Single-Use Bioreactors for Human Pluripotent and Adult Stem Cells: Towards Regenerative Medicine Applications
Abstract
:1. Introduction
2. Single-Use Bioreactors for Stem Cell Biomanufacturing
2.1. Single-Use Stirred-Tank Bioreactors
2.2. Fixed-Bed Bioreactors
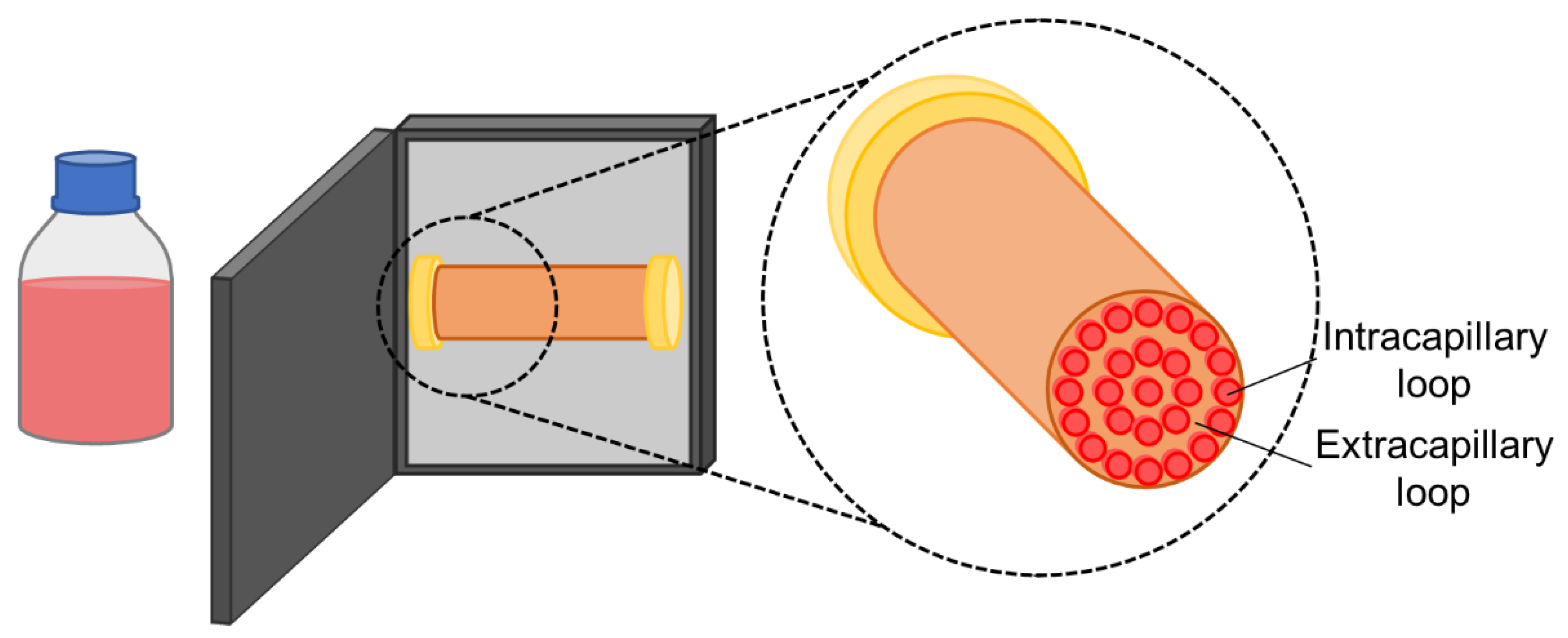
2.3. Hollow Fibre Bioreactors
2.4. Rotary Cell Culture Systems
2.5. Rotating Bed Bioreactors
2.6. Rocking Motion Bioreactors
2.7. Vertical-Wheel Bioreactors
3. Challenges of Single-Use Bioreactor-Based Processes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaiser, L.R. The future of multihospital systems. Top. Health Care Financ. 1992, 18, 32–45. [Google Scholar] [PubMed]
- Power, C.; Rasko, J.E. Whither prometheus’ liver? Greek myth and the science of regeneration. Ann. Intern. Med. 2008, 149, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.D. Regenerative medicine: Prometheus unbound. Nature 2004, 432, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Rowe, R.G.; Daley, G.Q. Induced pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Genet. 2019. [Google Scholar] [CrossRef]
- Dame, K.; Ribeiro, A.J. Microengineered systems with iPSC-derived cardiac and hepatic cells to evaluate drug adverse effects. Exp. Biol Med. 2021, 246, 317–331. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Huch, M. Disease modelling in human organoids. Dis Model. Mech 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Hass, R.; Kasper, C.; Bohm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Ren, H.; Han, Z. Mesenchymal stem cells: Immunomodulatory capability and clinical potential in immune diseases. J. Cell. Immunother. 2016, 2, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018, 2018, 3057624. [Google Scholar] [CrossRef]
- Gao, F.; Chiu, S.M.; Motan, D.A.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittenger, M.F.; Discher, D.E.; Peault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Fitzsimmons, R.E.B.; Mazurek, M.S.; Soos, A.; Simmons, C.A. Mesenchymal Stromal/Stem Cells in Regenerative Medicine and Tissue Engineering. Stem Cells Int. 2018, 2018, 8031718. [Google Scholar] [CrossRef]
- Moll, G.; Drzeniek, N.; Kamhieh-Milz, J.; Geissler, S.; Volk, H.D.; Reinke, P. MSC Therapies for COVID-19: Importance of Patient Coagulopathy, Thromboprophylaxis, Cell Product Quality and Mode of Delivery for Treatment Safety and Efficacy. Front. Immunol. 2020, 11, 1091. [Google Scholar] [CrossRef]
- Kabat, M.; Bobkov, I.; Kumar, S.; Grumet, M. Trends in mesenchymal stem cell clinical trials 2004-2018: Is efficacy optimal in a narrow dose range? Stem Cells Transl. Med. 2020, 9, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Melmed, G.Y.; Pandak, W.M.; Casey, K.; Abraham, B.; Valentine, J.; Schwartz, D.; Awais, D.; Bassan, I.; Lichtiger, S.; Sands, B.; et al. Human Placenta-derived Cells (PDA-001) for the Treatment of Moderate-to-severe Crohn’s Disease: A Phase 1b/2a Study. Inflamm. Bowel Dis. 2015, 21, 1809–1816. [Google Scholar] [CrossRef]
- Hess, D.C.; Wechsler, L.R.; Clark, W.M.; Savitz, S.I.; Ford, G.A.; Chiu, D.; Yavagal, D.R.; Uchino, K.; Liebeskind, D.S.; Auchus, A.P.; et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017, 16, 360–368. [Google Scholar] [CrossRef]
- Zweigerdt, R. Large scale production of stem cells and their derivatives. Eng. Stem Cells 2009, 114, 201–235. [Google Scholar] [CrossRef]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension: How 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef] [Green Version]
- Krtolica, A.; Genbacev, O.; Escobedo, C.; Zdravkovic, T.; Nordstrom, A.; Vabuena, D.; Nath, A.; Simon, C.; Mostov, K.; Fisher, S.J. Disruption of apical-basal polarity of human embryonic stem cells enhances hematoendothelial differentiation. Stem Cells 2007, 25, 2215–2223. [Google Scholar] [CrossRef]
- Schmideder, A.; Weuster-Botz, D. High-performance recombinant protein production with Escherichia coli in continuously operated cascades of stirred-tank reactors. J. Ind. Microbiol. Biotechnol. 2017, 44, 1021–1029. [Google Scholar] [CrossRef]
- van Lier, F.L.; van den End, E.J.; de Gooijer, C.D.; Vlak, J.M.; Tramper, J. Continuous production of baculovirus in a cascade of insect-cell reactors. Appl. Microbiol. Biotechnol. 1990, 33, 43–47. [Google Scholar] [CrossRef]
- Raven, N.; Rasche, S.; Kuehn, C.; Anderlei, T.; Klockner, W.; Schuster, F.; Henquet, M.; Bosch, D.; Buchs, J.; Fischer, R.; et al. Scaled-up manufacturing of recombinant antibodies produced by plant cells in a 200-L orbitally-shaken disposable bioreactor. Biotechnol. Bioeng. 2015, 112, 308–321. [Google Scholar] [CrossRef]
- Velez-Suberbie, M.L.; Tarrant, R.D.; Tait, A.S.; Spencer, D.I.; Bracewell, D.G. Impact of aeration strategy on CHO cell performance during antibody production. Biotechnol. Prog. 2013, 29, 116–126. [Google Scholar] [CrossRef]
- Rodrigues, C.A.; Fernandes, T.G.; Diogo, M.M.; da Silva, C.L.; Cabral, J.M. Stem cell cultivation in bioreactors. Biotechnol. Adv. 2011, 29, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Kropp, C.; Kempf, H.; Halloin, C.; Robles-Diaz, D.; Franke, A.; Scheper, T.; Kinast, K.; Knorpp, T.; Joos, T.O.; Haverich, A.; et al. Impact of Feeding Strategies on the Scalable Expansion of Human Pluripotent Stem Cells in Single-Use Stirred Tank Bioreactors. Stem Cells Transl. Med. 2016, 5, 1289–1301. [Google Scholar] [CrossRef] [Green Version]
- Kwok, C.K.; Ueda, Y.; Kadari, A.; Gunther, K.; Ergun, S.; Heron, A.; Schnitzler, A.C.; Rook, M.; Edenhofer, F. Scalable stirred suspension culture for the generation of billions of human induced pluripotent stem cells using single-use bioreactors. J. Tissue Eng. Regen. Med. 2018, 12, e1076–e1087. [Google Scholar] [CrossRef] [Green Version]
- Rafiq, Q.A.; Hanga, M.P.; Heathman, T.R.J.; Coopman, K.; Nienow, A.W.; Williams, D.J.; Hewitt, C.J. Process development of human multipotent stromal cell microcarrier culture using an automated high-throughput microbioreactor. Biotechnol. Bioeng. 2017, 114, 2253–2266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotondi, M.; Grace, N.; Betts, J.; Bargh, N.; Costariol, E.; Zoro, B.; Hewitt, C.J.; Nienow, A.W.; Rafiq, Q.A. Design and development of a new ambr250(R) bioreactor vessel for improved cell and gene therapy applications. Biotechnol. Lett. 2021. [Google Scholar] [CrossRef]
- Cunha, B.; Aguiar, T.; Carvalho, S.B.; Silva, M.M.; Gomes, R.A.; Carrondo, M.J.T.; Gomes-Alves, P.; Peixoto, C.; Serra, M.; Alves, P.M. Bioprocess integration for human mesenchymal stem cells: From up to downstream processing scale-up to cell proteome characterization. J. Biotechnol. 2017, 248, 87–98. [Google Scholar] [CrossRef]
- Schirmaier, C.; Jossen, V.; Kaiser, S.C.; Jüngerkes, F.; Brill, S.; Safavi-Nab, A.; Siehoff, A.; van den Bos, C.; Eibl, D.; Eibl, R. Scale-up of adipose tissue-derived mesenchymal stem cell production in stirred single-use bioreactors under low-serum conditions. Eng. Life Sci. 2014, 14, 292–303. [Google Scholar] [CrossRef]
- Grein, T.A.; Leber, J.; Blumenstock, M.; Petry, F.; Weidner, T.; Salzig, D.; Czermak, P. Multiphase mixing characteristics in a microcarrier-based stirred tank bioreactor suitable for human mesenchymal stem cell expansion. Process. Biochem. 2016, 51, 1109–1119. [Google Scholar] [CrossRef]
- Lawson, T.; Kehoe, D.E.; Schnitzler, A.C.; Rapiejko, P.J.; Der, K.A.; Philbrick, K.; Punreddy, S.; Rigby, S.; Smith, R.; Feng, Q. Process development for expansion of human mesenchymal stromal cells in a 50 L single-use stirred tank bioreactor. Biochem. Eng. J. 2017, 120, 49–62. [Google Scholar] [CrossRef]
- Ratcliffe, E.; Glen, K.E.; Workman, V.L.; Stacey, A.J.; Thomas, R.J. A novel automated bioreactor for scalable process optimisation of haematopoietic stem cell culture. J. Biotechnol. 2012, 161, 387–390. [Google Scholar] [CrossRef] [Green Version]
- Weber, C.; Pohl, S.; Portner, R.; Wallrapp, C.; Kassem, M.; Geigle, P.; Czermak, P. Cultivation and Differentiation of Encapsulated hMSC-TERT in a Disposable Small-Scale Syringe-Like Fixed Bed Reactor. Open Biomed. Eng. J. 2007, 1, 64–70. [Google Scholar] [CrossRef]
- Weber, C.; Freimark, D.; Pörtner, R.; Pino-Grace, P.; Pohl, S.; Wallrapp, C.; Geigle, P.; Czermak, P. Expansion of human mesenchymal stem cells in a fixed-bed bioreactor system based on non-porous glass carrier–part A: Inoculation, cultivation, and cell harvest procedures. Int. J. Artif. Organs. 2010, 33, 512–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizukami, A.; Orellana, M.D.; Caruso, S.R.; de Lima Prata, K.; Covas, D.T.; Swiech, K. Efficient expansion of mesenchymal stromal cells in a disposable fixed bed culture system. Biotechnol. Prog. 2013, 29, 568–572. [Google Scholar] [CrossRef]
- Roberts, I.; Baila, S.; Rice, R.B.; Janssens, M.E.; Nguyen, K.; Moens, N.; Ruban, L.; Hernandez, D.; Coffey, P.; Mason, C. Scale-up of human embryonic stem cell culture using a hollow fibre bioreactor. Biotechnol. Lett. 2012, 34, 2307–2315. [Google Scholar] [CrossRef]
- Paccola Mesquita, F.C.; Hochman-Mendez, C.; Morrissey, J.; Sampaio, L.C.; Taylor, D.A. Laminin as a Potent Substrate for Large-Scale Expansion of Human Induced Pluripotent Stem Cells in a Closed Cell Expansion System. Stem Cells Int. 2019, 2019, 9704945. [Google Scholar] [CrossRef] [Green Version]
- Tirughana, R.; Metz, M.Z.; Li, Z.; Hall, C.; Hsu, D.; Beltzer, J.; Annala, A.J.; Oganesyan, D.; Gutova, M.; Aboody, K.S. GMP Production and Scale-Up of Adherent Neural Stem Cells with a Quantum Cell Expansion System. Mol. Ther. Methods Clin. Dev. 2018, 10, 48–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, A.L.; Lefavor, R.C.; Zubair, A.C. Characterization and cost-benefit analysis of automated bioreactor-expanded mesenchymal stem cells for clinical applications. Transfusion 2018, 58, 2374–2382. [Google Scholar] [CrossRef] [PubMed]
- Haack-Sørensen, M.; Follin, B.; Juhl, M.; Brorsen, S.K.; Søndergaard, R.H.; Kastrup, J.; Ekblond, A. Culture expansion of adipose derived stromal cells. A closed automated Quantum Cell Expansion System compared with manual flask-based culture. J. Transl. Med. 2016, 14, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mennan, C.; Garcia, J.; Roberts, S.; Hulme, C.; Wright, K. A comprehensive characterisation of large-scale expanded human bone marrow and umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2019, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Mizukami, A.; Chilima, T.D.P.; Orellana, M.D.; Neto, M.A.; Covas, D.T.; Farid, S.S.; Swiech, K. Technologies for large-scale umbilical cord-derived MSC expansion: Experimental performance and cost of goods analysis. Biochem. Eng. J. 2018, 135, 36–48. [Google Scholar] [CrossRef]
- Mizukami, A.; de Abreu Neto, M.S.; Moreira, F.; Fernandes-Platzgummer, A.; Huang, Y.F.; Milligan, W.; Cabral, J.M.S.; da Silva, C.L.; Covas, D.T.; Swiech, K. A Fully-Closed and Automated Hollow Fiber Bioreactor for Clinical-Grade Manufacturing of Human Mesenchymal Stem/Stromal Cells. Stem Cell Rev. Rep. 2018, 14, 141–143. [Google Scholar] [CrossRef]
- Lambrechts, T.; Papantoniou, I.; Rice, B.; Schrooten, J.; Luyten, F.P.; Aerts, J.M. Large-scale progenitor cell expansion for multiple donors in a monitored hollow fibre bioreactor. Cytotherapy 2016, 18, 1219–1233. [Google Scholar] [CrossRef] [PubMed]
- Vymetalova, L.; Kucirkova, T.; Knopfova, L.; Pospisilova, V.; Kasko, T.; Lejdarova, H.; Makaturova, E.; Kuglik, P.; Oralova, V.; Matalova, E.; et al. Large-Scale Automated Hollow-Fiber Bioreactor Expansion of Umbilical Cord-Derived Human Mesenchymal Stromal Cells for Neurological Disorders. Neurochem. Res. 2020, 45, 204–214. [Google Scholar] [CrossRef]
- Haack-Sørensen, M.; Juhl, M.; Follin, B.; Harary Søndergaard, R.; Kirchhoff, M.; Kastrup, J.; Ekblond, A. Development of large-scale manufacturing of adipose-derived stromal cells for clinical applications using bioreactors and human platelet lysate. Scand. J. Clin. Lab. Investig. 2018, 78, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Nold, P.; Brendel, C.; Neubauer, A.; Bein, G.; Hackstein, H. Good manufacturing practice-compliant animal-free expansion of human bone marrow derived mesenchymal stroma cells in a closed hollow-fiber-based bioreactor. Biochem. Biophys. Res. Commun. 2013, 430, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.C.; Lin, H.; Cheng, Y.C.; Yen, C.H.; Huang, R.N.; Lin, K.H. Beta-adrenoceptor pathway enhances mitochondrial function in human neural stem cells via rotary cell culture system. J. Neurosci. Methods 2012, 207, 130–136. [Google Scholar] [CrossRef]
- Sakai, S.; Mishima, H.; Ishii, T.; Akaogi, H.; Yoshioka, T.; Ohyabu, Y.; Chang, F.; Ochiai, N.; Uemura, T. Rotating three-dimensional dynamic culture of adult human bone marrow-derived cells for tissue engineering of hyaline cartilage. J. Orthop. Res. 2009, 27, 517–521. [Google Scholar] [CrossRef]
- Neumann, A.; Lavrentieva, A.; Heilkenbrinker, A.; Loenne, M.; Kasper, C. Characterization and Application of a Disposable Rotating Bed Bioreactor for Mesenchymal Stem Cell Expansion. Bioengineering 2014, 1, 231–245. [Google Scholar] [CrossRef] [Green Version]
- Reichardt, A.; Polchow, B.; Shakibaei, M.; Henrich, W.; Hetzer, R.; Lueders, C. Large scale expansion of human umbilical cord cells in a rotating bed system bioreactor for cardiovascular tissue engineering applications. Open Biomed. Eng. J. 2013, 7, 50–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, B.M.; Loghin, E.R.; Conway, K.R.; Zhang, X. Automated Closed-System Expansion of Pluripotent Stem Cell Aggregates in a Rocking-Motion Bioreactor. SLAS Technol. 2018, 23, 364–373. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.; Bang, S.; Noh, I. Tissue Regeneration of Human Mesenchymal Stem Cells on Porous Gelatin Micro-Carriers by Long-Term Dynamic In Vitro Culture. Tissue Eng. Regen. Med. 2019, 16, 19–28. [Google Scholar] [CrossRef]
- da Silva, J.d.S.; Mizukami, A.; Gil, L.V.G.; de Campos, J.V.; Assis, O.B.; Covas, D.T.; Swiech, K.; Suazo, C.A.T. Improving wave-induced motion bioreactor performance for human mesenchymal stromal cell expansion. Process Biochem. 2019, 84, 143–152. [Google Scholar] [CrossRef]
- da Silva, J.d.S.; Severino, P.; Wodewotzky, T.I.; Covas, D.T.; Swiech, K.; Marti, L.C.; Suazo, C.A.T. Mesenchymal stromal cells maintain the major quality attributes when expanded in different bioreactor systems. Biochem. Eng. J. 2020, 161, 107693. [Google Scholar] [CrossRef]
- Timmins, N.E.; Athanasas, S.; Gunther, M.; Buntine, P.; Nielsen, L.K. Ultra-high-yield manufacture of red blood cells from hematopoietic stem cells. Tissue Eng. Part C Methods 2011, 17, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.P.; Sousa-Luis, R.; Fernandes, T.G.; Bekman, E.P.; Rodrigues, C.A.V.; Vaz, S.H.; Moreira, L.M.; Hashimura, Y.; Jung, S.; Lee, B.; et al. Transcriptome profiling of human pluripotent stem cell-derived cerebellar organoids reveals faster commitment under dynamic conditions. Biotechnol. Bioeng. 2021. [Google Scholar] [CrossRef]
- Nogueira, D.E.S.; Rodrigues, C.A.V.; Carvalho, M.S.; Miranda, C.C.; Hashimura, Y.; Jung, S.; Lee, B.; Cabral, J.M.S. Strategies for the expansion of human induced pluripotent stem cells as aggregates in single-use Vertical-Wheel bioreactors. J. Biol. Eng. 2019, 13, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.A.V.; Silva, T.P.; Nogueira, D.E.S.; Fernandes, T.G.; Hashimura, Y.; Wesselschmidt, R.; Diogo, M.M.; Lee, B.; Cabral, J.M.S. Scalable culture of human induced pluripotent cells on microcarriers under xeno-free conditions using single-use vertical-wheel (TM) bioreactors. J. Chem. Technol. Biotechnol. 2018, 93, 3597–3606. [Google Scholar] [CrossRef]
- Borys, B.S.; So, T.; Colter, J.; Dang, T.; Roberts, E.L.; Revay, T.; Larijani, L.; Krawetz, R.; Lewis, I.; Argiropoulos, B.; et al. Optimized serial expansion of human induced pluripotent stem cells using low-density inoculation to generate clinically relevant quantities in vertical-wheel bioreactors. Stem Cells Transl. Med. 2020, 9, 1036–1052. [Google Scholar] [CrossRef] [PubMed]
- Borys, B.S.; Dang, T.; So, T.; Rohani, L.; Revay, T.; Walsh, T.; Thompson, M.; Argiropoulos, B.; Rancourt, D.E.; Jung, S.; et al. Overcoming bioprocess bottlenecks in the large-scale expansion of high-quality hiPSC aggregates in vertical-wheel stirred suspension bioreactors. Stem Cell Res. Ther. 2021, 12, 1–19. [Google Scholar] [CrossRef]
- Yuan, X.; Tsai, A.C.; Farrance, I.; Rowley, J.; Ma, T. Aggregation of Culture Expanded Human Mesenchymal Stem Cells in Microcarrier-based Bioreactor. Biochem. Eng. J. 2018, 131, 39–46. [Google Scholar] [CrossRef]
- de Sousa Pinto, D.; Bandeiras, C.; de Almeida Fuzeta, M.; Rodrigues, C.A.V.; Jung, S.; Hashimura, Y.; Tseng, R.J.; Milligan, W.; Lee, B.; Ferreira, F.C.; et al. Scalable Manufacturing of Human Mesenchymal Stromal Cells in the Vertical-Wheel Bioreactor System: An Experimental and Economic Approach. Biotechnol. J. 2019, 14, e1800716. [Google Scholar] [CrossRef]
- de Almeida Fuzeta, M.; Bernardes, N.; Oliveira, F.D.; Costa, A.C.; Fernandes-Platzgummer, A.; Farinha, J.P.; Rodrigues, C.A.V.; Jung, S.; Tseng, R.J.; Milligan, W.; et al. Scalable Production of Human Mesenchymal Stromal Cell-Derived Extracellular Vesicles Under Serum-/Xeno-Free Conditions in a Microcarrier-Based Bioreactor Culture System. Front. Cell Dev. Biol. 2020, 8, 553444. [Google Scholar] [CrossRef]
- Lembong, J.; Kirian, R.; Takacs, J.D.; Olsen, T.R.; Lock, L.T.; Rowley, J.A.; Ahsan, T. Bioreactor Parameters for Microcarrier-Based Human MSC Expansion under Xeno-Free Conditions in a Vertical-Wheel System. Bioengineering 2020, 7. [Google Scholar] [CrossRef]
- Sousa, M.F.; Silva, M.M.; Giroux, D.; Hashimura, Y.; Wesselschmidt, R.; Lee, B.; Roldao, A.; Carrondo, M.J.; Alves, P.M.; Serra, M. Production of oncolytic adenovirus and human mesenchymal stem cells in a single-use, Vertical-Wheel bioreactor system: Impact of bioreactor design on performance of microcarrier-based cell culture processes. Biotechnol. Prog. 2015, 31, 1600–1612. [Google Scholar] [CrossRef] [PubMed]
- Stolberg, S.; McCloskey, K.E. Can shear stress direct stem cell fate? Biotechnol. Prog. 2009, 25, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Earls, J.K.; Jin, S.; Ye, K. Mechanobiology of human pluripotent stem cells. Tissue Eng. Part B Rev. 2013, 19, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.W.; Chen, A.; Choo, A.B.; Reuveny, S.; Oh, S.K. Agitation can induce differentiation of human pluripotent stem cells in microcarrier cultures. Tissue Eng. Part C Methods 2011, 17, 165–172. [Google Scholar] [CrossRef]
- Yourek, G.; McCormick, S.M.; Mao, J.J.; Reilly, G.C. Shear stress induces osteogenic differentiation of human mesenchymal stem cells. Regen. Med. 2010, 5, 713–724. [Google Scholar] [CrossRef] [Green Version]
- Özbek, B.; Gayik, S. The studies on the oxygen mass transfer coefficient in a bioreactor. Process Biochem. 2001, 36, 729–741. [Google Scholar] [CrossRef]
- Nienow, A.W. Scale-up, Stirred Tank Reactors. Encyclopedia of Industrial Biotechnology: Bioprocess., Bioseparation, and Cell Technology; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 1–38. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.-J.; Xia, J.-Y.; Haringa, C.; Liu, Y.-P.; Chu, J.; Zhuang, Y.-P.; Zhang, S.-L. Application of Euler-Lagrange CFD for quantitative evaluating the effect of shear force on Carthamus tinctorius L. cell in a stirred tank bioreactor. Biochem. Eng. J. 2016, 114, 209–217. [Google Scholar] [CrossRef]
- Halloin, C.; Schwanke, K.; Lobel, W.; Franke, A.; Szepes, M.; Biswanath, S.; Wunderlich, S.; Merkert, S.; Weber, N.; Osten, F.; et al. Continuous WNT Control Enables Advanced hPSC Cardiac Processing and Prognostic Surface Marker Identification in Chemically Defined Suspension Culture. Stem Cell Rep. 2019, 13, 366–379. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, Y.; Shimada, Y.; Sugihara, A.; Tominaga, Y. Enzymatic conversion of waste edible oil to biodiesel fuel in a fixed-bed bioreactor. J. Am. Oil. Chem. Soc. 2001, 78, 703–707. [Google Scholar] [CrossRef]
- Salum, T.F.C.; Villeneuve, P.; Barea, B.; Yamamoto, C.I.; Côcco, L.C.; Mitchell, D.A.; Krieger, N. Synthesis of biodiesel in column fixed-bed bioreactor using the fermented solid produced by Burkholderia cepacia LTEB11. Process Biochem. 2010, 45, 1348–1354. [Google Scholar] [CrossRef]
- Chang, J.-S.; Lee, K.-S.; Lin, P.-J. Biohydrogen production with fixed-bed bioreactors. Int. J. Hydrogen Energy 2002, 27, 1167–1174. [Google Scholar] [CrossRef]
- Kim, K.; Logan, B.E. Fixed-bed bioreactor treating perchlorate-contaminated waters. Environ. Eng. Sci 2000, 17, 257–265. [Google Scholar] [CrossRef]
- Chen, B.-Y.; Chen, S.-Y.; Chang, J.-S. Immobilized cell fixed-bed bioreactor for wastewater decolorization. Process Biochem. 2005, 40, 3434–3440. [Google Scholar] [CrossRef]
- Rajendran, R.; Lingala, R.; Vuppu, S.K.; Bandi, B.O.; Manickam, E.; Macherla, S.R.; Dubois, S.; Havelange, N.; Maithal, K. Assessment of packed bed bioreactor systems in the production of viral vaccines. AMB Express 2014, 4, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valkama, A.J.; Leinonen, H.M.; Lipponen, E.M.; Turkki, V.; Malinen, J.; Heikura, T.; Yla-Herttuala, S.; Lesch, H.P. Optimization of lentiviral vector production for scale-up in fixed-bed bioreactor. Gene Ther. 2018, 25, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Powers, A.D.; Piras, B.A.; Clark, R.K.; Lockey, T.D.; Meagher, M.M. Development and Optimization of AAV hFIX Particles by Transient Transfection in an iCELLis((R)) Fixed-Bed Bioreactor. Hum. Gene Ther. Methods 2016, 27, 112–121. [Google Scholar] [CrossRef]
- Meissner, P.; Schroder, B.; Herfurth, C.; Biselli, M. Development of a fixed bed bioreactor for the expansion of human hematopoietic progenitor cells. Cytotechnology 1999, 30, 227–234. [Google Scholar] [CrossRef]
- Simonsen, J.L.; Rosada, C.; Serakinci, N.; Justesen, J.; Stenderup, K.; Rattan, S.I.; Jensen, T.G.; Kassem, M. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat. Biotechnol. 2002, 20, 592–596. [Google Scholar] [CrossRef]
- Barckhausen, C.; Rice, B.; Baila, S.; Sensebe, L.; Schrezenmeier, H.; Nold, P.; Hackstein, H.; Rojewski, M.T. GMP-Compliant Expansion of Clinical-Grade Human Mesenchymal Stromal/Stem Cells Using a Closed Hollow Fiber Bioreactor. Methods Mol. Biol. 2016, 1416, 389–412. [Google Scholar] [CrossRef]
- MacDonald, J.M.; Wolfe, S.P.; Roy-Chowdhury, I.; Kubota, H.; Reid, L.M. Effect of flow configuration and membrane characteristics on membrane fouling in a novel multicoaxial hollow-fiber bioartificial liver. Ann. N. Y. Acad. Sci. 2001, 944, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Tapia, F.; Vogel, T.; Genzel, Y.; Behrendt, I.; Hirschel, M.; Gangemi, J.D.; Reichl, U. Production of high-titer human influenza A virus with adherent and suspension MDCK cells cultured in a single-use hollow fiber bioreactor. Vaccine 2014, 32, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, P.J.; Shively, L.; Clark, C.; Cheung, C.-W.; Le, W.; Szpikowska, B.; Shively, J.E.; Raubitschek, A.A.; Wu, A.M. Mammalian expression and hollow fiber bioreactor production of recombinant anti-CEA diabody and minibody for clinical applications. J. Immunol. Methods 2001, 253, 195–208. [Google Scholar] [CrossRef]
- Jardin, B.A.; Zhao, Y.; Selvaraj, M.; Montes, J.; Tran, R.; Prakash, S.; Elias, C.B. Expression of SEAP (secreted alkaline phosphatase) by baculovirus mediated transduction of HEK 293 cells in a hollow fiber bioreactor system. J. Biotechnol. 2008, 135, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Pankhania, M.; Stephenson, T.; Semmens, M.J. Hollow fibre bioreactor for wastewater treatment using bubbleless membrane aeration. Water Res. 1994, 28, 2233–2236. [Google Scholar] [CrossRef]
- Gimenez, J.B.; Robles, A.; Carretero, L.; Duran, F.; Ruano, M.V.; Gatti, M.N.; Ribes, J.; Ferrer, J.; Seco, A. Experimental study of the anaerobic urban wastewater treatment in a submerged hollow-fibre membrane bioreactor at pilot scale. Bioresour. Technol. 2011, 102, 8799–8806. [Google Scholar] [CrossRef]
- Wang, Y.-z.; Zhao, Z.-p.; Li, M.-f.; Chen, Y.-z.; Liu, W.-f. Development of a hollow fiber membrane micro-reactor for biocatalytic production of formate from CO2. J. Membr. Sci. 2016, 514, 44–52. [Google Scholar] [CrossRef]
- Krastanov, A.; Blazheva, D.; Stanchev, V. Sucrose conversion into palatinose with immobilized Serratia plymuthica cells in a hollow-fibre bioreactor. Process Biochem. 2007, 42, 1655–1659. [Google Scholar] [CrossRef]
- Bieback, K.; Fernandez-Munoz, B.; Pati, S.; Schafer, R. Gaps in the knowledge of human platelet lysate as a cell culture supplement for cell therapy: A joint publication from the AABB and the International Society for Cell & Gene Therapy. Transfusion 2019, 59, 3448–3460. [Google Scholar] [CrossRef] [Green Version]
- Wnorowski, A.; Sharma, A.; Chen, H.; Wu, H.; Shao, N.Y.; Sayed, N.; Liu, C.; Countryman, S.; Stodieck, L.S.; Rubins, K.H.; et al. Effects of Spaceflight on Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Structure and Function. Stem Cell Rep. 2019, 13, 960–969. [Google Scholar] [CrossRef] [Green Version]
- Lelkes, P.I.; Galvan, D.L.; Hayman, G.T.; Goodwin, T.J.; Chatman, D.Y.; Cherian, S.; Garcia, R.M.; Unsworth, B.R. Simulated microgravity conditions enhance differentiation of cultured PC12 cells towards the neuroendocrine phenotype. In Vitro Cell Dev. Biol. Anim. 1998, 34, 316–325. [Google Scholar] [CrossRef]
- Tsao, Y.D.; Goodwin, T.J.; Wolf, D.A.; Spaulding, G.F. Responses of gravity level variations on the NASA/JSC bioreactor system. Physiologist 1992, 35, S49–S50. [Google Scholar] [PubMed]
- Hammond, T.G.; Hammond, J.M. Optimized suspension culture: The rotating-wall vessel. Am. J. Physiol. Ren. Physiol. 2001, 281, F12–F25. [Google Scholar] [CrossRef] [PubMed]
- Cowger, N.L.; O’Connor, K.C.; Hammond, T.G.; Lacks, D.J.; Navar, G.L. Characterization of bimodal cell death of insect cells in a rotating-wall vessel and shaker flask. Biotechnol. Bioeng. 1999, 64, 14–26. [Google Scholar] [CrossRef] [Green Version]
- Abdella, A.; Mazeed, T.E.-S.; El-Baz, A.F.; Yang, S.-T. Production of β-glucosidase from wheat bran and glycerol by Aspergillus niger in stirred tank and rotating fibrous bed bioreactors. Process Biochem. 2016, 51, 1331–1337. [Google Scholar] [CrossRef]
- Lan, T.Q.; Wei, D.; Yang, S.T.; Liu, X. Enhanced cellulase production by Trichoderma viride in a rotating fibrous bed bioreactor. Bioresour. Technol. 2013, 133, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Pourakbar, M.; Moussavi, G.; Yaghmaeian, K. Enhanced biodegradation of phenol in a novel cyclic activated sludge integrated with a rotating bed bioreactor in anoxic and peroxidase-mediated conditions. RSC Advances 2018, 8, 6293–6305. [Google Scholar] [CrossRef] [Green Version]
- Jafari, S.J.; Moussavi, G.; Yaghmaeian, K. High-rate biological denitrification in the cyclic rotating-bed biological reactor: Effect of COD/NO3(-), nitrate concentration and salinity and the phylogenetic analysis of denitrifiers. Bioresour. Technol. 2015, 197, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Suck, K.; Behr, L.; Fischer, M.; Hoffmeister, H.; van Griensven, M.; Stahl, F.; Scheper, T.; Kasper, C. Cultivation of MC3T3-E1 cells on a newly developed material (Sponceram) using a rotating bed system bioreactor. J. Biomed. Mater. Res. Part A 2007, 80, 268–275. [Google Scholar] [CrossRef]
- Singh, V. Disposable bioreactor for cell culture using wave-induced agitation. Cytotechnology 1999, 30, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Kalmbach, A.; Bordas, R.; Oncul, A.A.; Thevenin, D.; Genzel, Y.; Reichl, U. Experimental characterization of flow conditions in 2- and 20-L bioreactors with wave-induced motion. Biotechnol. Prog. 2011, 27, 402–409. [Google Scholar] [CrossRef]
- Marsh, D.T.J.; Lye, G.J.; Micheletti, M.; Odeleye, A.O.O.; Ducci, A.; Osborne, M.D. Fluid dynamic characterization of a laboratory scale rocked bag bioreactor. AIChE J. 2017, 63, 4177–4187. [Google Scholar] [CrossRef] [Green Version]
- Zhan, C.; Hagrot, E.; Brandt, L.; Chotteau, V. Study of hydrodynamics in wave bioreactors by computational fluid dynamics reveals a resonance phenomenon. Chem. Eng. Sci. 2019, 193, 53–65. [Google Scholar] [CrossRef]
- Croughan, M.S.; Giroux, D.; Fang, D.; Lee, B. Novel Single-Use Bioreactors for Scale-Up of Anchorage-Dependent Cell Manufacturing for Cell Therapies In Stem Cell Manufacturing; da Silva, C.L., Chase, L.G., Diogo, M.M., Eds.; Elsevier: Cambridge, UK, 2016; pp. 105–139. [Google Scholar] [CrossRef]
- Dee, K.U.; Shuler, M.L.; Wood, H.A. Inducing single-cell suspension of BTI-TN5B1-4 insect cells: I. The use of sulfated polyanions to prevent cell aggregation and enhance recombinant protein production. Biotechnol. Bioeng. 1997, 54, 191–205. [Google Scholar] [CrossRef]
- Zanghi, J.A.; Renner, W.A.; Bailey, J.E.; Fussenegger, M. The growth factor inhibitor suramin reduces apoptosis and cell aggregation in protein-free CHO cell batch cultures. Biotechnol. Prog. 2000, 16, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Hou, L.; Huang, N.F. Vascularization of three-dimensional engineered tissues for regenerative medicine applications. Acta Biomater 2016, 41, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marghitoiu, L.; Liu, J.; Lee, H.; Perez, L.; Fujimori, K.; Ronk, M.; Hammond, M.R.; Nunn, H.; Lower, A.; Rogers, G.; et al. Extractables analysis of single-use flexible plastic biocontainers. PDA J. Pharm. Sci. Technol. 2015, 69, 49–58. [Google Scholar] [CrossRef]
- Hammond, M.; Marghitoiu, L.; Lee, H.; Perez, L.; Rogers, G.; Nashed-Samuel, Y.; Nunn, H.; Kline, S. A cytotoxic leachable compound from single-use bioprocess equipment that causes poor cell growth performance. Biotechnol. Prog. 2014, 30, 332–337. [Google Scholar] [CrossRef]
- Hammond, M.; Nunn, H.; Rogers, G.; Lee, H.; Marghitoiu, A.L.; Perez, L.; Nashed-Samuel, Y.; Anderson, C.; Vandiver, M.; Kline, S. Identification of a leachable compound detrimental to cell growth in single-use bioprocess containers. PDA J. Pharm. Sci. Technol. 2013, 67, 123–134. [Google Scholar] [CrossRef]
- Liu, J.; Kennedy, J.H.; Ronk, M.; Marghitoiu, L.; Lee, H.; Nashed-Samuel, Y. Ambient analysis of leachable compounds from single-use bioreactors with desorption electrospray ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 2285–2291. [Google Scholar] [CrossRef] [PubMed]
- Jurkiewicz, E.; Husemann, U.; Greller, G.; Barbaroux, M.; Fenge, C. Verification of a new biocompatible single-use film formulation with optimized additive content for multiple bioprocess applications. Biotechnol. Prog. 2014, 30, 1171–1176. [Google Scholar] [CrossRef] [Green Version]
- Pietrzykowski, M.; Flanagan, W.; Pizzi, V.; Brown, A.; Sinclair, A.; Monge, M. An environmental life cycle assessment comparison of single-use and conventional process technology for the production of monoclonal antibodies. J. Clean. Prod. 2013, 41, 150–162. [Google Scholar] [CrossRef]
- Levine, H.L.; Stock, R.; Lilja, J.; Gaasvik, A.; Hummel, H.; Ransohoff, T.C.; Jones, S.D. Single-use technology and modular construction. BioProcess Int. 2013, 11, 40–45. [Google Scholar]
- Rogge, P.; Müller, D.; Schmidt, S.R. The single-use or stainless steel decision process. BioProcess Int. 2015, 13. [Google Scholar]
- Lipsitz, Y.Y.; Timmins, N.E.; Zandstra, P.W. Quality cell therapy manufacturing by design. Nat. Biotechnol. 2016, 34, 393–400. [Google Scholar] [CrossRef] [PubMed]







| Bioreactor Type | Cell Type | Working Volume/Area | Culture Time (days) | Maximum Final Cell Density | Ref. |
|---|---|---|---|---|---|
| Stirred tank | hiPSCs | 125 mL | 7 | (2.9 ± 0.3) × 10 6 cells∙mL–1 | [29] |
| 1.0-1.5 L | 7 | (1.99 ± 0.09) × 10 6 cells∙mL–1 | [30] | ||
| hMSCs | 15 mL | 8 | 8.1 × 10 5 cells∙mL–1 | [31] | |
| 100–200 mL | 10 | 1.8 × 10 5 cells∙mL–1 | [32] | ||
| 1.0–2.0 L | 7 | 4.1 × 10 5 cells∙mL–1 | [33] | ||
| 2.0 L | 7 | (2.7 ± 0.2) × 10 5 cells∙mL–1 | [34] | ||
| 1.0–2.4 L | 14 | ~ 1 × 10 5 cells∙mL–1 | [35] | ||
| 35 L | 7 | 3.1 × 10 5 cells∙mL–1 | [34] | ||
| 50 L | 11 | 2.6 × 10 5 cells∙mL–1 | [36] | ||
| hHSPCs | 10 mL | 10 | 1.4 × 10 7 cells∙mL–1 | [37] | |
| Fixed bed | hMSCs | 3 mL | 20.8 | N/A (1) | [38] |
| 14.2 mL | 5.6 | (2.9 ± 0.1) × 10 6 cells∙mL–1 | [39] | ||
| 60 mL | 7.0 | 1.75 × 10 6 cells∙mL–1 | [39] | ||
| 300 mL | 6.9 | 2.05 × 10 6 cells∙mL–1 | [39] | ||
| 500 mL | 7 | (8.3 ± 1.6) × 10 5 cells∙mL–1 | [40] | ||
| Hollow fibre | hESCs | 2.1 m2 | 5 | 3.4 × 10 4 cells∙cm–2 | [41] |
| hiPSCs | 2.1 m2 | 6–7 | (3.3 ± 0.4) × 10 4 cells∙cm–2 | [42] | |
| hNSCs | 2.1 m2 | 7–11 | 1.5 × 10 5 cells∙cm–2 | [43] | |
| hMSCs | 2.1 m2 | 7–9 | N/A (2) | [44] | |
| 2.1 m2 | 17 ± 6 | (4.7 ± 0.6) × 10 3 cells∙cm–2 | [45] | ||
| 2.1 m2 | 8 ± 2 | (8.0 ± 2.5) × 10 3 cells∙cm–2 | [46] | ||
| 2.1 m2 | 5 | (9.8 ± 1.0) × 10 3 cells∙cm–2 | [47] | ||
| 2.1 m2 | 5 | (1.1 ± 0.2) × 10 4 cells∙cm–2 | [48] | ||
| 2.1 m2 | 7.9–9.9 | (1.8 ± 0.2) × 10 4 cells∙cm–2 | [49] | ||
| 2.1 m2 | 6 | (1.9 ± 0.3) × 10 4 cells∙cm–2 | [50] | ||
| 2.1 m2 | 6 | 2.9 × 10 4 cells∙cm–2 | [51] | ||
| 2.1 m2 | 6–13 | 4.7 × 10 4 cells∙cm–2 | [52] | ||
| Rotary cell culture system | hNSCs | 4 mL | 3 | ~ 5 × 10 5 cells∙mL–1 | [53] |
| hMSCs | 10 mL | 14 | N/A (1) | [54] | |
| Rotating bed | hMSCs | 2000 cm2 | 5 | (1.2 ± 0.1) × 10 4 cells∙cm–2 | [55] |
| 6000 cm2 | 9 | (5.8 ± 0.9) × 10 4 cells∙cm–2 | [56] | ||
| Rocking motion | hESCs | 150 mL (3) | 4 | 2.8 × 10 6 cells∙mL–1 | [57] |
| 400 mL (3) | 4 | 1.4 × 10 6 cells∙mL–1 | [57] | ||
| 1.0 L (3) | 4 | 1.3 × 10 6 cells∙mL–1 | [57] | ||
| hMSCs | 50–200 mL | 100 | (1.32 ± 0.09) × 10 6 cells∙mL–1 | [58] | |
| 50–600 mL | 10 | 4.4 × 10 4 cells∙mL–1 | [59] | ||
| 50–600 mL | 11 | 2.2 × 10 5 cells∙mL–1 | [60] | ||
| hHSPC-RBCs | 200 mL–1 L | 33 | 4.5 × 10 12 cells∙mL–1 | [61] | |
| Vertical-Wheel | hiPSCs | 60 mL | 80 | N/A (1) | [62] |
| 60 mL | 7 | (2.3 ± 0.2) × 10 6 cells∙mL–1 | [63] | ||
| 60–73 mL | 7 | (1.79 ± 0.03) × 10 6 cells∙mL–1 | [63] | ||
| 80 mL | 9 | (1.21 ± 0.02) × 10 6 cells∙mL–1 | [64] | ||
| 300 mL | 8 | (8.6 ± 1.5) × 10 5 cells∙mL–1 | [64] | ||
| 100 mL | 6 | (6.3 ± 0.4) × 10 5 cells∙mL–1 | [65] | ||
| 100 mL | 6 | (6.5 ± 0.6) × 10 5 cells∙mL–1 | [66] | ||
| 500 mL | 6 | ~4 × 10 5 cells∙mL–1 | [66] | ||
| hMSCs | 60 mL | 4 | 1.1 × 10 5 cells∙mL–1 | [67] | |
| 60–100 mL | 7 | (5.3 ± 0.4) × 10 5 cells∙mL–1 | [68] | ||
| 60–100 mL | 7–11 | 5.3 × 10 5 cells∙mL–1 | [69] | ||
| 90–92 mL | 5 | ~ 6 × 10 5 cells∙mL–1 | [70] | ||
| 2.2 L | 14 | ~ 3 × 10 5 cells∙mL–1 | [71] |
| Bioreactor | Company | Impeller | Working Volume Range |
|---|---|---|---|
| BioBLU® | Eppendorf | Eight-blade or pitched-blade | 100 mL–40 L |
| Mobius® CellReady | Merck | Marine (scoping) | 1.0–2.4 L |
| Ambr® | Sartorius Stedim Biotech | Pitched-blade or Rushton | 10–250 mL |
| BIOSTAT® CultiBag STR Plus | Sartorius Stedim Biotech | Three- or six-blade | 12.5–200 L |
| UniVessel® SU | Sartorius Stedim Biotech | Three-blade | 600 mL–2.0 L |
| Platform | Advantages | Drawbacks/Limitations |
|---|---|---|
| Single-use bioreactors |
|
|
| Stirred tank |
|
|
| Fixed bed |
|
|
| Hollow fibre |
|
|
| Rotary cell culture system |
|
|
| Rotating bed |
|
|
| Rocking motion |
|
|
| Vertical-Wheel |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogueira, D.E.S.; Cabral, J.M.S.; Rodrigues, C.A.V. Single-Use Bioreactors for Human Pluripotent and Adult Stem Cells: Towards Regenerative Medicine Applications. Bioengineering 2021, 8, 68. https://doi.org/10.3390/bioengineering8050068
Nogueira DES, Cabral JMS, Rodrigues CAV. Single-Use Bioreactors for Human Pluripotent and Adult Stem Cells: Towards Regenerative Medicine Applications. Bioengineering. 2021; 8(5):68. https://doi.org/10.3390/bioengineering8050068
Chicago/Turabian StyleNogueira, Diogo E.S., Joaquim M.S. Cabral, and Carlos A.V. Rodrigues. 2021. "Single-Use Bioreactors for Human Pluripotent and Adult Stem Cells: Towards Regenerative Medicine Applications" Bioengineering 8, no. 5: 68. https://doi.org/10.3390/bioengineering8050068
APA StyleNogueira, D. E. S., Cabral, J. M. S., & Rodrigues, C. A. V. (2021). Single-Use Bioreactors for Human Pluripotent and Adult Stem Cells: Towards Regenerative Medicine Applications. Bioengineering, 8(5), 68. https://doi.org/10.3390/bioengineering8050068







