TCA Cycle and Its Relationship with Clavulanic Acid Production: A Further Interpretation by Using a Reduced Genome-Scale Metabolic Model of Streptomyces clavuligerus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Cultivations Conditions
2.2. Bottom-Up Reconstruction of a Reduced Genome-Scale Metabolic Model of S. clavuligerus
2.3. Validation of the Sclav_Red Model Using Experimental Data
2.4. FBA and Shadow Prices as Tools for Sensitivity Analysis of Metabolic Networks
3. Results and Discussion
3.1. Fed-Batch Cultivation of S. clavuligerus
3.2. Construction of a Reduced Model of S. clavuligerus Metabolism
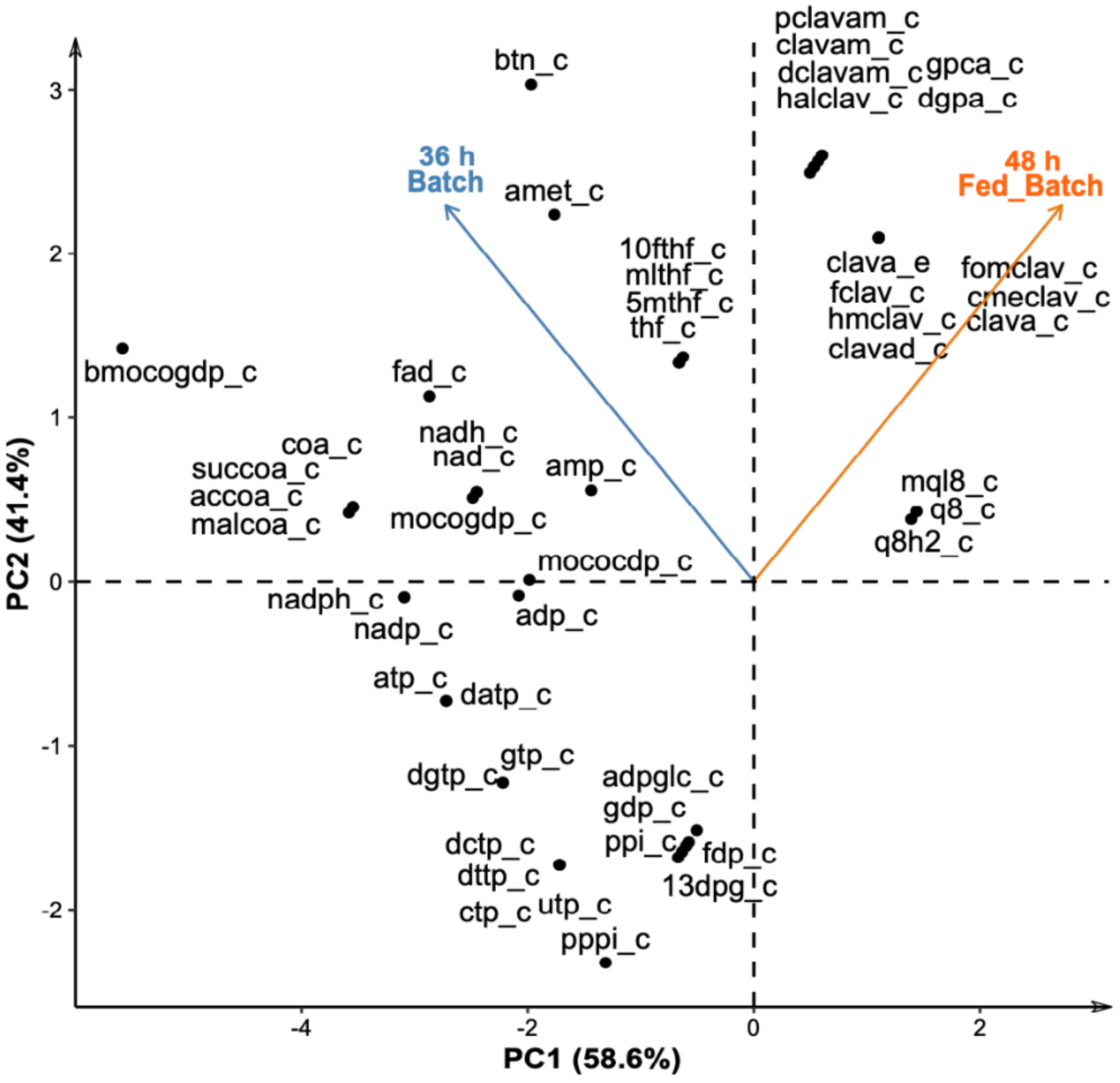
3.3. Flux Distributions in S. clavuligerus at Two Different Metabolic States during Fed-Batch Cultivations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, A.; Butterworth, D.; Cole, M.; Hanscomb, G.; Hood, J.; Reading, C.; Rolinson, G. Naturally occurring β-lactamase inhibitors with actibacterial activity. J. Antibiot. 1976, 29, 668–669. [Google Scholar] [CrossRef] [PubMed]
- Elander, R.P. Industrial production of β-lactam antibiotics. Appl. Microbiol. Biotechnol. 2003, 61, 385–392. [Google Scholar] [CrossRef]
- Llarrull, L.I.; Testero, S.A.; Fisher, J.F.; Mobashery, S. The future of the β-lactams. Curr. Opin. Microbiol. 2010, 13, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Malule, H. Bibliometric analysis of global research on clavulanic acid. Antibiotics 2018, 7, 102. [Google Scholar] [CrossRef]
- Ser, H.-L.; Law, J.W.-F.; Chaiyakunapruk, N.; Jacob, S.A.; Palanisamy, U.D.; Chan, K.-G.; Goh, B.-H.; Lee, L.-H. Fermentation conditions that affect clavulanic acid production in Streptomyces clavuligerus: A systematic review. Front. Microbiol. 2016, 7, 522. [Google Scholar] [CrossRef] [PubMed]
- Hamed, R.B.; Gomez-Castellanos, J.R.; Henry, L.; Ducho, C.; McDonough, M.A.; Schofield, C.J. The enzymes of β-lactam biosynthesis. Nat. Prod. Rep. 2013, 30, 21–107. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, N.; Jeong, Y.; Lee, Y.; Kim, W.; Cho, S.; Palsson, B.O.; Cho, B.-K. Primary transcriptome and translatome analysis determines transcriptional and translational regulatory elements encoded in the Streptomyces clavuligerus genome. Nucleic Acids Res. 2019, 47, 6114–6129. [Google Scholar] [CrossRef]
- Martínez-Burgo, Y.; Santos-Aberturas, J.; Rodríguez-García, A.; Barreales, E.G.; Tormo, J.R.; Truman, A.W.; Reyes, F.; Aparicio, J.F.; Liras, P. Activation of secondary metabolite gene clusters in Streptomyces clavuligerus by the PimM regulator of Streptomyces natalensis. Front. Microbiol. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- López-Agudelo, V.A.; Gómez-Ríos, D.; Ramirez-Malule, H. Clavulanic acid production by Streptomyces clavuligerus: Insights from systems biology, strain engineering, and downstream processing. Antibiotics 2021, 10, 84. [Google Scholar] [CrossRef]
- Qin, R.; Zhong, C.; Zong, G.; Fu, J.; Pang, X.; Cao, G. Improvement of clavulanic acid production in Streptomyces clavuligerus F613-1 by using a claR-neo reporter strategy. Electron. J. Biotechnol. 2017, 28, 41–46. [Google Scholar] [CrossRef]
- Kizildoğan, A.K.; Jaccard, G.V.; Mutlu, A.; Sertdemir, I.; Özcengiz, G. Genetic engineering of an industrial strain of Streptomyces clavuligerus for further enhancement of clavulanic acid production. Turkish J. Biol. 2017, 41, 342–353. [Google Scholar] [CrossRef]
- Ramirez-Malule, H.; Restrepo, A.; Cardona, W.; Junne, S.; Neubauer, P.; Rios-Estepa, R. Inversion of the stereochemical configuration (3S,5S)-clavaminic acid into (3R,5R)-clavulanic acid: A computationally-assisted approach based on experimental evidence. J. Theor. Biol. 2016, 395, 40–50. [Google Scholar] [CrossRef]
- Arulanantham, H.; Kershaw, N.J.; Hewitson, K.S.; Hughes, C.E.; Thirkettle, J.E.; Schofield, C.J. ORF17 from the clavulanic acid biosynthesis gene cluster catalyzes the ATP-dependent formation of N-glycyl-clavaminic acid. J. Biol. Chem. 2006, 281, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Fulston, M.; Davison, M.; Elson, S.W.; Tyler, J.W.; Woroniecki, S.R. Clavulanic acid biosynthesis; the final steps. J. Chem. Soc. Perkin Trans. 1 2001, 1, 1122–1130. [Google Scholar] [CrossRef]
- Iqbal, A.; Arunlanantham, H.; Brown, T.; Chowdhury, R.; Clifton, I.J.; Kershaw, N.J.; Hewitson, K.S.; McDonough, M.A.; Schofield, C.J. Crystallographic and mass spectrometric analyses of a tandem GNAT protein from the clavulanic acid biosynthesis pathway. Proteins 2010, 78, 1398–1407. [Google Scholar] [CrossRef]
- Valegård, K.; Iqbal, A.; Kershaw, N.J.; Ivison, D.; Généreux, C.; Dubus, A.; Blikstad, C.; Demetriades, M.; Hopkinson, R.J.; Lloyd, A.J.; et al. Structural and mechanistic studies of the orf12 gene product from the clavulanic acid biosynthesis pathway. Acta Crystallogr. Sect. D Biol. Crystallogr. 2013, 69, 1567–1579. [Google Scholar] [CrossRef]
- Gómez, S.; Ramírez-Malule, H.; Cardona-G, W.; Osorio, E.; Restrepo, A. Double-ring epimerization in the biosynthesis of clavulanic acid. J. Phys. Chem. A 2020, 124, 9413–9426. [Google Scholar] [CrossRef] [PubMed]
- Paradkar, A. Clavulanic acid production by Streptomyces clavuligerus: Biogenesis, regulation and strain improvement. J. Antibiot. 2013, 66, 411–420. [Google Scholar] [CrossRef]
- Hodgson, D. A Primary metabolism and its control in streptomycetes: A most unusual group of bacteria. Adv. Microb. Physiol. 2000, 42, 47–238. [Google Scholar] [PubMed]
- Ramirez-Malule, H.; Junne, S.; Nicolás Cruz-Bournazou, M.; Neubauer, P.; Ríos-Estepa, R. Streptomyces clavuligerus shows a strong association between TCA cycle intermediate accumulation and clavulanic acid biosynthesis. Appl. Microbiol. Biotechnol. 2018, 102, 4009–4023. [Google Scholar] [CrossRef]
- Gómez-Ríos, D.; López-Agudelo, V.A.; Ramírez-Malule, H.; Neubauer, P.; Junne, S.; Ochoa, S.; Ríos-Estepa, R. A Genome-scale insight into the effect of shear stress during the fed-batch production of clavulanic acid by Streptomyces clavuligerus. Microorganisms 2020, 8, 1255. [Google Scholar] [CrossRef] [PubMed]
- Bellão, C.; Antonio, T.; Araujo, M.L.G.C.; Badino, A.C. Production of clavulanic acid and cephamycin c by Streptomyces clavuligerus under different fed-batch conditions. Brazilian J. Chem. Eng. 2013, 30, 257–266. [Google Scholar] [CrossRef]
- Teodoro, J.C.; Araujo, M.L.G.C.; Hokka, C.O.; Badino, A.C. Influence of glycerol and ornithine feeding on clavulanic acid production by Streptomyces clavuligerus. Brazilian J. Chem. Eng. 2010, 27, 499–506. [Google Scholar] [CrossRef]
- O’Brien, E.J.; Monk, J.M.; Palsson, B.O. Using genome-scale models to predict biological capabilities. Cell 2015, 161, 971–987. [Google Scholar] [CrossRef]
- López-Agudelo, V.A.; Baena, A.; Ramirez-Malule, H.; Ochoa, S.; Barrera, L.F.; Ríos-Estepa, R. Metabolic adaptation of two in silico mutants of Mycobacterium tuberculosis during infection. BMC Syst. Biol. 2017, 11, 107. [Google Scholar] [CrossRef]
- Orth, J.D.; Thiele, I.; Palsson, B.O. What is flux balance analysis? Nat. Biotechnol. 2010, 28, 245–248. [Google Scholar] [CrossRef]
- Medema, M.H.; Alam, M.T.; Heijne, W.H.M.; Van Den Berg, M.A.; Müller, U.; Trefzer, A.; Bovenberg, R.A.L.; Breitling, R.; Takano, E. Genome-wide gene expression changes in an industrial clavulanic acid overproduction strain of Streptomyces clavuligerus. Microb. Biotechnol. 2011, 4, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cerón, S.; Galindo-Betancur, D.; Ramírez-Malule, H. Data set of in silico simulation for the production of clavulanic acid and cephamycin C by Streptomyces clavuligerus using a genome scale metabolic model. Data Br. 2019, 24, 103992. [Google Scholar] [CrossRef]
- Toro, L.; Pinilla, L.; Avignone-Rossa, C.; Ríos-Estepa, R. An enhanced genome-scale metabolic reconstruction of Streptomyces clavuligerus identifies novel strain improvement strategies. Bioprocess. Biosyst. Eng. 2018, 41, 657–669. [Google Scholar] [CrossRef]
- Thiele, I.; Palsson, B.Ø. A protocol for generating a high-quality genome-scale metabolic reconstruction. Nat. Protoc. 2010, 5, 93. [Google Scholar] [CrossRef]
- Arnold, A.; Nikoloski, Z. Bottom-up metabolic reconstruction of arabidopsis and its application to determining the metabolic costs of enzyme production. Plant. Physiol. 2014, 165, 1380–1391. [Google Scholar] [CrossRef]
- Tokic, M.; Hatzimanikatis, V.; Miskovic, L. Large-scale kinetic metabolic models of Pseudomonas putida KT2440 for consistent design of metabolic engineering strategies. Biotechnol. Biofuels 2020, 13, 33. [Google Scholar] [CrossRef]
- Ataman, M.; Hernandez Gardiol, D.F.; Fengos, G.; Hatzimanikatis, V. redGEM: Systematic reduction and analysis of genome-scale metabolic reconstructions for development of consistent core metabolic models. PLoS Comput. Biol. 2017, 13, e1005444. [Google Scholar] [CrossRef]
- Masid, M.; Ataman, M.; Hatzimanikatis, V. Analysis of human metabolism by reducing the complexity of the genome-scale models using redHUMAN. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Hameri, T.; Fengos, G.; Ataman, M.; Miskovic, L.; Hatzimanikatis, V. Kinetic models of metabolism that consider alternative steady-state solutions of intracellular fluxes and concentrations. Metab. Eng. 2019, 52, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Kirk, S.; Avignone-rossa, C.A.; Bushell, M.E. Growth limiting substrate affects antibiotic production and associated metabolic fluxes in Streptomyces clavuligerus. Biotechnol. Lett. 2000, 22, 1803–1809. [Google Scholar] [CrossRef]
- Bushell, M.E.; Kirk, S.; Zhao, H.-J.; Avignone-Rossa, C.A. Manipulation of the physiology of clavulanic acid biosynthesis with the aid of metabolic flux analysis. Enzyme Microb. Technol. 2006, 39, 149–157. [Google Scholar] [CrossRef]
- Gómez-Ríos, D.; Ramírez-Malule, H.; Neubauer, P.; Junne, S.; Ríos-Estepa, R. Data of clavulanic acid and clavulanate-imidazole stability at low temperatures. Data Br. 2019, 23, 103775. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ríos, D.; Ramírez-Malule, H.; Neubauer, P.; Junne, S.; Ríos-Estepa, R. Degradation kinetics of clavulanic acid in fermentation broths at low temperatures. Antibiotics 2019, 8, 6. [Google Scholar] [CrossRef]
- Bersanetti, P.A.; Almeida, R.M.R.G.; Barboza, M.; Araújo, M.L.G.C.; Hokka, C.O. Kinetic studies on clavulanic acid degradation. Biochem. Eng. J. 2005, 23, 31–36. [Google Scholar] [CrossRef]
- Beyß, M.; Parra-Peña, V.D.; Ramirez-Malule, H.; Nöh, K. Robustifying experimental tracer design for13C-Metabolic flux analysis. Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef]
- Reznik, E.; Mehta, P.; Segrè, D. Flux imbalance analysis and the sensitivity of cellular growth to changes in metabolite pools. PLoS Comput. Biol. 2013, 9, e1003195. [Google Scholar] [CrossRef]
- Gómez-Ríos, D.; Junne, S.; Neubauer, P.; Ochoa, S.; Ríos-Estepa, R.; Ramírez-Malule, H. Characterization of the metabolic response of Streptomyces clavuligerus to shear stress in stirred tanks and single-use 2d rocking motion bioreactors for clavulanic acid production. Antibiotics 2019, 8, 168. [Google Scholar] [CrossRef]
- Ramirez-Malule, H.; Junne, S.; López, C.; Zapata, J.; Sáez, A.; Neubauer, P.; Rios-Estepa, R. An improved HPLC-DAD method for clavulanic acid quantification in fermentation broths of Streptomyces clavuligerus. J. Pharm. Biomed. Anal. 2016, 120, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Junne, S.; Klingner, A.; Kabisch, J.; Schweder, T.; Neubauer, P. A two-compartment bioreactor system made of commercial parts for bioprocess scale-down studies: Impact of oscillations on Bacillus subtilis fed-batch cultivations. Biotechnol. J. 2011, 6, 1009–1017. [Google Scholar] [CrossRef]
- King, Z.A.; Lu, J.; Dräger, A.; Miller, P.; Federowicz, S.; Lerman, J.A.; Ebrahim, A.; Palsson, B.O.; Lewis, N.E. BiGG Models: A platform for integrating, standardizing and sharing genome-scale models. Nucleic Acids Res. 2015, 44, D515–D522. [Google Scholar] [CrossRef] [PubMed]
- Norsigian, C.J.; Pusarla, N.; McConn, J.L.; Yurkovich, J.T.; Dräger, A.; Palsson, B.O.; King, Z. BiGG Models 2020: Multi-strain genome-scale models and expansion across the phylogenetic tree. Nucleic Acids Res. 2020, 48, D402–D406. [Google Scholar] [CrossRef] [PubMed]
- Ataman, M.; Hatzimanikatis, V. lumpGEM: Systematic generation of subnetworks and elementally balanced lumped reactions for the biosynthesis of target metabolites. PLoS Comput. Biol. 2017, 13, e1005513. [Google Scholar] [CrossRef]
- Noor, E. Removing both internal and unrealistic energy-generating cycles in flux balance analysis. arXiv 2018, arXiv:1803.04999. [Google Scholar]
- Fritzemeier, C.J.; Hartleb, D.; Szappanos, B.; Papp, B.; Lercher, M.J. Erroneous energy-generating cycles in published genome scale metabolic networks: Identification and removal. PLoS Comput. Biol. 2017, 13, e1005494. [Google Scholar] [CrossRef]
- Schellenberger, J.; Lewis, N.E.; Palsson, B. Elimination of thermodynamically infeasible loops in steady-state metabolic models. Biophys. J. 2011, 100, 544–553. [Google Scholar] [CrossRef]
- López-Agudelo, V.A.; Mendum, T.A.; Laing, E.; Wu, H.H.; Baena, A.; Barrera, L.F.; Beste, D.J.V.; Rios-Estepa, R. A systematic evaluation of Mycobacterium tuberculosis genome-scale metabolic networks. PLoS Comput. Biol. 2020, 16, e1007533. [Google Scholar] [CrossRef]
- Flamholz, A.; Noor, E.; Bar-Even, A.; Milo, R. eQuilibrator—the biochemical thermodynamics calculator. Nucleic Acids Res. 2011, 40, D770–D775. [Google Scholar] [CrossRef] [PubMed]
- Heirendt, L.; Arreckx, S.; Pfau, T.; Mendoza, S.N.; Richelle, A.; Heinken, A.; Haraldsdóttir, H.S.; Wachowiak, J.; Keating, S.M.; Vlasov, V.; et al. Creation and analysis of biochemical constraint-based models using the COBRA Toolbox v.3.0. Nat. Protoc. 2019, 14, 639–702. [Google Scholar] [CrossRef] [PubMed]
- Stephanopoulos, G.; Aristidou, A.; Nielsen, J. Metabolic Engineering: Principles and Methodologies; Academic Press: San Diego, CA, USA, 1998; ISBN 0126662606. [Google Scholar]
- Villadsen, J.; Nielsen, J.; Lidén, G. Bioreaction Engineering Principles, 3rd ed.; Springer: New York, NY, USA, 2011; ISBN 9781441996879. [Google Scholar]
- Palsson, B. Systems Biology: Properties of Reconstructed Networks; Cambridge University Press: New York, NY, USA, 2005; ISBN 9780521859035. [Google Scholar]
- Grafahrend-Belau, E.; Schreiber, F.; Koschützki, D.; Junker, B.H. Flux balance analysis of barley seeds: A computational approach to study systemic properties of central metabolism. Plant. Physiol. 2009, 149, 585–598. [Google Scholar] [CrossRef]
- Haraldsdóttir, H.S.; Cousins, B.; Thiele, I.; Fleming, R.M.T.; Vempala, S. CHRR: Coordinate hit-and-run with rounding for uniform sampling of constraint-based models. Bioinformatics 2017, 33, 1741–1743. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Wang, H.; Marcišauskas, S.; Sánchez, B.J.; Domenzain, I.; Hermansson, D.; Agren, R.; Nielsen, J.; Kerkhoven, E.J. RAVEN 2.0: A versatile toolbox for metabolic network reconstruction and a case study on Streptomyces coelicolor. PLoS Comput. Biol. 2018, 14, e1006541. [Google Scholar] [CrossRef]
- Junne, S.; Solymosi, T.; Oosterhuis, N.; Neubauer, P. Cultivation of cells and microorganisms in wave-mixed disposable bag bioreactors at different scales. Chem. Ingeniur Tech. 2012, 85, 57–66. [Google Scholar] [CrossRef]
- Ozcengiz, G.; Demain, A.L. Recent advances in the biosynthesis of penicillins, cephalosporins and clavams and its regulation. Biotechnol. Adv. 2013, 31, 287–311. [Google Scholar] [CrossRef] [PubMed]
- Townsend, C. A New reactions in clavulanic acid biosynthesis. Curr. Opin. Chem. Biol. 2002, 6, 583–589. [Google Scholar] [CrossRef]
- Zhang, Z.; Ren, J.S.; Harlos, K.; McKinnon, C.H.; Clifton, I.J.; Schofield, C.J. Crystal structure of a clavaminate synthase-Fe(II)-2-oxoglutarate-substrate-NO complex: Evidence for metal centered rearrangements. FEBS Lett. 2002, 517, 7–12. [Google Scholar] [CrossRef]
- Elson, S.; Gillett, J.; Nicholson, N.H.; Tyler, J.W. N-Acyl derivatives of clavaminic acid produced by a mutant of Streptomyces clavuligerus. J. Am. Chem. Soc. Chem. Commun. 1988, 979–980. [Google Scholar] [CrossRef]
- Jensen, S.E.; Paradkar, A.S.; Mosher, R.H.; Anders, C.; Beatty, P.H.; Brumlik, M.J.; Griffin, A.; Barton, B. Five additional genes are involved in clavulanic acid biosynthesis in Streptomyces clavuligerus. Antimicrob. Agents Chemother. 2004, 48, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.A.; Dyson, B.C.; Vass, L.; Johnson, G.N.; Schwartz, J.-M. Flux sampling is a powerful tool to study metabolism under changing environmental conditions. NPJ Syst. Biol. Appl. 2019, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Niebel, B.; Leupold, S.; Heinemann, M. An upper limit on Gibbs energy dissipation governs cellular metabolism. Nat. Metab. 2019, 1, 125–132. [Google Scholar] [CrossRef] [PubMed]
- van Rosmalen, R.P.; Smith, R.W.; Martins dos Santos, V.A.P.; Fleck, C.; Suarez-Diez, M. Model reduction of genome-scale metabolic models as a basis for targeted kinetic models. Metab. Eng. 2021, 64, 74–84. [Google Scholar] [CrossRef]
- Shiio, I.; Ôtsuka, S.I.; Katsuya, N. Effect of biotin on the bacterial formation of glutamic acid: II. metabolism of glucose. J. Biochem. 1962, 52, 108–116. [Google Scholar] [CrossRef]
- Agrawal, S.; Agrawal, A.; Said, H.M. Biotin deficiency enhances the inflammatory response of human dendritic cells. Am. J. Physiol. Cell Physiol. 2016, 311, C386–C391. [Google Scholar] [CrossRef]
- Virolle, M.-J. A Challenging View: Antibiotics play a role in the regulation of the energetic metabolism of the producing bacteria. Antibiotics 2020, 9, 83. [Google Scholar] [CrossRef]



| Reaction | D = 0.045 | D = 0.035 | D = 0.050 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FBA | Exp. | SE | FBA | Exp. | SE | FBA | Exp. | SE | |
| Growth (h−1) | 0.039 | 0.045 | 0 | 0.034 | 0.035 | 0 | 0.044 | 0.050 | 0 |
| O2 | −0.375 | −1.665 | 1.662 | −0.322 | −1.621 | 1.686 | −0.511 | −1.848 | 1.787 |
| Glycerol | −0.695 | −1.110 | 0.172 | −0.639 | −0.968 | 0.108 | −0.728 | −0.728 | 0 |
| CO2 | 0.067 | 0.067 | 0 | 0.023 | 0.023 | 0 | 0.253 | 0.253 | 0 |
| Clavulanate | 0.017 | 0.398 | 0.145 | 0.015 | 0.357 | 0.117 | 0.020 | 0.435 | 0.172 |
| Phosphate | 0.000 | N/A | N/A | 0.000 | N/A | N/A | 0.000 | N/A | N/A |
| Glutamate | −0.350 | N/A | N/A | −0.310 | N/A | N/A | −0.400 | N/A | N/A |
| MSE | 0.396 | 0.382 | 0.392 | ||||||
| Reaction | Batch (36 h) | Fed-Batch (48 h) | ||||
|---|---|---|---|---|---|---|
| FBA | Exp. | SE | FBA | Exp. | SE | |
| Growth (h−1) | 0.042 | 0.042 ± 0.004 | 0 | 0.031 | 0.031 ± 0.003 | 0 |
| O2 | −0.512 | −1.350 | 0.702 | −0.776 | −1.2 | 0.180 |
| Glycerol | −0.182 | −0.182 | 0 | −0.457 | −0.47 | 0 |
| CO2 | 0.639 | 1.640 | 1.002 | 0.610 | 1.4 | 0.623 |
| Clavulanate | 0.002 | 0.002 | 0 | 0.004 | 0.004 | 0 |
| Succinate | 0.014 | 0.014 | 0 | 0.257 | 0.007 | 0.063 |
| Oxaloacetate | 0.004 | 0.004 | 0 | 0 | 0.003 | 0 |
| Malate | 0 | 0.001 | 0 | 0 | 0 | 0 |
| Pyruvate | 0 | 0 | 0 | 0.038 | 0.005 | 0.001 |
| Acetate | 0 | 0 | 0 | 0 | 0 | 0 |
| MSE | 1.700 | 0.860 | ||||
| Metabolites | Shadow Prices (CPLEX) | ||
|---|---|---|---|
| Batch (36 h) | Fed-Batch (48 h) | Pathway | |
| -ketoglutarate | 0 | 0.05 | Glycolysis and TCA cycle |
| Pyruvate | 0 | 0.02 | |
| Citrate | 0 | 0.05 | |
| Isocitrate | 0 | 0.05 | |
| Fumarate | 0 | 0.02 | |
| Acetate | 0 | 0.02 | |
| Succinate | 0 | 0.02 | |
| Oxaloacetate | 0 | 0.02 | |
| Malate | 0 | 0.02 | |
| Erythrose 4 phosphate | 0 | −0.40 | Pentose phosphate pathway |
| Fructose 6 phosphate | 0 | −0.38 | |
| Xylulose 5 phosphate | 0 | −0.39 | |
| Sedoheptulose 7-phosphate | 0 | −0.37 | |
| L-glutamate | 0.5 | 0.05 | Amino acids synthesis |
| L-glutamin | 0.5 | 0.05 | |
| L-arginine | 1.5 | 0.05 | |
| L-asparagin | 0.5 | 0.02 | |
| L-aspartate | 0.5 | 0.02 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez-Malule, H.; López-Agudelo, V.A.; Gómez-Ríos, D.; Ochoa, S.; Ríos-Estepa, R.; Junne, S.; Neubauer, P. TCA Cycle and Its Relationship with Clavulanic Acid Production: A Further Interpretation by Using a Reduced Genome-Scale Metabolic Model of Streptomyces clavuligerus. Bioengineering 2021, 8, 103. https://doi.org/10.3390/bioengineering8080103
Ramirez-Malule H, López-Agudelo VA, Gómez-Ríos D, Ochoa S, Ríos-Estepa R, Junne S, Neubauer P. TCA Cycle and Its Relationship with Clavulanic Acid Production: A Further Interpretation by Using a Reduced Genome-Scale Metabolic Model of Streptomyces clavuligerus. Bioengineering. 2021; 8(8):103. https://doi.org/10.3390/bioengineering8080103
Chicago/Turabian StyleRamirez-Malule, Howard, Víctor A. López-Agudelo, David Gómez-Ríos, Silvia Ochoa, Rigoberto Ríos-Estepa, Stefan Junne, and Peter Neubauer. 2021. "TCA Cycle and Its Relationship with Clavulanic Acid Production: A Further Interpretation by Using a Reduced Genome-Scale Metabolic Model of Streptomyces clavuligerus" Bioengineering 8, no. 8: 103. https://doi.org/10.3390/bioengineering8080103
APA StyleRamirez-Malule, H., López-Agudelo, V. A., Gómez-Ríos, D., Ochoa, S., Ríos-Estepa, R., Junne, S., & Neubauer, P. (2021). TCA Cycle and Its Relationship with Clavulanic Acid Production: A Further Interpretation by Using a Reduced Genome-Scale Metabolic Model of Streptomyces clavuligerus. Bioengineering, 8(8), 103. https://doi.org/10.3390/bioengineering8080103








