J.; Cheng, D.; Martin, J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2016, 6, e009857. [Google Scholar] [CrossRef] [PubMed]
- Rapp, W.; Brauner, T.; Weber, L.; Grau, S.; Mündermann, A.; Horstmann, T. Improvement of walking speed and gait symmetry in older patients after hip arthroplasty: A prospective cohort study. BMC Musculoskelet. Disord. 2015, 16, 291. [Google Scholar] [CrossRef] [Green Version]



| OA Group (5F, 6M) | Control Group (5F, 6M) | |
|---|---|---|
| Age (years) | 68.3 ± 5.8 | 67.8 ± 5.4 |
| Body mass (kg) | 75.4 ± 16.9 | 75.9 ± 10.1 |
| Height (cm) | 162.0 ± 5.4 | 165.9 ± 7.6 |
| Control Group | OA Unaffected Limb | OA Affected Limb | |
| Stance Phase | 60.17 ± 2.57 | 67.39 ± 2.62 * | 62.50 ± 4.83 † |
| Swing Phase | 39.08 ± 1.95 | 32.63 ± 2.61 * | 36.03 ± 4.70 *,† |
| Double Support Phase | 11.10 ± 1.99 | 17.07 ± 7.4 * | 16.22 ± 2.90 * |
| Step Length (m) | 0.60 ± 0.06 | 0.40 ± 0.07 * | 0.40 ± 0.06 * |
| Control Group | OA Group | ||
| Mean Velocity (m/s) | 1.05 ± 0.19 | 0.56 ± 0.12 * | |
| Cadence (step/min) | 105.94 ± 11.12 | 84.69 ± 12.00 * | |
| Step Width (m) | 0.21 ± 0.02 | 0.24 ± 0.03 | |
| Parameter | Control Group | OA Unaffected Limb | OA Affected Limb |
|---|---|---|---|
| GPS | 6.9 ± 1.7 | 10.0 ± 2.6 * | 9.5 ± 2.6 * |
| Pelvic Obliquity GVS | 2.2 ± 0.6 | 4.2 ± 1.5 * | 3.8 ± 1.0 * |
| Pelvic Tilt GVS | 4.7 ± 3.0 | 9.3 ± 6.6 | 8.6 ± 6.2 |
| Pelvic Rotation GVS | 3.7 ± 1.2 | 6.0 ± 2.3 * | 5.3 ± 1.6 |
| Hip Ab-Add GVS | 3.7 ± 1.0 | 6.1 ± 2.3* | 4.1 ± 1.4 |
| Hip Flex-Ext GVS | 7.7 ± 2.9 | 13.4 ± 7.0 | 15.5 ± 6.9 * |
| Hip Rotation GVS | 9.8 ± 3.3 | 9.5 ± 4.8 | 7.5 ± 3.0 |
| Knee Flex-Ext GVS | 9.0 ± 3.4 | 12.9 ± 3.0 | 11.6 ± 4.6 |
| Ankle Dorsi-Plantarflex GVS | 5.6 ± 1.4 | 10.5 ± 3.9 * | 8.9 ± 3.6 |
| Foot Progression GVS | 8.1 ± 4.0 | 7.9 ± 4.2 | 9.0 ± 3.8 |
| Control Group | OA Unaffected Limb | OA Affected Limb | |
|---|---|---|---|
| Hip | 42.8 ± 4.6 | 43.0 ± 8.4 | 20.9 ± 7.5 *,† |
| Knee | 54.2 ± 4.6 | 48.0 ± 7.1 * | 43.7 ± 7.2 * |
| Ankle | 26.5 ± 7.3 | 22.2 ± 3.9 | 21.0 ± 2.7 * |
| Cyclogram Parameter | Control Group | OA Group | |
|---|---|---|---|
| Hip | Area | 96.87 ± 79.6 | 169.60 ± 117.14 |
| Orientation ϕ | 3.53 ±3.95 | 20.25 ± 9.49 * | |
| Trend Symmetry | 0.37 ± 0.35 | 1.78 ± 1.24 * | |
| Knee | Area | 188.38 ± 232.21 | 677.38 ± 304.48 * |
| Orientation ϕ | 2.17 ± 2.48 | 5.11 ± 7.02 | |
| Trend Symmetry | 0.90 ± 1.33 | 8.42 ± 5.16 * | |
| Ankle | Area | 106.16 ± 72.88 | 80.46 ± 54.51 |
| Orientation ϕ | 3.36 ± 3.16 | 3.13 ± 1.52 | |
| Trend Symmetry | 3.30 ± 4.86 | 5.22 ± 4.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porta, M.; Pau, M.; Leban, B.; Deidda, M.; Sorrentino, M.; Arippa, F.; Marongiu, G. Lower Limb Kinematics in Individuals with Hip Osteoarthritis during Gait: A Focus on Adaptative Strategies and Interlimb Symmetry. Bioengineering 2021, 8, 47. https://doi.org/10.3390/bioengineering8040047
Porta M, Pau M, Leban B, Deidda M, Sorrentino M, Arippa F, Marongiu G. Lower Limb Kinematics in Individuals with Hip Osteoarthritis during Gait: A Focus on Adaptative Strategies and Interlimb Symmetry. Bioengineering. 2021; 8(4):47. https://doi.org/10.3390/bioengineering8040047
Chicago/Turabian StylePorta, Micaela, Massimiliano Pau, Bruno Leban, Michela Deidda, Marco Sorrentino, Federico Arippa, and Giuseppe Marongiu. 2021. "Lower Limb Kinematics in Individuals with Hip Osteoarthritis during Gait: A Focus on Adaptative Strategies and Interlimb Symmetry" Bioengineering 8, no. 4: 47. https://doi.org/10.3390/bioengineering8040047
APA StylePorta, M., Pau, M., Leban, B., Deidda, M., Sorrentino, M., Arippa, F., & Marongiu, G. (2021). Lower Limb Kinematics in Individuals with Hip Osteoarthritis during Gait: A Focus on Adaptative Strategies and Interlimb Symmetry. Bioengineering, 8(4), 47. https://doi.org/10.3390/bioengineering8040047








