Motor Nerve Conduction Block Estimation in Demyelinating Neuropathies by Deconvolution
Abstract
:1. Introduction
2. Methods
2.1. Mathematical Model and Processing
- seven parameters related to the AH functions (the scaling factor and the amplitude of the six functions; these are the only parameters considered in [19]);
- two parameters defining the descending exponential , namely, the amplitude A and the time constant
- two parameters defining the sigmoid function , i.e., the time instant c in which and the rate of change a
2.2. Experimental Data
2.3. Statistical Analysis
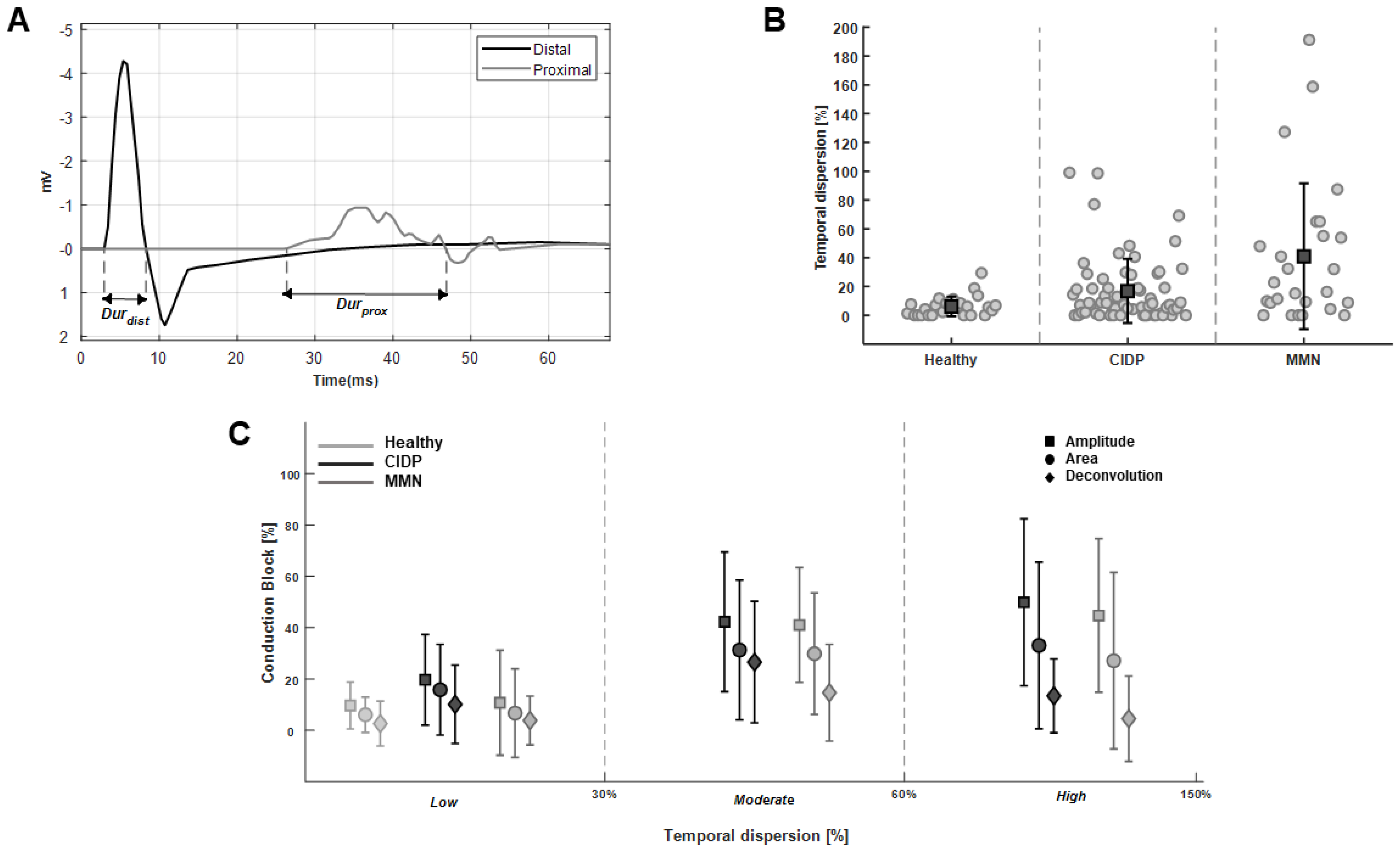
3. Results
4. Discussion
4.1. Significance
4.2. Limitations
4.3. Future Perspectives
5. Conclusions and Further Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AP | Action Potential |
| CB | Conduction Block |
| CIDP | Chronic Inflammatory Demyelinating Polyneuropathy |
| CMAP | Compound Muscle Action Potential |
| EDX | Electrodiagnostic |
| EMG | Electromyogram |
| MMN | Multifocal Motor Neuropathy |
| MU | Motor Unit |
| MUAP | Motor Unit Action Potential |
| NEE | Needle Electrode Examination |
| NCS | Nerve Conduction Study |
| PNS | Peripheral Nervous System |
| SAW | Slow AfterWave |
| TD | Temporal Dispersion |
References
- Eftimov, F.; Lucke, I.M.; Querol, L.A.; Rajabally, Y.A.; Verhamme, C. Diagnostic challenges in chronic inflammatory demyelinating polyradiculoneuropathy. Brain 2020, 143, 3214–3224. [Google Scholar] [CrossRef]
- Ferrante, M.A.; Spiegelberg, B.T.; Tsao, B.E. Principles of Nerve Conduction Studies and Needle EMG; American Association of Neuromuscular & Electrodiagnostic Medicine: Rochester, NY, USA, 2014; pp. 1–14. [Google Scholar]
- Feasby, T.E.; Brown, W.F.; Gilbert, J.J.; Hahn, A.F. The pathological basis of conduction block in human neuropathies. J. Neurol. Neurosurg. Psychiatry 1985, 48, 239–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, J. Consequences of peripheral nerve demyelination: Basic and clinical aspects. Can. J. Neurol. Sci. 1993, 20, 263–270. [Google Scholar] [PubMed]
- Coleman, M.P.; Freeman, M.R. Wallerian degeneration, wld(s), and nmnat. Annu. Rev. Neurosci. 2010, 33, 245–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stathopoulos, P.; Alexopoulos, H.; Dalakas, M.C. Autoimmune antigenic targets at the node of Ranvier in demyelinating disorders. Nat. Rev. Neurol. 2015, 11, 143–156. [Google Scholar] [CrossRef]
- Spina, E.; Doneddu, P.E.; Liberatore, G.; Cocito, D.; Fazio, R.; Briani, C.; Filosto, M.; Benedetti, L.; Antonini, G.; Cosentino, G.; et al. Prolonged distal motor latency of median nerve does not improve diagnostic accuracy for CIDP. J. Neurol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Mattler, W.J.; Jakob, M.; Zierz, S. Assessment of temporal dispersion in motor nerves with normal conduction velocity. Clin. Neurophysiol. 1999, 110, 740–747. [Google Scholar] [CrossRef]
- Ad Hoc subcommittee of the American Academy of Neurology AIDS Task Force. Research criteria for diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP). Neurology 1991, 41, 617–618. [Google Scholar] [CrossRef]
- Schulte-Mattler, W.J.; Muller, T.; Georgiadis, D.; Kornhuber, M.E.; Zierz, S. Length dependence of variables associated with temporal dispersion in human motor nerves. Muscle Nerve 2001, 24, 527–533. [Google Scholar] [CrossRef]
- Reutskiy, S.; Rossoni, E.; Tirozzi, B. Conduction in bundles of demyelinated nerve fibers: Computer simulation. Biol. Cybern. 2003, 89, 439–448. [Google Scholar] [CrossRef]
- Rhee, E.K.; England, J.D.; Sumner, A.J. A computer simulation of conduction block: Effects produced by actual block versus interphase cancellation. Ann. Neurol. 1990, 28, 146–156. [Google Scholar] [CrossRef]
- Stalberg, E.; Karlsson, L. The motor nerve simulator. Clin. Neurophysiol. 2001, 112, 2118–2132. [Google Scholar] [CrossRef]
- Tani, T.; Ushida, T.; Yamamoto, H.; Okuhara, Y. Waveform changes due to conduction block and their underlying mechanism in spinal somatosensory evoked potential: A computer simulation. J. Neurosurg. 1997, 86, 303–310. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, D.E.; Kuruoglu, H.R. What is the best diagnostic index of conduction block and temporal dispersion? Muscle Nerve 1994, 17, 489–493. [Google Scholar] [CrossRef]
- Van Asseldonk, J.T.; Van den Berg, L.H.; Wieneke, G.H.; Wokke, J.H.; Franssen, H. Criteria for conduction block based on computer simulation studies of nerve conduction with human data obtained in the forearm segment of the median nerve. Brain 2006, 129, 2447–2460. [Google Scholar] [CrossRef]
- Fuglsang-Frederiksen, A.; Pugdahl, K. Current status on electrodiagnostic standards and guidelines in neuromuscular disorders. Clin. Neurophysiol. 2011, 122, 440–455. [Google Scholar] [CrossRef]
- Olney, R.K.; Lewis, R.A.; Putnam, T.D.; Campellone, J.V., Jr.; American Association of Electrodiagnostic Medicine. Consensus criteria for the diagnosis of multifocal motor neuropathy. Muscle Nerve 2003, 27, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Mesin, L.; Cocito, D. A new method for the estimation of motor nerve conduction block. Clin. Neurophysiol. 2007, 118, 730–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimitrova, N.A. Model of the extracellular potential field of a single striated muscle fibre. Electromyogr. Clin. NeurOphysiol. 1974, 14, 53–66. [Google Scholar] [PubMed]
- Lateva, Z.C.; McGill, K.C.; Burgar, C.G. Anatomical and electrophysiological determinants of the human thenar compound muscle action potential. Muscle Nerve 1996, 19, 1457–1468. [Google Scholar] [CrossRef]
- Lateva, Z.C.; McGill, K.C. The physiological origin of the slow afterwave in muscle action potentials. Electroencephalogr. Clin. Neurophysiol. 1998, 109, 462–469. [Google Scholar] [CrossRef]
- McGill, K.C.; Lateva, Z.C. Slow repolarization phase of the intracellular action potential influences the motor unit action potential. Muscle Nerve 2000, 23, 826–828. [Google Scholar] [CrossRef]
- McGill, K.C.; Lateva, Z.C.; Xiao, S. A model of the muscle action potential for describing the leading edge, terminal wave, and slow afterwave. IEEE Trans. Biomed. Eng. 2001, 48, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- McGill, K.C.; Lateva, Z.C. A model of the muscle-fiber intracellular action potential waveform, including the slow repolarization phase. IEEE Trans. Biomed. Eng. 2001, 48, 1480–1483. [Google Scholar] [CrossRef] [PubMed]
- Benoit, C.; Svahn, J.; Debs, R.; Vandendries, C.; Lenglet, T.; Zyss, J.; Maisonobe, T.; Viala, K. Focal chronic inflammatory demyelinating polyradiculoneuropathy: Onset, course, and distinct features. J. Peripher. Nerv. Syst. 2021, 26, 193–201. [Google Scholar] [CrossRef]
- Spina, E.; Doneddu, P.E.; Liberatore, G.; Cocito, D.; Fazio, R.; Briani, C.; Filosto, M.; Benedetti, L.; Antonini, G.; Cosentino, G.; et al. The neurophysiological lesson from the Italian CIDP database. Neurol. Sci. 2021, 43, 573–582. [Google Scholar] [CrossRef]
- Topakian, R.; Muller, P.; Ciovica-Oel, I.C.; Trenkler, J. In the borderland of multifocal motor neuropathy and chronic inflammatory demyelinating polyradiculopathy. Neurol. Sci. 2021, 42, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Tracy, J.A.; Taylor, B.V.; Kiernan, M.; Dyck, P.J.; Crum, B.A.; Mauermann, M.L.; Amrami, K.K.; Spinner, R.J.; Dyck, P.J.B. Nerve Pathology Distinguishes Focal Motor Chronic Inflammatory Demyelinating Polyradiculoneuropathy From Multifocal Motor Neuropathy. J. Clin. Neuromuscul. Dis. 2020, 22, 1–10. [Google Scholar] [CrossRef]
- Van den Bergh, P.Y.K.; van Doorn, P.A.; Hadden, R.D.M.; Avau, B.; Vankrunkelsven, P.; Allen, J.A.; Attarian, S.; Blomkwist-Markens, P.H.; Cornblath, D.R.; Eftimov, F.; et al. European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint Task Force-Second revision. J. Peripher. Nerv. Syst. 2021, 26, 242–268. [Google Scholar] [CrossRef]
- Corazza, G.; Le Corroller, T.; Grapperon, A.M.; Salort-Campana, E.; Verschueren, A.; Attarian, S.; Delmont, E. Comparison of MRI and motor evoked potential with triple stimulation technique for the detection of brachial plexus abnormalities in multifocal motor neuropathy. Muscle Nerve 2020, 61, 325–329. [Google Scholar] [CrossRef]
- Li, Y.; Niu, J.; Liu, T.; Ding, Q.; Wu, S.; Guan, Y.; Cui, L.; Liu, M. Conduction Block and Nerve Cross-Sectional Area in Multifocal Motor Neuropathy. Front. Neurol. 2019, 10, 1055. [Google Scholar] [CrossRef]
- Mesin, L. Volume conductor models in surface electromyography: Computational techniques. Comput. Biol. Med. 2013, 43, 942–952. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D. Review of Methods for Solving the EEG Inverse Problem. Int. J. Bioelectromagn. 1999, 1, 75–86. [Google Scholar]
- Schoonhoven, R.; Stegeman, D.F. Models and analysis of compound nerve action potentials. Crit. Rev. Biomed. Eng. 1991, 19, 47–111. [Google Scholar] [PubMed]
- Tikhonov, A.N.; Arsenin, V. Solution of Ill-Posed Problems; Wiley: Hoboken, NJ, USA, 1977; Volume 21, pp. 266–267. [Google Scholar]
- Tu, Y.X.; Wernsdorfer, A.; Honda, S.; Tomita, Y. Estimation of conduction velocity distribution by regularized-least-squares method. IEEE Trans. Biomed. Eng. 1997, 44, 1102–1106. [Google Scholar] [CrossRef]
- Kimura, J. Electrodiagnosis in Disease of the Nerve and Muscle: Principles and Practice, 2nd ed.; Oxford University Press: Oxford, UK, 1989; pp. 103–138. [Google Scholar]
- Olney, R.K.; Budingen, H.J.; Miller, R.G. The effect of temporal dispersion on compound action potential area in human peripheral nerve. Muscle Nerve 1987, 10, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, P.Y.K.; Hadden, R.D.; Bouche, P.; Cornblath, D.R.; Hahn, A.; Illa, I.; Koski, C.L.; Léger, J.M.; Nobile-Orazio, E.; Pollard, J.; et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society-first revision. Eur. J. Neurol. 2010, 17, 356–363, Erratum in: Eur. J. Neurol. 2011, 18, 796. [Google Scholar]
- Joint Task Force of the EFNS and the PNS. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of multifocal motor neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society–first revision. J. Peripher. Nerv. Syst. 2010, 15, 295–301. [Google Scholar] [CrossRef]
- Di Stefano, V.; Gagliardo, A.; Barbone, F.; Vitale, M.; Ferri, L.; Lupica, A.; Iacono, S.; Di Muzio, A.; Brighina, F. Median-to-Ulnar Nerve Communication in Carpal Tunnel Syndrome: An Electrophysiological Study. Neurol. Int. 2021, 13, 304–314. [Google Scholar] [CrossRef]
- Scheirer, C.J.; Ray, W.S.; Hare, N. The analysis of ranked data derived from completely randomized factorial designs. Biometrics 1976, 32, 429–434. [Google Scholar] [CrossRef]
- American Association of Electrodiagnostic Medicine; Olney, R.K. Guidelines in electrodiagnostic medicine. Consensus criteria for the diagnosis of partial conduction block. Muscle Nerve Suppl. 1999, 8, S225–S229. [Google Scholar] [PubMed]
- Allen, J.A.; Ney, J.; Lewis, R.A. Electrodiagnostic errors contribute to chronic inflammatory demyelinating polyneuropathy misdiagnosis. Muscle Nerve 2018, 57, 542–549. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesin, L.; Lingua, E.; Cocito, D. Motor Nerve Conduction Block Estimation in Demyelinating Neuropathies by Deconvolution. Bioengineering 2022, 9, 23. https://doi.org/10.3390/bioengineering9010023
Mesin L, Lingua E, Cocito D. Motor Nerve Conduction Block Estimation in Demyelinating Neuropathies by Deconvolution. Bioengineering. 2022; 9(1):23. https://doi.org/10.3390/bioengineering9010023
Chicago/Turabian StyleMesin, Luca, Edoardo Lingua, and Dario Cocito. 2022. "Motor Nerve Conduction Block Estimation in Demyelinating Neuropathies by Deconvolution" Bioengineering 9, no. 1: 23. https://doi.org/10.3390/bioengineering9010023
APA StyleMesin, L., Lingua, E., & Cocito, D. (2022). Motor Nerve Conduction Block Estimation in Demyelinating Neuropathies by Deconvolution. Bioengineering, 9(1), 23. https://doi.org/10.3390/bioengineering9010023







