A Bioprinted Heart-on-a-Chip with Human Pluripotent Stem Cell-Derived Cardiomyocytes for Drug Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Hydrogel Materials
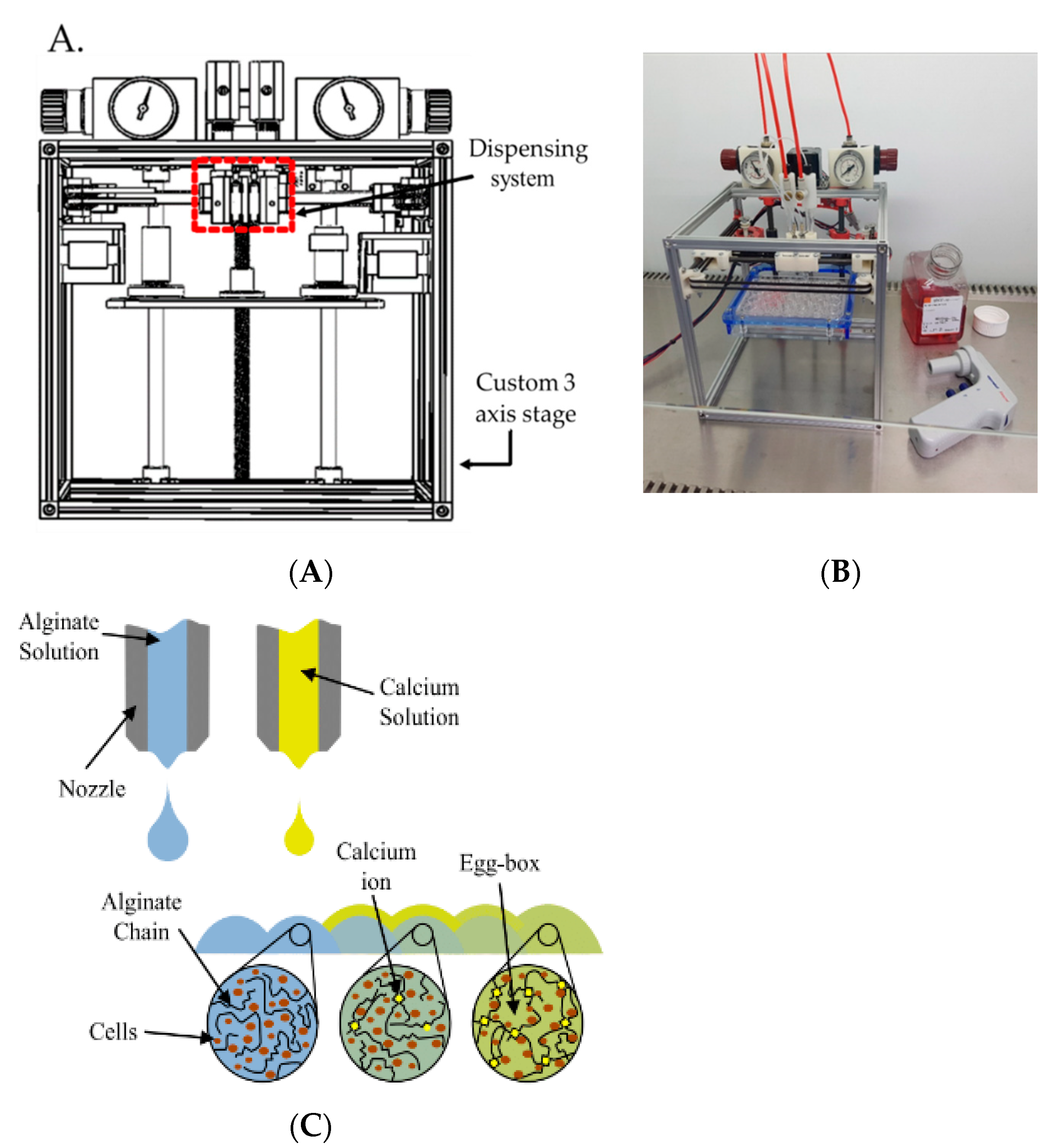
2.3. Bioprinting Platform
2.4. Substrate Modification
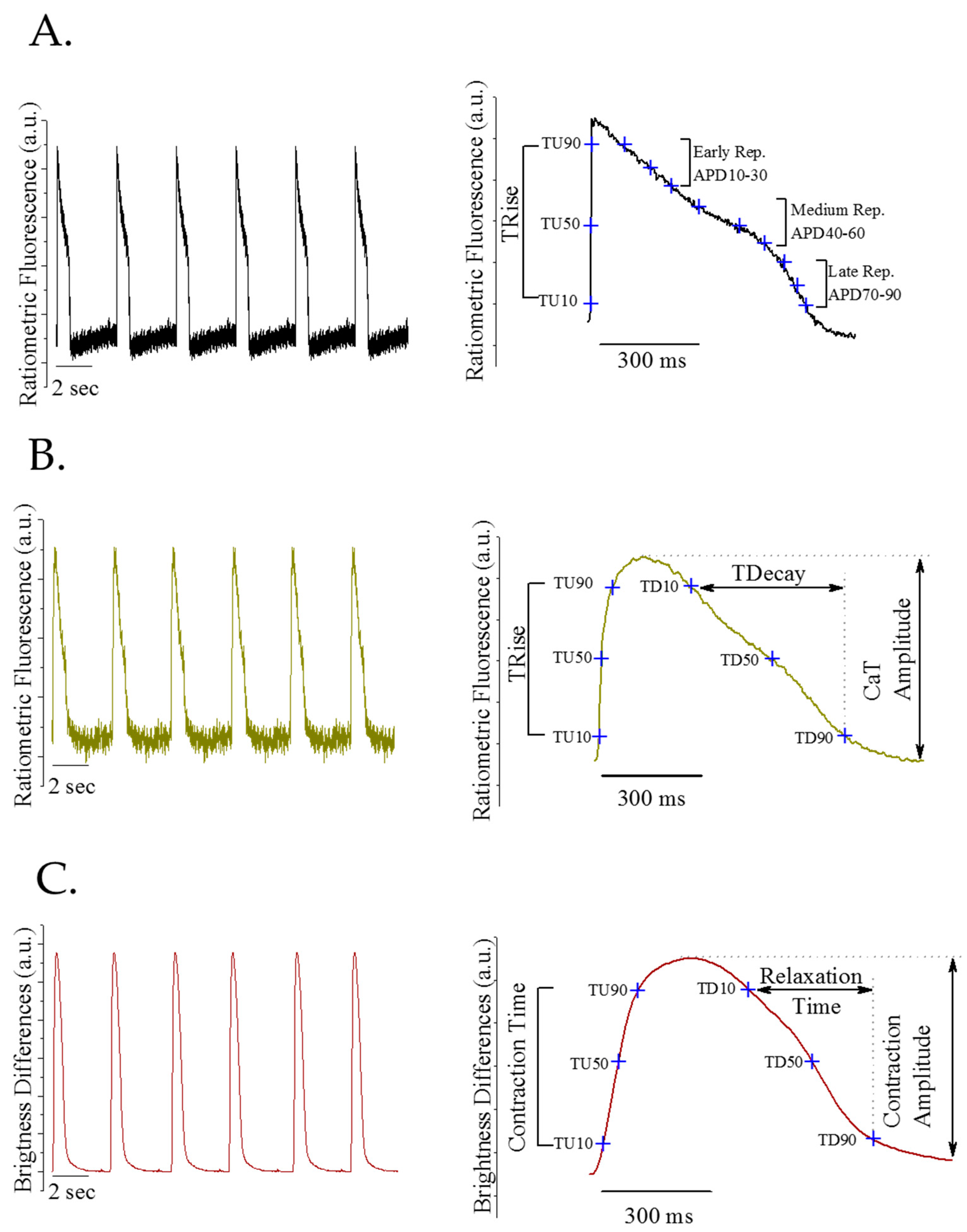
2.5. High Sensitivity Physiological Function Measurement
2.6. Cardiotoxicity Screening of Hydrogels and Their Cross-Linkers
2.7. Electrophysiology Assessment
2.8. Bioprinting of Alginate Barriers for Reduced Cellular Numbers
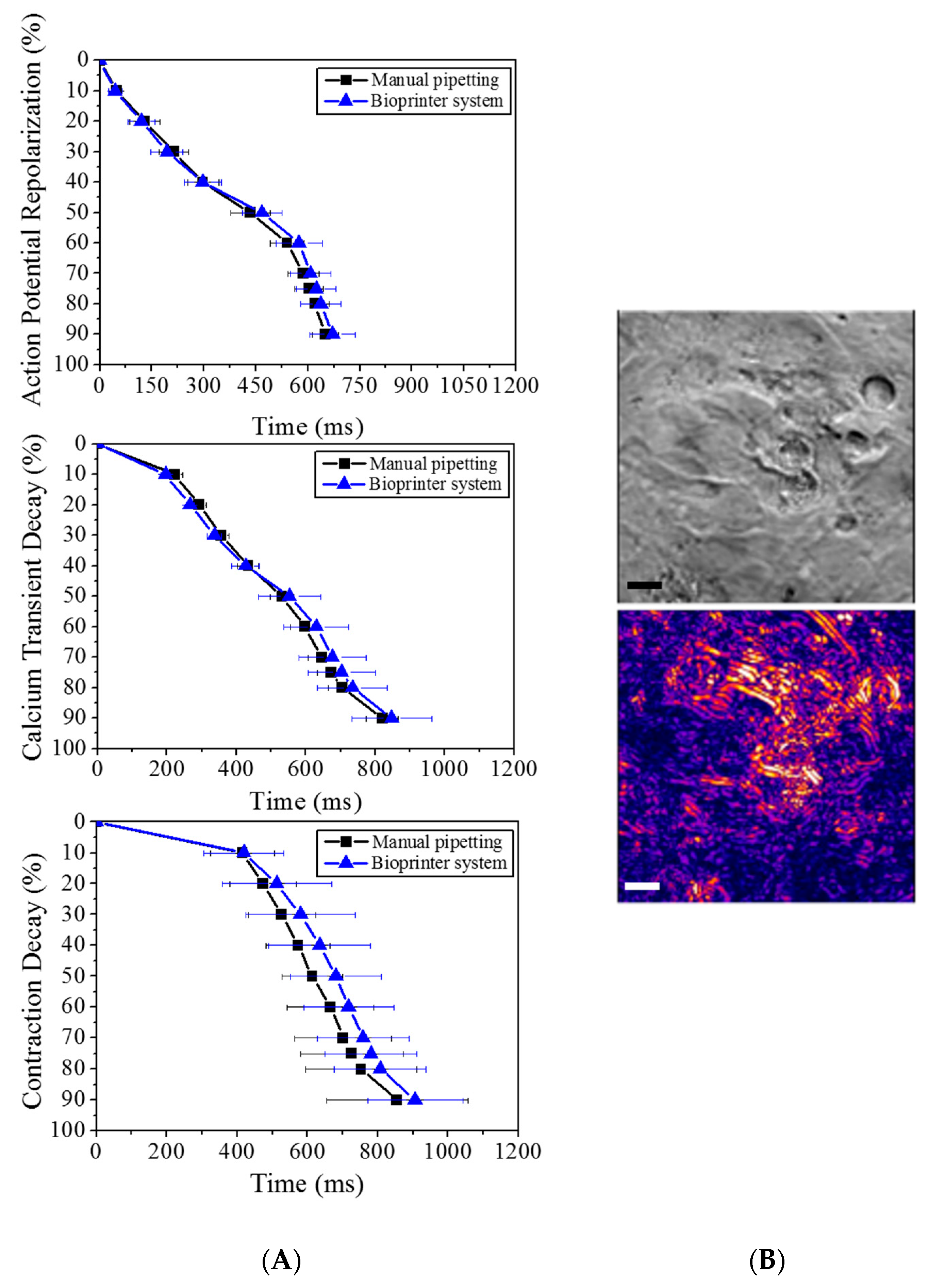
2.9. Drug Testing Process Flow Integration and Validation
2.10. Data Analysis and Statistics
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dickson, M.; Gagnon, J.P. The cost of new drug discovery and development. Discov. Med. 2004, 4, 172–179. [Google Scholar]
- Kaitin, K. Deconstructing the Drug Development Process: The New Face of Innovation Current Challenges for the Research-Based Industry. Clin. Pharmocol. Ther. 2010, 3, 356–361. [Google Scholar] [CrossRef]
- Paul, S.M.; Mytelka, D.S.; Dunwiddie, C.T.; Persinger, C.C.; Munos, B.H.; Lindborg, S.R.; Schacht, A.L. How to improve R&D productivity: The pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 2010, 9, 203–214. [Google Scholar]
- Scott, C.W.; Peters, M.F.; Dragan, Y.P. Human induced pluripotent stem cells and their use in drug discovery for toxicity testing. Toxicol. Lett. 2013, 219, 49–58. [Google Scholar] [CrossRef]
- Ferri, N.; Siegl, P.; Corsini, A.; Herrmann, J.; Lerman, A.; Benghozi, R. Drug attrition during pre-clinical and clinical development: Understanding and managing drug-induced cardiotoxicity. Pharmacol. Ther. 2013, 138, 470–484. [Google Scholar] [CrossRef]
- Ho, D.; Zhao, X.; Gao, S.; Hong, C.; Vatner, D.E.; Vatner, S.F. Heart Rate and Electrocardiography Monitoring in Mice. Curr. Protoc. Mouse Biol. 2011, 1, 123–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huyer, L.D.; Montgomery, M.; Zhao, Y.; Xiao, Y.; Conant, G.; Korolj, A.; Radisic, M. Biomaterial based cardiac tissue engineering and its applications. Biomed. Mater. 2015, 10, 034004. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, J.C.; Linartz, R.R.; Hamlin, R.L.; Stoll, R.E. Noninvasive measurement of systemic arterial blood pressure in the conscious beagle dog. Fundam. Appl. Toxicol. 1988, 10, 89–97. [Google Scholar] [CrossRef]
- Kline, D.D.; Hasser, E.M.; Heesch, C.M. Regulation of the heart. In Dukes’ Physiology of Domestic Animals; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 341–351. ISBN 9781118501399. [Google Scholar]
- Lin, X.; Tang, J.; Lou, Y.R. Human pluripotent stem-cell-derived models as a missing link in drug discovery and development. Pharmaceuticals 2021, 14, 525. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Kiyokawa, J.; Tabo, M.; Inoue, T. Electrophysiological characterization of cardiomyocytes derived from human induced pluripotent stem cells. J. Pharmacol. Sci. 2011, 117, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Del Álamo, J.C.; Lemons, D.; Serrano, R.; Savchenko, A.; Cerignoli, F.; Bodmer, R.; Mercola, M. High throughput physiological screening of iPSC-derived cardiomyocytes for drug development. Biochim. Biophys. Acta 2016, 1863, 1717–1727. [Google Scholar] [CrossRef]
- Smith, A.S.T.; Macadangdang, J.; Leung, W.; Laflamme, M.A.; Kim, D.-H. Human iPSC-derived cardiomyocytes and tissue engineering strategies for disease modeling and drug screening. Biotechnol. Adv. 2017, 35, 77–94. [Google Scholar] [CrossRef] [Green Version]
- Jensen, J.; Hyllner, J.; Björquist, P. Human Embryonic Stem Cell Technologies and Drug Discovery. J. Cell. Physiol. 2009, 219, 513–519. [Google Scholar] [CrossRef]
- Yamada, K.M.; Cukierman, E. Modeling Tissue Morphogenesis and Cancer in 3D. Cell 2007, 130, 601–610. [Google Scholar] [CrossRef] [Green Version]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell. Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Trounson, A.; Dewitt, N.D. Stem cell biology: Towards the reality of cell therapeutics. Nat. Cell Biol. 2012, 14, 331. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-based drug screen: Considerations and practical approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Breslin, S.; O’Driscoll, L. Three-dimensional cell culture: The missing link in drug discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Kolaja, K. Stem cells and stem cell-derived tissues and their use in safety assessment. J. Biol. Chem. 2014, 289, 4555–4561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Yuan Hsin, H.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [Green Version]
- Chang, R.; Emami, K.; Wu, H.; Sun, W. Biofabrication of a three-dimensional liver micro-organ as an in vitro drug metabolism model. Biofabrication 2010, 2, 045004. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Karoly, J.; Marga, F.; Norotte, C.; Murphy, K.; Vunjak-Novakovic, G.; Forgacs, G. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication 2010, 2, 022001. [Google Scholar]
- Derby, B. Printing and prototyping of tissues and scaffolds. Science 2012, 338, 921–926. [Google Scholar] [CrossRef] [Green Version]
- Nair, K.; Gandhi, M.; Khalil, S.; Yan, K.C.; Marcolongo, M.; Barbee, K.; Sun, W. Characterization of cell viability during bioprinting processes. Biotechnol. J. 2009, 4, 1168–1177. [Google Scholar] [CrossRef]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling Shear Stress in 3D Bioprinting is a Key Factor to Balance Printing Resolution and Stem Cell Integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef]
- Hendriks, J.; Willem Visser, C.; Henke, S.; Leijten, J.; Saris, D.B.F.; Sun, C.; Lohse, D.; Karperien, M. Optimizing cell viability in droplet-based cell deposition. Sci. Rep. 2015, 5, 11304. [Google Scholar] [CrossRef]
- Donderwinkel, I.; Van Hest, J.C.M.; Cameron, N.R. Bio-inks for 3D bioprinting: Recent advances and future prospects. Polym. Chem. 2017, 8, 4451–4471. [Google Scholar] [CrossRef] [Green Version]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Axpe, E.; Oyen, M.L. Applications of alginate-based bioinks in 3D bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Cha, C. Advanced Polymer-Based Bioink Technology for Printing Soft Biomaterials. Macromol. Res. 2020, 28, 689–702. [Google Scholar] [CrossRef]
- Hortigon-Vinagre, M.P.; Zamora, V.; Burton, F.L.; Smith, G.L. The use of voltage sensitive dye di-4-ANEPPS and video-based contractility measurements to assess drug effects on excitation-contraction coupling in human induced pluripotent stem cell-derived cardiomyocytes. J. Cardiovasc. Pharmacol. 2020, 77, 280–290. [Google Scholar] [CrossRef]
- Faulkner-Jones, L.; Greenhough, S.; King, J.A.; Gardner, J.; Courtney, A.; Shu, W. Development of a valve-based cell printer for the formation of human embryonic stem cell spheroid aggregates. Biofabrication 2013, 5, 015013. [Google Scholar] [CrossRef] [PubMed]
- Faulkner-Jones, A.; Catherine, F.; Cornelissen, D.-J.; Gardner, J.; King, J.; Courtney, A.; Shu, W. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication 2015, 7, 044102. [Google Scholar] [CrossRef]
- Hortigon-Vinagre, M.P.; Zamora, V.; Burton, F.L.; Green, J.; Gintant, G.A.; Smith, G.L. The use of ratiometric fluorescence measurements of the voltage sensitive dye Di-4-ANEPPS to examine action potential characteristics and drug effects on human induced pluripotent stem cell-derived cardiomyocytes. Toxicol. Sci. 2016, 154, 320–331. [Google Scholar] [CrossRef] [Green Version]
- Blinova, K.; Stohlman, J.; Vicente, J.; Chan, D.; Johannesen, L.; Hortigon-Vinagre, M.P.; Zamora, V.; Smith, G.; Crumb, W.J.; Pang, L.; et al. Comprehensive translational assessment of human-induced pluripotent stem cell derived cardiomyocytes for evaluating drug-induced arrhythmias. Toxicol. Sci. 2017, 155, 234–247. [Google Scholar] [CrossRef]
- Lamore, S.D.; Ahlberg, E.; Boyer, S.; Lamb, M.L.; Hortigon-Vinagre, M.P.; Rodriguez, V.; Smith, G.L.; Sagemark, J.; Carlsson, L.; Bates, S.M.; et al. Deconvoluting kinase inhibitor induced cardiotoxicity. Toxicol. Sci. 2017, 158, 213–226. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.R.; Hortigon-Vinagre, M.P.; Zamora, V.; Kopljar, I.; De Bondt, A.; Gallacher, D.J.; Smith, G. Application of optical action potentials in human induced pluripotent stem cells-derived cardiomyocytes to predict drug-induced cardiac arrhythmias. J. Pharmacol. Toxicol. Methods 2017, 87, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Feaster, T.K.; Casciola, M.; Narkar, A.; Blinova, K. Acute effects of cardiac contractility modulation on human induced pluripotent stem cell–derived cardiomyocytes. Physiol. Rep. 2021, 9, e15085. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, C.; Passini, E.; Hyttinen, J.; Paci, M.; Severi, S. Simulation of the Effects of Extracellular Calcium Changes Leads to a Novel Computational Model of Human Ventricular Action Potential with a Revised Calcium Handling. Front. Physiol. 2020, 11, 314. [Google Scholar] [CrossRef] [PubMed]

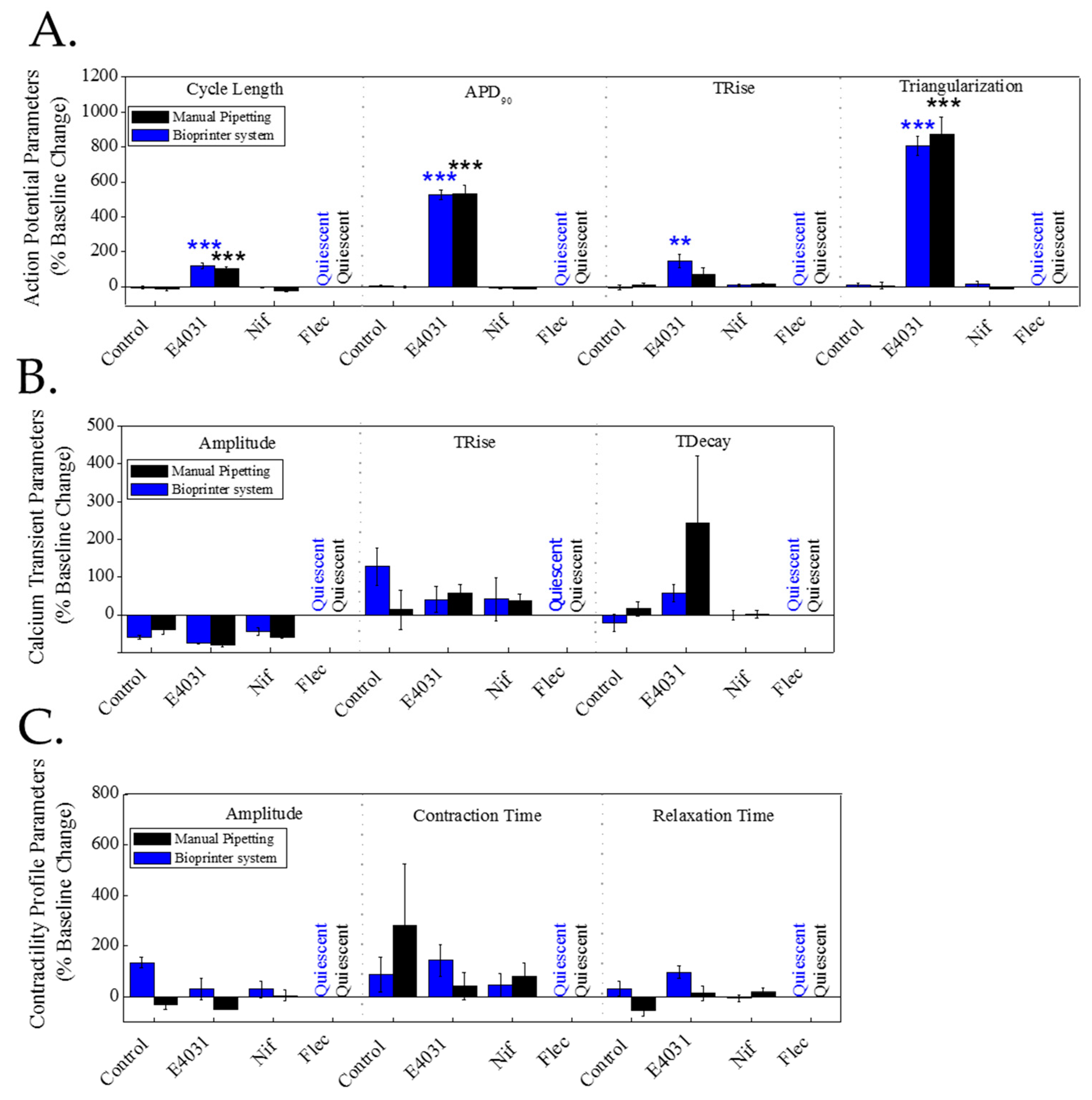
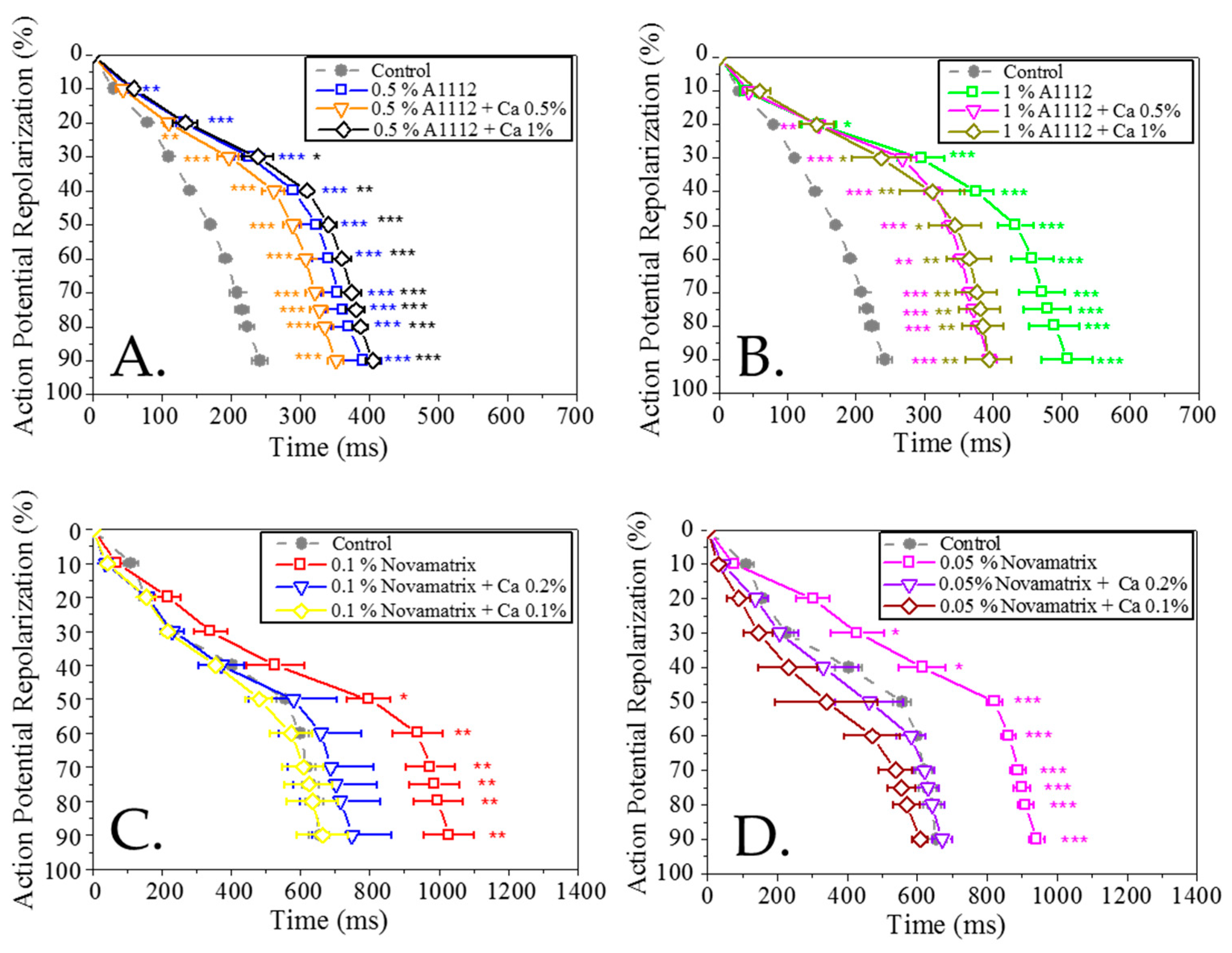

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faulkner-Jones, A.; Zamora, V.; Hortigon-Vinagre, M.P.; Wang, W.; Ardron, M.; Smith, G.L.; Shu, W. A Bioprinted Heart-on-a-Chip with Human Pluripotent Stem Cell-Derived Cardiomyocytes for Drug Evaluation. Bioengineering 2022, 9, 32. https://doi.org/10.3390/bioengineering9010032
Faulkner-Jones A, Zamora V, Hortigon-Vinagre MP, Wang W, Ardron M, Smith GL, Shu W. A Bioprinted Heart-on-a-Chip with Human Pluripotent Stem Cell-Derived Cardiomyocytes for Drug Evaluation. Bioengineering. 2022; 9(1):32. https://doi.org/10.3390/bioengineering9010032
Chicago/Turabian StyleFaulkner-Jones, Alan, Victor Zamora, Maria P. Hortigon-Vinagre, Wenxing Wang, Marcus Ardron, Godfrey L. Smith, and Wenmiao Shu. 2022. "A Bioprinted Heart-on-a-Chip with Human Pluripotent Stem Cell-Derived Cardiomyocytes for Drug Evaluation" Bioengineering 9, no. 1: 32. https://doi.org/10.3390/bioengineering9010032
APA StyleFaulkner-Jones, A., Zamora, V., Hortigon-Vinagre, M. P., Wang, W., Ardron, M., Smith, G. L., & Shu, W. (2022). A Bioprinted Heart-on-a-Chip with Human Pluripotent Stem Cell-Derived Cardiomyocytes for Drug Evaluation. Bioengineering, 9(1), 32. https://doi.org/10.3390/bioengineering9010032