Dual Network Composites of Poly(vinyl alcohol)-Calcium Metaphosphate/Alginate with Osteogenic Ions for Bone Tissue Engineering in Oral and Maxillofacial Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dual Network Composite (DNC)
2.3. X-ray Diffraction (XRD) Spectroscopy
13C-Nuclear Magnetic Resonance Spectroscopy (13C-NMR)
2.4. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.5. Raman Spectroscopy
2.6. Compression Tests
2.6.1. Static Compression Tests
2.6.2. Cyclic Compression Tests
2.7. Water Uptake Study
2.8. Differential Scanning Calorimetry (DSC)
2.9. Scanning Electron Microscopy (SEM)
2.10. Micro-Computed Tomography (µCT)
2.11. In Vitro Biological Evaluation
2.11.1. MTT Assay for Cytotoxicity Evaluation
2.11.2. Protein Adsorption
2.11.3. Alamarblue™ Cell Proliferation Assay
2.11.4. Cell Lysate Preparation
2.11.5. RUNX2, BMP2, COL1A1, OPN and IBSP Gene Expression
2.11.6. Total Protein Production
2.11.7. Alkaline Phosphatase (ALP) Production
2.11.8. Osteocalcin (OCN) Production
2.12. Statistical Analysis
3. Results
3.1. Physicochemical Properties
3.2. Compressive Properties
3.3. Water Uptake
3.4. Thermal Properties
3.5. Morphological Properties
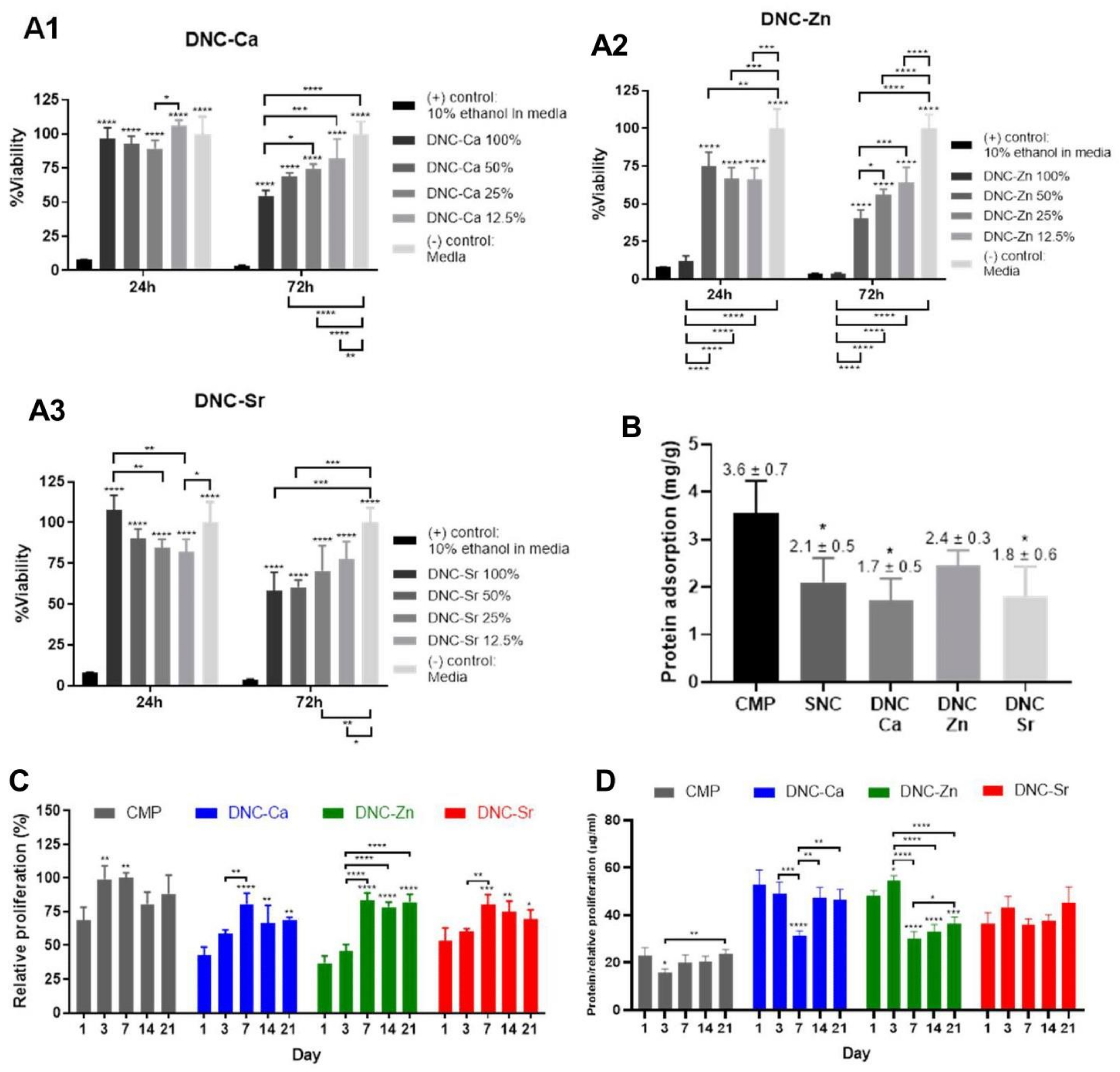
3.6. In Vitro Biological Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Young, S.; Kasper, F.K.; Melville, J.; Donahue, R.; Athanasiou, K.A.; Mikos, A.G.; Wong, M.E.-K. Chapter 65—Tissue Engineering in Oral and Maxillofacial Surgery. In Principles of Tissue Engineering, 5th ed.; Lanza, R., Langer, R., Vacanti, J.P., Atala, A., Eds.; Academic Press: London, UK, 2020; pp. 1201–1220. [Google Scholar] [CrossRef]
- Kinaci, A.; Neuhaus, V.; Ring, D.C. Trends in bone graft use in the United States. Orthopedics 2014, 37, e783–e788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, E.; Smith, B.T.; Smoak, M.M.; Tatara, A.M.; Shah, S.R.; Pearce, H.A.; Hogan, J.C.; Shum, J.; Melville, J.C.; Hanna, I.A. Localized mandibular infection affects remote in vivo bioreactor bone generation. Biomaterials 2020, 256, 120185. [Google Scholar] [CrossRef]
- Velasco, M.A.; Narváez-Tovar, C.A.; Garzón-Alvarado, D.A. Design, materials, and mechanobiology of biodegradable scaffolds for bone tissue engineering. Biomed. Res. Int. 2015, 2015, 729076. [Google Scholar] [CrossRef]
- Spicer, P.; Young, S.; Kasper, F.K.; Athanasiou, K.A.; Mikos, A.G.; Wong, M.E.-K. Tissue Engineering in Oral and Maxillofacial Surgery. In Principles of Tissue Engeering., 4th ed.; Lanza, R., Langer, R., Vacanti, J., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 1487–1506. [Google Scholar] [CrossRef]
- Rambhia, K.J.; Ma, P.X. Controlled drug release for tissue engineering. J. Contr. Release 2015, 219, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, L.M.C.; Silva, S.M.; Moulton, S.E. Growth factor delivery: Defining the next generation platforms for tissue engineering. J. Contr. Release 2019, 306, 40–58. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.; Awale, G.; Daneshmandi, L.; Umerah, O.; Lo, K.W.H. The roles of ions on bone regeneration. Drug Discov. Today 2018, 23, 879–890. [Google Scholar] [CrossRef]
- Kyzioł, A.; Khan, W.; Sebastian, V.; Kyzioł, K. Tackling microbial infections and increasing resistance involving formulations based on antimicrobial polymers. Chem. Eng. J. 2020, 385, 123888. [Google Scholar] [CrossRef]
- Tommasi, G.; Perni, S.; Prokopovich, P. An Injectable Hydrogel as Bone Graft Material with Added Antimicrobial Properties. Tissue Eng. A. 2016, 22, 862–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nkhwa, S.; Kemal, E.; Gurav, N.; Deb, S. Dual polymer networks: A new strategy in expanding the repertoire of hydrogels for biomedical applications. J. Mater. Sci. Mater. Med. 2019, 30, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Nkhwa, S.; Iskandar, L.; Gurav, N.; Deb, S. Combinatorial design of calcium meta phosphate poly(vinyl alcohol) bone-like biocomposites. J. Mater. Sci. Mater. Med. 2018, 29, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özarslan, A.C.; Yücel, S. Fabrication and characterization of strontium incorporated 3-D bioactive glass scaffolds for bone tissue from biosilica. Mater. Sci. Eng. C 2016, 68, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Ke, D.; Bose, S. Doped tricalcium phosphate bone tissue engineering scaffolds using sucrose as template and microwave sintering: Enhancement of mechanical and biological properties. Mater. Sci. Eng. C 2017, 78, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, L.; Rojo, L.; Di Silvio, L.; Deb, S. The effect of chelation of sodium alginate with osteogenic ions, calcium, zinc, and strontium. J. Biomater. Appl. 2019, 34, 573–584. [Google Scholar] [CrossRef]
- Nkhwa, S. Hydrogel Biocomposites for Bone Tissue Regeneration; King’s College London: London, UK, 2016. [Google Scholar]
- Yang, H.; Xu, S.; Jiang, L.; Dan, Y. Thermal Decomposition Behavior of Poly (Vinyl Alcohol) with Different Hydroxyl Content. J. Macromol. Sci. B 2012, 51, 464–480. [Google Scholar] [CrossRef]
- Bai, R.; Yang, J.; Suo, Z. Fatigue of hydrogels. Eur. J. Mech. A Solids 2019, 74, 337–370. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Qi, X.; Hu, X.; Wei, W.; Yu, H.; Li, J.; Zhang, J.; Dong, W. Investigation of Salecan/poly(vinyl alcohol) hydrogels prepared by freeze/thaw method. Carbohydr. Polym. 2015, 118, 9–60. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Kouvelos, E.P.; Favvas, E.P.; Sapalidis, A.A.; Romanos, G.E.; Katsaros, F.K. Metal-carboxylate interactions in metal-alginate complexes studied with FTIR spectroscopy. Carbohydr. Res. 2010, 345, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Karampas, I.A.; Kontoyannis, C.G. Characterization of calcium phosphates mixtures. Vibrational Spectrosc. 2013, 64, 126–133. [Google Scholar] [CrossRef]
- Kudo, K.; Ishida, J.; Syuu, G.; Sekine, Y.; Ikeda-Fukazawa, T. Structural changes of water in poly(vinyl alcohol) hydrogel during dehydration. J. Chem. Phys. 2014, 140, 44909. [Google Scholar] [CrossRef]
- Campos-Vallette, M.M.; Chandía, N.P.; Clavijo, E.; Leal, D.; Matsuhiro, B.; Osorio-Román, I.O.; Torres, S. Characterization of sodium alginate and its block fractions by surface-enhanced Raman spectroscopy. J. Raman Spectrosc. 2010, 41, 758–763. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ando, I.; Ishii, T.; Amiya, S. Structural and dynamical studies of poly(vinyl alcohol) gels by high-resolution solid-state 13C NMR spectroscopy. J. Mol. Struct. 1998, 440, 155–164. [Google Scholar] [CrossRef]
- Brus, J.; Urbanova, M.; Czernek, J.; Pavelkova, M.; Kubova, K.; Vyslouzil, J.; Abbrent, S.; Konefal, R.; Horský, J.; Vetchy, D.; et al. Structure and Dynamics of Alginate Gels Cross-Linked by Polyvalent Ions Probed via Solid State NMR Spectroscopy. Biomacromol. J. 2017, 18, 2478–2488. [Google Scholar] [CrossRef] [Green Version]
- Zamani, D.; Moztarzadeh, F.; Bizari, D. Alginate-bioactive glass containing Zn and Mg composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019, 137, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Liu, J.Z.; Chang, S.L.Y.; Wu, Y.; Li, D. Biomimetic superelastic graphene-based cellular monoliths. Nat Commun. 2012, 3, 1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Sou, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef]
- Martínez-Gómez, F.; Guerrero, J.; Matsuhiro, B.; Pavez, J. In vitro release of metformin hydrochloride from sodium alginate/polyvinyl alcohol hydrogels. Carbohydr. Polym. 2017, 155, 182–191. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, N.; Zhu, D. Endothelial Cellular Responses to Biodegradable Metal Zinc. ACS Biomater. Sci. Eng. 2015, 1, 1174–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeno, S.; Niki, Y.; Matsumoto, H.; Morioka, H.; Yatabe, T.; Funayama, A.; Toyama, Y.; Taguchi, T.; Tanaka, J. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials 2005, 26, 4847–4855. [Google Scholar] [CrossRef]
- Er, K.; Polat, Z.A.; Özan, F.; Taşdemir, T.; Sezer, U.; Siso, Ş.H. Cytotoxicity Analysis of Strontium Ranelate on Cultured Human Periodontal Ligament Fibroblasts: A Preliminary Report. J. Formos. Med. Assoc. 2008, 107, 609–615. [Google Scholar] [CrossRef] [Green Version]
- Brash, J.L.; Horbett, T.A. Chapter 1–Proteins at Interfaces. In Proteins at Interfaces II.; Brash, J.L., Horbett, T.A., Eds.; American Chemical Society: Washington, DC, USA, 1995; Volume 602, pp. 1–23. [Google Scholar] [CrossRef] [Green Version]
- Swartzlander, M.D.; Barnes, C.A.; Blakney, A.K.; Kaar, J.L.; Kyriakides, T.R.; Bryant, S.J. Linking the foreign body response and protein adsorption to PEG-based hydrogels using proteomics. Biomaterials 2015, 41, 26–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoker, M.G.P.; Rubin, H. Density Dependent Inhibition of Cell Growth in Culture. Nature 1967, 215, 171–172. [Google Scholar] [CrossRef]
- Lian, J.B.; Stein, G.S. Development of the osteoblast phenotype: Molecular mechanisms mediating osteoblast growth and differentiation. Iowa Orthop. J. 1995, 15, 118–140. Available online: https://pubmed.ncbi.nlm.nih.gov/7634023 (accessed on 4 January 2021).
- Aubin, J.E. Mesenchymal Stem Cells and Osteoblast Differentiation. Princ. Bone Biol. 2008, 1, 85–107. [Google Scholar] [CrossRef]
- Sadtler, K.; Singh, A.; Wolf, M.; Wang, X.; Pardoll, D.; Elisseeff, J. Design, clinical translation and immunological response of biomaterials in regenerative medicine. Nat. Rev. Mater. 2016, 1, 16040. [Google Scholar] [CrossRef]
- Marrazzo, P.; O’Leary, C. Repositioning Natural Antioxidants for Therapeutic Applications in Tissue Engineering. Bioengineering 2020, 7, 104. [Google Scholar] [CrossRef] [PubMed]








| No. | Action | Target Temperature | Duration/Rate | Purpose |
|---|---|---|---|---|
| 1 | Hold | 110 °C | 10 min | To evaporate the water molecules in the sample without decomposing its constituents (reported temperature of PVA thermal decomposition was 280–290 °C [18]) |
| 2 | Cool | 0 °C | 100 °C/min | Rapid cooling |
| 3 | Heat | 110 °C | 10 °C/min | To erase the thermal history of the sample |
| 4 | Cool | −10 °C | 100 °C/min | Rapid cooling |
| 5 | Hold | −10 °C | 5 min | To stabilise the baseline, avoiding disrupted curve |
| 6 | Heat | 300 °C | 10 °C/min | To get a clean curve for phase examination |
| 7 | Cool | 30 °C | 100 °C/min | Rapid cooling to finish the test |
| Gene | Forward Primer (5′ 3′) | Reverse Primer (5′ 3′) |
|---|---|---|
| GAPDH | ACAGTTGCCATGTAGACC | TTGAGCACAGGGTACTTTA |
| RUNX2 | AAGCTTGATGACTCTAAACC | TCTGTAATCTGACTCTGTCC |
| BMP2 | TCCACCATGAAGAATCTTTG | TAATTCGGTGATGGAAACTG |
| COL1A1 | GCTATGATGAGAAATCAACCG | TCATCTCCATTCTTTCCAGG |
| OPN | GACCAAGGAAAACTCACTAC | CTGTTTAACTGGTATGGCAC |
| IBSP | GGAGACTTCAAATGAAGGAG | CAGAAAGTGTGGTATTCTCAG |
| Composite | EWC (%) | Statistical Significance | ||
|---|---|---|---|---|
| DNC-Ca | DNC-Zn | DNC-Sr | ||
| SNC | 34.4 ± 1.8 | 0.0755 (ns) | 0.0033 (**) | 0.0319 (*) |
| DNC-Ca | 37.3 ± 0.9 | 0.1606 (ns) | 0.9252 (ns) | |
| DNC-Zn | 39.6 ± 0.3 | 0.3572 (ns) | ||
| DNC-Sr | 37.6 ± 1.2 | |||
| A | Tg (°C) | Statistical Significance (p-Value) | ||
| DNC-Ca | DNC-Zn | DNC-Sr | ||
| SNC | 124.2 ± 0.7 | 0.0098 (**) | 0.0014 (**) | 0.0008 (***) |
| DNC-Ca | 132.0 ± 0.9 | 0.4200 (ns) | 0.2046 (ns) | |
| DNC-Zn | 134.9 ± 3.6 | 0.9375 (ns) | ||
| DNC-Sr | 135.9 ± 2.2 | |||
| B | Tm (°C) | Statistical Significance (p-Value) | ||
| DNC-Ca | DNC-Zn | DNC-Sr | ||
| SNC | 228.8 ± 0.6 | 0.8569 (ns) | 0.1270 (ns) | 0.1631 (ns) |
| DNC-Ca | 228.6 ± 0.4 | 0.3806 (ns) | 0.4448 (ns) | |
| DNC-Zn | 228.1 ± 0.1 | 0.9977 (ns) | ||
| DNC-Sr | 228.1 ± 0.1 | |||
| A | Obj.V/TV (%) | Statistical Significance (p-Value) | ||
| DNC-Ca | DNC-Zn | DNC-Sr | ||
| SNC | 60.7 ± 7.9 | 0.1088 (ns) | 0.0243 (*) | 0.0062 (**) |
| DNC-Ca | 72.5 ± 7.9 | 0.7152 (ns) | 0.2289 (ns) | |
| DNC-Zn | 77.2 ± 6.8 | 0.7336 (ns) | ||
| DNC-Sr | 81.8 ± 2.0 | |||
| B | Total Porosity (%) | Statistical Significance (p-Value) | ||
| DNC-Ca | DNC-Zn | DNC-Sr | ||
| SNC | 39.3 ± 7.9 | 0.1088 (ns) | 0.0243 (*) | 0.0062 (**) |
| DNC-Ca | 27.5 ± 2.5 | 0.7152 (ns) | 0.2289 (ns) | |
| DNC-Zn | 22.8 ± 6.8 | 0.7336 (ns) | ||
| DNC-Sr | 18.2 ± 2.0 | |||
| C | Open Porosity (%) | Statistical Significance (p-Value) | ||
| DNC-Ca | DNC-Zn | DNC-Sr | ||
| SNC | 36.5 ± 8.7 | 0.1450 (ns) | 0.0276 (*) | 0.0075 (**) |
| DNC-Ca | 24.3 ± 3.3 | 0.6539 (ns) | 0.2132 (ns) | |
| DNC-Zn | 18.4 ± 7.7 | 0.7639 (ns) | ||
| DNC-Sr | 13.4 ± 2.1 | |||
| D | Open/Total Porosity | Statistical Significance (p-Value) | ||
| DNC-Ca | DNC-Zn | DNC-Sr | ||
| SNC | 0.93 ± 0.04 | 0.9945 (ns) | 0.9978 (ns) | 0.1162 (ns) |
| DNC-Ca | 0.88 ± 1.31 | 0.9732 (ns) | 0.0825 (ns) | |
| DNC-Zn | 0.81 ± 1.14 | 0.1489 (ns) | ||
| DNC-Sr | 0.74 ± 1.07 | |||
| E | Degree of Anisotropy | Statistical Significance (p-Value) | ||
| DNC-Ca | DNC-Zn | DNC-Sr | ||
| SNC | 0.10 ± 0.04 | 0.9875 (ns) | 0.9945 (ns) | 0.1167 (ns) |
| DNC-Ca | 0.08 ± 0.03 | 0.9393 (ns) | 0.0740 (ns) | |
| DNC-Zn | 0.12 ± 0.03 | 0.1636 (ns) | ||
| DNC-Sr | 0.26 ± 0.14 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iskandar, L.; DiSilvio, L.; Acheson, J.; Deb, S. Dual Network Composites of Poly(vinyl alcohol)-Calcium Metaphosphate/Alginate with Osteogenic Ions for Bone Tissue Engineering in Oral and Maxillofacial Surgery. Bioengineering 2021, 8, 107. https://doi.org/10.3390/bioengineering8080107
Iskandar L, DiSilvio L, Acheson J, Deb S. Dual Network Composites of Poly(vinyl alcohol)-Calcium Metaphosphate/Alginate with Osteogenic Ions for Bone Tissue Engineering in Oral and Maxillofacial Surgery. Bioengineering. 2021; 8(8):107. https://doi.org/10.3390/bioengineering8080107
Chicago/Turabian StyleIskandar, Lilis, Lucy DiSilvio, Jonathan Acheson, and Sanjukta Deb. 2021. "Dual Network Composites of Poly(vinyl alcohol)-Calcium Metaphosphate/Alginate with Osteogenic Ions for Bone Tissue Engineering in Oral and Maxillofacial Surgery" Bioengineering 8, no. 8: 107. https://doi.org/10.3390/bioengineering8080107
APA StyleIskandar, L., DiSilvio, L., Acheson, J., & Deb, S. (2021). Dual Network Composites of Poly(vinyl alcohol)-Calcium Metaphosphate/Alginate with Osteogenic Ions for Bone Tissue Engineering in Oral and Maxillofacial Surgery. Bioengineering, 8(8), 107. https://doi.org/10.3390/bioengineering8080107






