MicroRNA Expression Profile Analysis of Chlamydomonas reinhardtii during Lipid Accumulation Process under Nitrogen Deprivation Stresses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Lipid Analysis
2.2.1. Nile Red Fluorescence Determination of Neutral Lipid
2.2.2. Lipid Extraction
2.2.3. Fatty Acid Determination
2.3. Small RNA Library Construction and Deep Sequencing
2.4. Sequence Analysis
2.5. The Nitrogen Deprivation-Responsive miRNAs in C. reinhardtii
2.6. The Target Nitrogen Deprivation-Responsive miRNAs and KOGs Analysis
3. Results
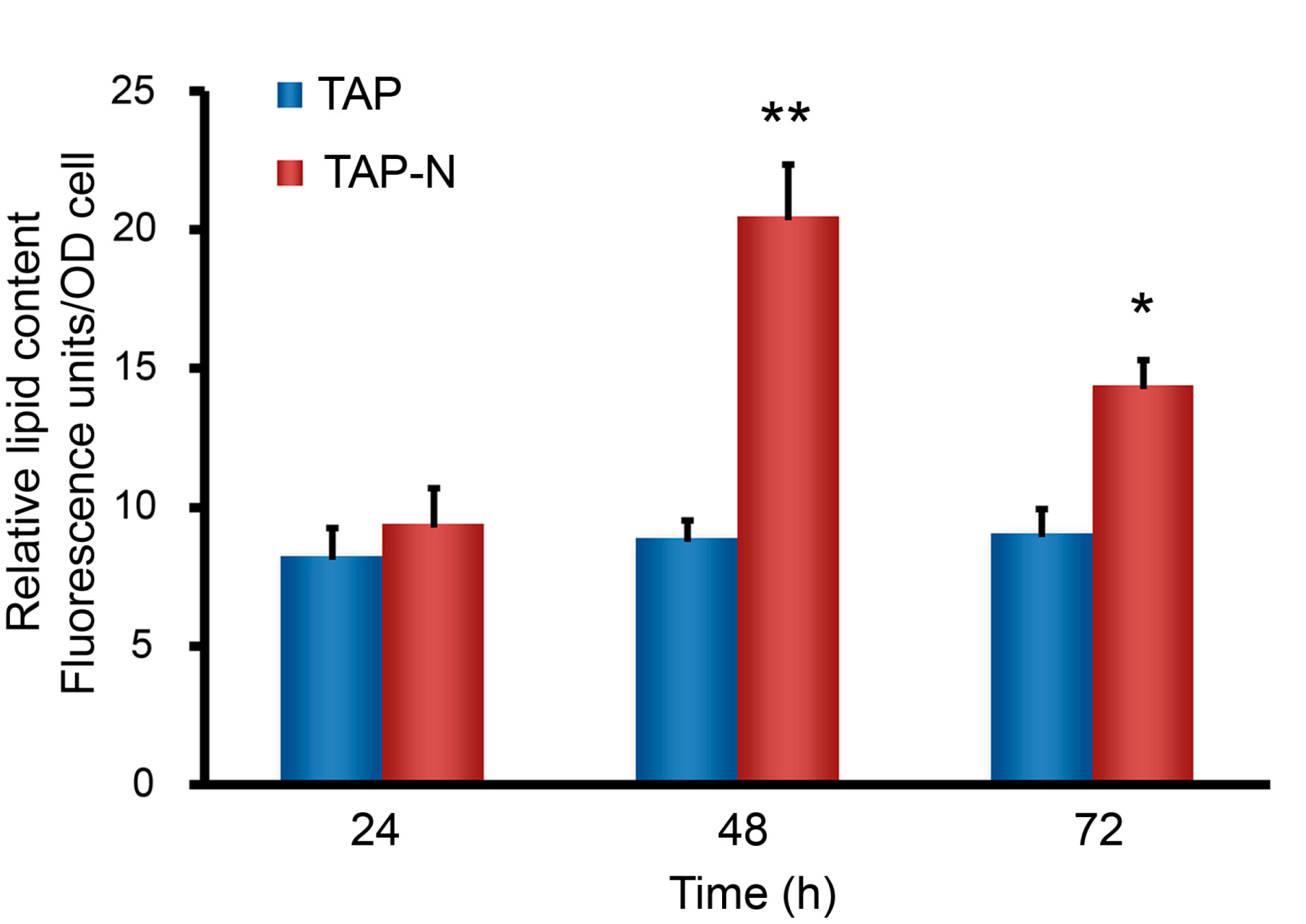
3.1. Analysis on Lipid and Fatty Acid Composition of C. reinhardtii under Nitrogen-Replete and Nitrogen-Deprived Conditions
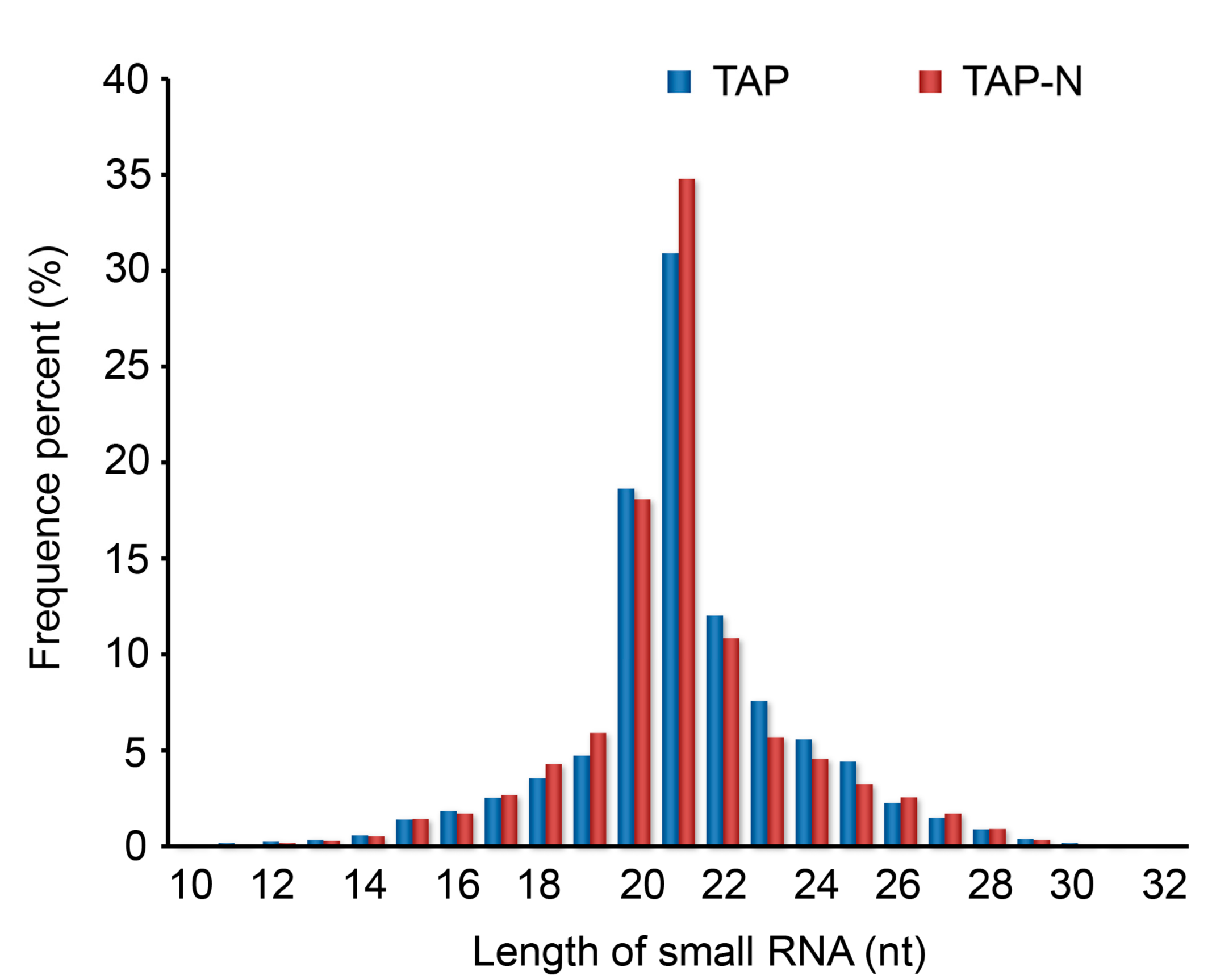
3.2. Deep Sequencing of C. reinhardtii Small RNAs
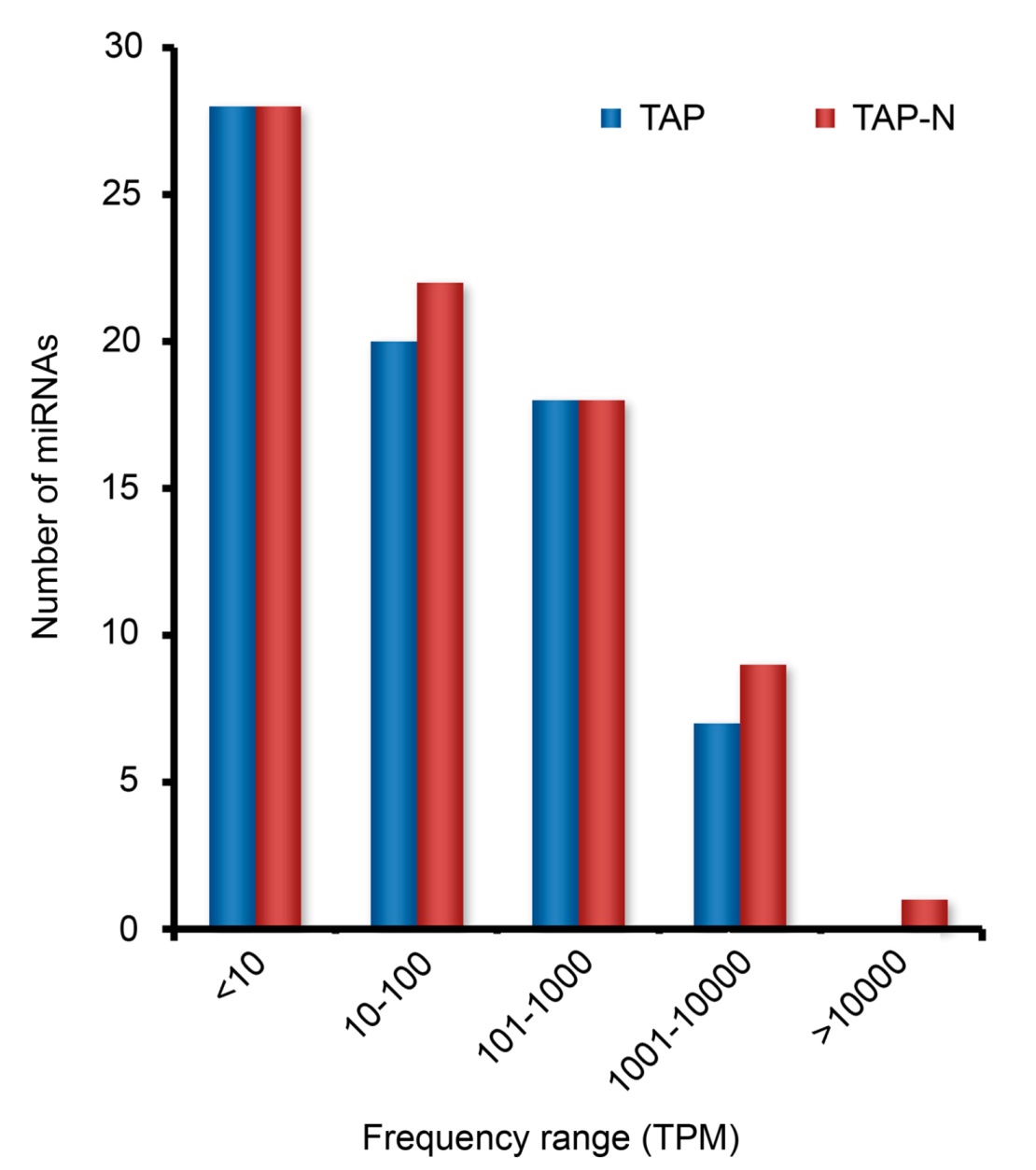
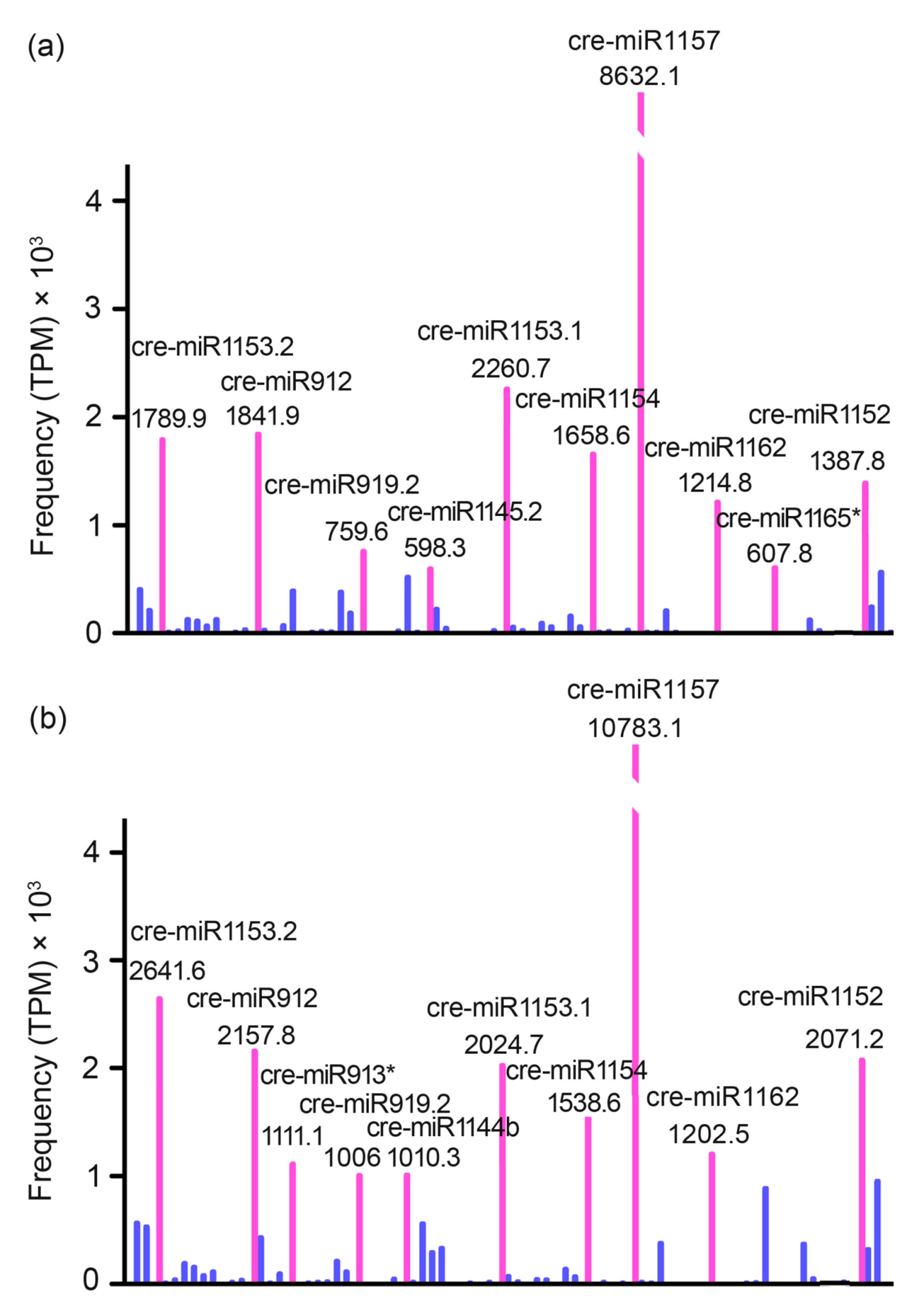
3.3. Identification and Profiling of Known miRNAs
3.4. Potential Novel miRNAs
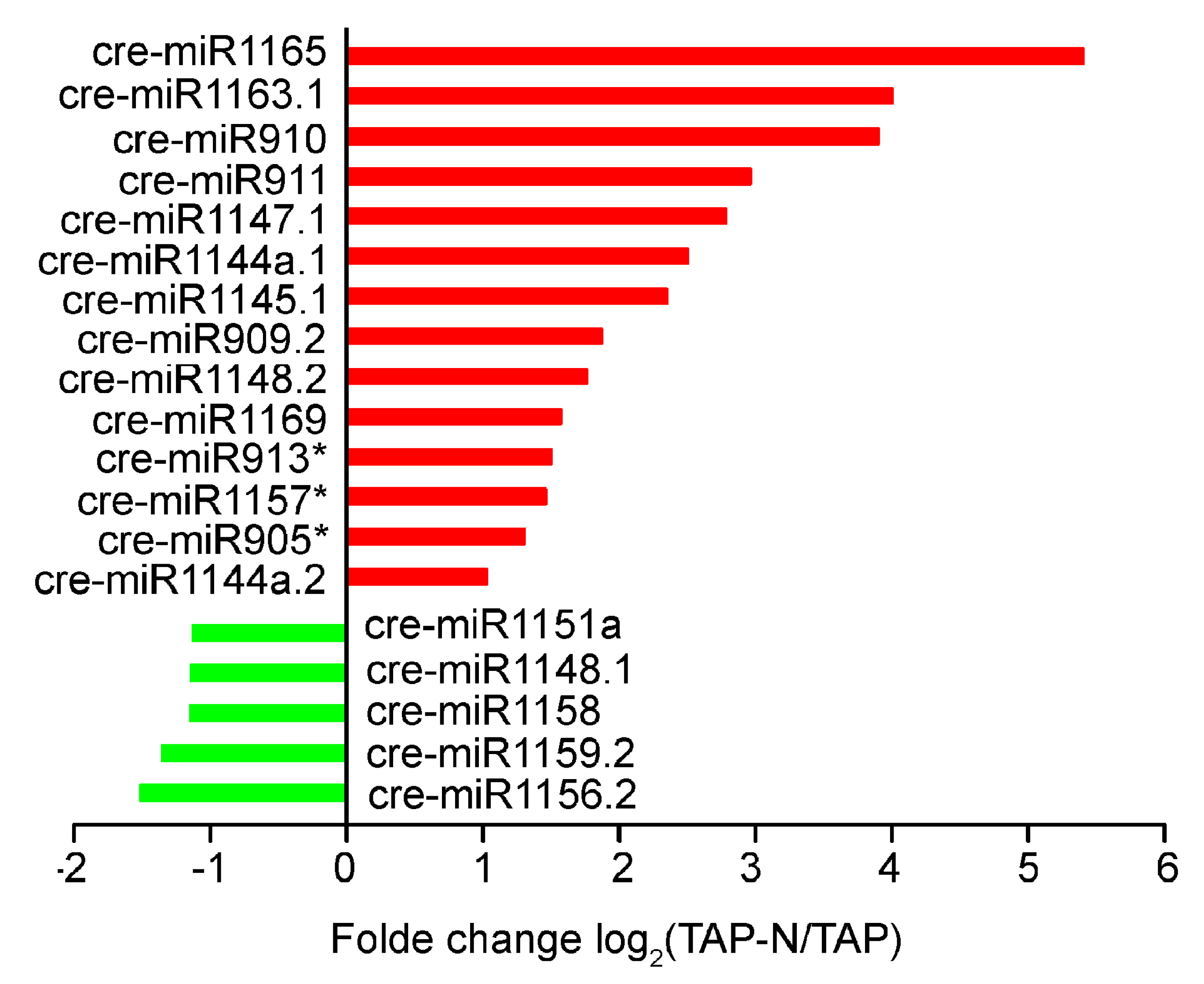
3.5. Response of C. reinhardtii miRNAs to Nitrogen Deprivation Stress
3.6. The Target of Nitrogen Deficiency-Responsive miRNAs and KOGs Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Zhou, W.; Cheng, W.; Gao, L.; Liu, T. Strategy study on enhancing lipid productivity of filamentous oleaginous microalgae Tribonema. Bioresour. Technol. 2016, 218, 161–166. [Google Scholar] [CrossRef]
- Gargouri, M.; Bates, P.D.; Park, J.J.; Kirchhoff, H.; Gang, D.R. Functional photosystem I maintains proper energy balance during nitrogen depletion in Chlamydomonas reinhardtii, promoting triacylglycerol accumulation. Biotechnol. Biofuels 2017, 10, 89. [Google Scholar] [CrossRef] [Green Version]
- Tanvir, R.U.; Zhang, J.; Canter, T.; Chen, D.; Lu, J.; Hu, Z. Harnessing Solar Energy using Phototrophic Microorganisms: A Sustainable Pathway to Bioenergy, Biomaterials, and Environmental Solutions. Renew. Sustain. Energy Rev. 2021, 146, 111181. [Google Scholar] [CrossRef]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Ferreira, V.S.; Pinto, R.F.; Sant’Anna, C. The green microalga S. dimorphus (UTEX 1237) showed an increase in body lipids in N-limited cultures. J. Appl. Microbiol. 2016, 120, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Illman, A.M.; Scragg, A.H.; Shales, S.W. Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzym. Microb. Technol. 2000, 27, 631–635. [Google Scholar] [CrossRef]
- Adamakis, I.D.; Lazaridis, P.A.; Terzopoulou, E.; Torofias, S.; Valari, M.; Kalaitzi, P.; Rousonikolos, V.; Gkoutzikostas, D.; Zouboulis, A.; Zalidis, G.; et al. Cultivation, characterization, and properties of Chlorella vulgaris microalgae with different lipid contents and effect on fast pyrolysis oil composition. Environ. Sci. Pollut. Res. Int. 2018, 25, 23018–23032. [Google Scholar] [CrossRef] [PubMed]
- Charria-Girón, E.; Amazo, V.; De Angulo, D.; Hidalgo, E.; Villegas-Torres, M.F.; Baganz, F.; Caicedo Ortega, N.H. Strategy for Managing Industrial Anaerobic Sludge through the Heterotrophic Cultivation of Chlorella sorokiniana: Effect of Iron Addition on Biomass and Lipid Production. Bioengineering 2021, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Salomé, P.A.; Merchant, S.S. A Series of Fortunate Events: Introducing Chlamydomonas as a Reference Organism. Plant Cell 2019, 31, 1682–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaby, I.K.; Glaesener, A.G.; Mettler, T.; Fitz-Gibbon, S.T.; Gallaher, S.D.; Liu, B.; Boyle, N.R.; Kropat, J.; Stitt, M.; Johnson, S.; et al. Systems-level analysis of nitrogen starvation-induced modifications of carbon metabolism in a Chlamydomonas reinhardtii starchless mutant. Plant Cell 2013, 25, 4305–4323. [Google Scholar] [CrossRef] [Green Version]
- Park, J.J.; Wang, H.; Gargouri, M.; Deshpande, R.R.; Skepper, J.N.; Holguin, F.O.; Juergens, M.T.; Shachar-Hill, Y.; Hicks, L.M.; Gang, D.R. The response of Chlamydomonas reinhardtii to nitrogen deprivation: A systems biology analysis. Plant J. 2015, 81, 611–624. [Google Scholar] [CrossRef]
- Hernández-Torres, A.; Zapata-Morales, A.L.; Ochoa Alfaro, A.E.; Soria-Guerra, R.E. Identification of gene transcripts involved in lipid biosynthesis in Chlamydomonas reinhardtii under nitrogen, iron and sulfur deprivation. World J. Microbiol. Biotechnol. 2016, 32, 55. [Google Scholar] [CrossRef]
- Takeuchi, T.; Sears, B.B.; Lindeboom, C.; Lin, Y.T.; Fekaris, N.; Zienkiewicz, K.; Zienkiewicz, A.; Poliner, E.; Benning, C. Chlamydomonas CHT7 Is Required for an Effective Quiescent State by Regulating Nutrient-Responsive Cell Cycle Gene Expression. Plant Cell 2020, 32, 1240–1269. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Kong, F.; Lee, J.; Choi, B.Y.; Wang, P.; Gao, P.; Yamano, T.; Fukuzawa, H.; Kang, B.H.; Lee, Y. CrABCA2 Facilitates Triacylglycerol Accumulation in Chlamydomonas reinhardtii under Nitrogen Starvation. Mol. Cells 2020, 43, 48–57. [Google Scholar] [CrossRef]
- Miller, R.; Wu, G.; Deshpande, R.R.; Vieler, A.; Gärtner, K.; Li, X.; Moellering, E.R.; Zäuner, S.; Cornish, A.J.; Liu, B.; et al. Changes in transcript abundance in Chlamydomonas reinhardtii following nitrogen deprivation predict diversion of metabolism. Plant Physiol. 2010, 154, 1737–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Q.; Song, W.; Li, Y.; Wang, C.; Hu, Z. Flagella-Associated WDR-Containing Protein CrFAP89 Regulates Growth and Lipid Accumulation in Chlamydomonas reinhardtii. Front. Plant Sci. 2018, 9, 691. [Google Scholar] [CrossRef] [Green Version]
- Philipps, G.; Happe, T.; Hemschemeier, A. Nitrogen deprivation results in photosynthetic hydrogen production in Chlamydomonas reinhardtii. Planta 2012, 235, 729–745. [Google Scholar] [CrossRef]
- Zienkiewicz, K.; Du, Z.Y.; Ma, W.; Vollheyde, K.; Benning, C. Stress-induced neutral lipid biosynthesis in microalgae—Molecular, cellular and physiological insights. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 1269–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Meng, Y.; Chu, Y.; Fan, Y.; Cao, X.; Xue, S.; Chi, Z. Triacylglycerol accumulates exclusively outside the chloroplast in short-term nitrogen-deprived Chlamydomonas reinhardtii. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1478–1487. [Google Scholar] [CrossRef]
- Jones-Rhoades, M.W.; Bartel, D.P.; Bartel, B. MicroRNAS and their regulatory roles in plants. Annu. Rev. Plant Biol. 2006, 57, 19–53. [Google Scholar] [CrossRef]
- Zhao, T.; Li, G.; Mi, S.; Li, S.; Hannon, G.J.; Wang, X.J.; Qi, Y. A complex system of small RNAs in the unicellular green alga Chlamydomonas reinhardtii. Genes Dev. 2007, 21, 1190–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Li, Z.; Song, X.; Han, L.; Wang, L.; Zhang, J.; Long, Y.; Pei, X. Identification and Characterization of microRNAs in the Developing Seed of Linseed Flax (Linum usitatissimum L.). Int. J. Mol. Sci. 2020, 21, 2708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Wu, G.; Yuan, H.; Cheng, L.; Zhao, D.; Huang, W.; Zhang, S.; Zhang, L.; Chen, H.; Zhang, J.; et al. Identification and characterization of miRNAs and targets in flax (Linum usitatissimum) under saline, alkaline, and saline-alkaline stresses. BMC Plant Biol. 2016, 16, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunkar, R.; Chinnusamy, V.; Zhu, J.; Zhu, J.K. Small RNAs as big players in plant abiotic stress responses and nutrient deprivation. Trends Plant Sci. 2007, 12, 301–309. [Google Scholar] [CrossRef]
- Li, T.; Li, H.; Zhang, Y.X.; Liu, J.Y. Identification and analysis of seven H₂O₂-responsive miRNAs and 32 new miRNAs in the seedlings of rice (Oryza sativa L. ssp. indica). Nucleic Acids Res. 2011, 39, 2821–2833. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Vernooy, S.Y.; Guo, M.; Hay, B.A. The Drosophila microRNA Mir-14 suppresses cell death and is required for normal fat metabolism. Curr. Biol. 2003, 13, 790–795. [Google Scholar] [CrossRef] [Green Version]
- Goedeke, L.; Rotllan, N.; Canfrán-Duque, A.; Aranda, J.F.; Ramírez, C.M.; Araldi, E.; Lin, C.S.; Anderson, N.N.; Wagschal, A.; de Cabo, R.; et al. MicroRNA-148a regulates LDL receptor and ABCA1 expression to control circulating lipoprotein levels. Nat. Med. 2015, 21, 1280–1289. [Google Scholar] [CrossRef] [Green Version]
- Horie, T.; Nishino, T.; Baba, O.; Kuwabara, Y.; Nakao, T.; Nishiga, M.; Usami, S.; Izuhara, M.; Nakazeki, F.; Ide, Y.; et al. MicroRNA-33b knock-in mice for an intron of sterol regulatory element-binding factor 1 (Srebf1) exhibit reduced HDL-C in vivo. Sci. Rep. 2014, 4, 5312. [Google Scholar] [CrossRef] [Green Version]
- Barvkar, V.T.; Pardeshi, V.C.; Kale, S.M.; Qiu, S.; Rollins, M.; Datla, R.; Gupta, V.S.; Kadoo, N.Y. Genome-wide identification and characterization of microRNA genes and their targets in flax (Linum usitatissimum): Characterization of flax miRNA genes. Planta 2013, 237, 1149–1161. [Google Scholar] [CrossRef]
- Escorcia, W.; Ruter, D.L.; Nhan, J.; Curran, S.P. Quantification of Lipid Abundance and Evaluation of Lipid Distribution in Caenorhabditis elegans by Nile Red and Oil Red O Staining. J. Vis. Exp. 2018, 133, 57352. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, C.; Song, L.; Sommerfeld, M.; Hu, Q. A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J. Microbiol. Methods 2009, 77, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tang, H.; Ma, H.; Holland, T.C.; Ng, K.Y.; Salley, S.O. Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresour. Technol. 2011, 102, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.; Xie, Z.; Gustafson, A.M.; Carrington, J.C. MicroRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 2005, 121, 207–221. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Li, Y.; Li, X.; Ning, X.; Li, M.; Yang, G. MicroRNA identity and abundance in developing swine adipose tissue as determined by Solexa sequencing. J. Cell Biochem. 2011, 112, 1318–1328. [Google Scholar] [CrossRef]
- Xin, M.; Wang, Y.; Yao, Y.; Xie, C.; Peng, H.; Ni, Z.; Sun, Q. Diverse set of microRNAs are responsive to powdery mildew infection and heat stress in wheat (Triticum aestivum L.). BMC Plant Biol. 2010, 10, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwab, R.; Palatnik, J.F.; Riester, M.; Schommer, C.; Schmid, M.; Weigel, D. Specific effects of microRNAs on the plant transcriptome. Dev. Cell 2005, 8, 517–527. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Huang, J.; Sun, Z.; Zhong, Y.; Jiang, Y.; Chen, F. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: Assessment of algal oils for biodiesel production. Bioresour. Technol. 2011, 102, 106–110. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Zou, J.; Xu, D.; Su, F.; Ye, N. Identification of miRNA from Porphyra yezoensis by high-throughput sequencing and bioinformatics analysis. PLoS ONE 2010, 5, e10698. [Google Scholar] [CrossRef]
- González-Ballester, D.; Casero, D.; Cokus, S.; Pellegrini, M.; Merchant, S.S.; Grossman, A.R. RNA-seq analysis of sulfur-deprived Chlamydomonas cells reveals aspects of acclimation critical for cell survival. Plant Cell 2010, 22, 2058–2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, J.; Jin, P.; Wang, E.; Marincola, F.M.; Stroncek, D.F. MicroRNA and gene expression patterns in the differentiation of human embryonic stem cells. J. Transl. Med. 2009, 7, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molnár, A.; Schwach, F.; Studholme, D.J.; Thuenemann, E.C.; Baulcombe, D.C. miRNAs control gene expression in the single-cell alga Chlamydomonas reinhardtii. Nature 2007, 447, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Voshall, A.; Kim, E.J.; Ma, X.; Yamasaki, T.; Moriyama, E.N.; Cerutti, H. miRNAs in the alga Chlamydomonas reinhardtii are not phylogenetically conserved and play a limited role in responses to nutrient deprivation. Sci. Rep. 2017, 7, 5462. [Google Scholar] [CrossRef] [Green Version]
- Valli, A.A.; Santos, B.A.; Hnatova, S.; Bassett, A.R.; Molnar, A.; Chung, B.Y.; Baulcombe, D.C. Most microRNAs in the single-cell alga Chlamydomonas reinhardtii are produced by Dicer-like 3-mediated cleavage of introns and untranslated regions of coding RNAs. Genome Res. 2016, 26, 519–529. [Google Scholar] [CrossRef] [Green Version]
- Azaman, S.N.A.; Satharasinghe, D.A.; Tan, S.W.; Nagao, N.; Yusoff, F.M.; Yeap, S.K. Identification and Analysis of microRNAs in Chlorella sorokiniana Using High-Throughput Sequencing. Genes 2020, 11, 1131. [Google Scholar] [CrossRef]
- Deng, X.-Y.; Hu, X.-L.; Li, D.; Wang, L.; Cheng, J.; Gao, K. Identification and analysis of microRNAs in Botryococcus braunii using high-throughput sequencing. Aquat. Biol. 2017, 26, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Siomi, H.; Siomi, M.C. Expanding RNA physiology: MicroRNAs in a unicellular organism. Genes Dev. 2007, 21, 1153–1156. [Google Scholar] [CrossRef] [Green Version]
- Sunkar, R.; Zhu, J.K. Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 2004, 16, 2001–2019. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Tai, H.; Sun, S.; Zhang, F.; Xu, Y.; Li, W.X. Cloning and characterization of maize miRNAs involved in responses to nitrogen deficiency. PLoS ONE 2012, 7, e29669. [Google Scholar] [CrossRef] [Green Version]
- Shu, L.; Hu, Z. Characterization and differential expression of microRNAs elicited by sulfur deprivation in Chlamydomonas reinhardtii. BMC Genom. 2012, 13, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smythers, A.L.; McConnell, E.W.; Lewis, H.C.; Mubarek, S.N.; Hicks, L.M. Photosynthetic Metabolism and Nitrogen Reshuffling Are Regulated by Reversible Cysteine Thiol Oxidation Following Nitrogen Deprivation in Chlamydomonas. Plants 2020, 9, 784. [Google Scholar] [CrossRef] [PubMed]
- Warakanont, J.; Li-Beisson, Y.; Benning, C. LIP4 Is Involved in Triacylglycerol Degradation in Chlamydomonas reinhardtii. Plant Cell Physiol. 2019, 60, 1250–1259. [Google Scholar] [CrossRef] [PubMed]

| Fatty Acid (%) | C. reinhardtii Based Biodiesel | |
|---|---|---|
| TAP | TAP-N | |
| C14:0 | 1.40 ± 0.43 | 3.95 ± 0.13 |
| C16:0 | 17.05 ± 0.28 | 18.07 ± 0.70 |
| C16:1ω7 | 1.47 ± 0.39 | 3.00 ± 0.24 |
| C18:0 | 12.17 ± 0.46 | 11.75 ± 0.69 |
| C16:1ω9 | 8.27 ± 0.91 | 10.59 ± 0.14 |
| C18:2ω6 | 4.49 ± 0.94 | 7.87 ± 1.35 |
| C20:0 | 1.56 ± 0.48 | 3.52 ± 0.59 |
| C18:3ω3 | 32.63 ± 1.79 | 24.40 ± 0.63 |
| C21:0 | 1.25 ± 0.34 | 2.19 ± 0.38 |
| C20:2ω6 | 13.45 ± 0.72 | 9.22 ± 0.87 |
| C20:3ω6 | 2.19 ± 0.11 | 2.24 ± 0.11 |
| C22:4ω6 | 0.94 ± 0.43 | 0.33 ± 0.43 |
| Other | 3.13 ± 1.14 | 2.88 ± 0.25 |
| ∑Unsaturat | 65.74 ± 2.58 | 58.04 ± 1.88 |
| ∑Saturat | 34.26 ± 1.23 | 41.96 ± 1.52 |
| Novel miRNA Name | Normalized Expressio (TPM) | Fold-Change (log2(TAP-N/TAP)) | p-Value | Sig-Lable | |
|---|---|---|---|---|---|
| TAP | TAP-N | ||||
| cre-miR-new1 | 0.65 | 1.17 | 0.8381 | 0.163 | |
| cre-miR-new2 | 64.68 | 33.15 | −0.9644 | 7.98 × 10−34 | |
| cre-miR-new3 | 34.08 | 22.85 | −0.5765 | 1.89 × 10−8 | |
| cre-miR-new4 | 129.80 | 88.87 | −0.5464 | 1.38 × 10−25 | |
| cre-miR-new5 | 2.47 | 16.33 | 2.7280 | 2.96 × 10−13 | ** |
| cre-miR-new6 | 1.23 | 1.51 | 0.2926 | 0.542 | |
| cre-miR-new7 | 95.14 | 123.33 | 0.3744 | 0 | |
| cre-miR-new8 | 0.87 | 4.60 | 2.4017 | 3.45 × 10−7 | ** |
| cre-miR-new9 | 0.65 | 1.44 | 1.1430 | 0.046 | * |
| cre-miR-new10 | 0.87 | 1.10 | 0.3356 | 0.553 | |
| cre-miR-new11 | 88.39 | 139.45 | 0.6578 | 1.41 × 10−13 | |
| cre-miR-new12 | 1.89 | 3.23 | 0.7748 | 0.026 | |
| cre-miR-new13 | 7.90 | 7.89 | −0.0021 | 0.989 | |
| cre-miR-new14 | 33.28 | 9.95 | −1.7418 | 5.51 × 10−43 | ** |
| cre-miR-new15 | 179.25 | 170.61 | −0.0712 | 0.08 | |
| cre-miR-new16 | 3.55 | 7.76 | 1.1261 | 2.23 × 10−6 | ** |
| cre-miR-new17 | 0.65 | 3.02 | 2.2101 | 4.22 × 10−6 | ** |
| cre-miR-new18 | 8.77 | 20.11 | 1.1965 | 3.55 × 10−14 | ** |
| cre-miR-new19 | 365.68 | 132.11 | −1.4688 | 0 | ** |
| cre-miR-new20 | 5.80 | 73.84 | 3.6701 | 0 | ** |
| cre-miR-new21 | 18.20 | 23.95 | 0.3961 | 8.60 × 10−4 | |
| cre-miR-new22 | 72.66 | 142.06 | 0.9674 | 2.63 × 10−13 | |
| cre-miR-new23 | 24.58 | 50.79 | 1.0468 | 1.79 × 10−13 | ** |
| cre-miR-new24 | 0.00 | 4.25 | -1 | 2.22 × 10−16 | |
| cre-miR-new25 | 0.00 | 12.63 | -1 | 2.22 × 10−16 | |
| cre-miR-new26 | 7.18 | 0.00 | +2 | 9.70 × 10−32 | |
| cre-miR-new27 | 5.44 | 0.00 | +2 | 3.18 × 10−24 | |
| cre-miR-new28 | 17.98 | 0.00 | +2 | 2.13 × 10−78 | |
| cre-miR-new29 | 29.80 | 0.00 | +2 | 1.93 × 10−129 | |
| cre-miR-new30 | 44.88 | 0.00 | +2 | 1.42 × 10−194 | |
| miRNA Expression | Target Gene Expression | |||||
|---|---|---|---|---|---|---|
| miRNA | Fold Change | Expression | Gene Product | Fold Change | Expression | p-Value |
| cre-miR1156.2 | 0.35 | Down | Prosaposin | 2.17 | Up | 0 |
| cre-miR1144a.2 | 2.04 | Up | SAM-dependent methyltransferases | 0.07 | Down | 2.12 × 10−38 |
| Animal-type fatty acid synthase and related proteins | 0.24 | Down | 0.302 | |||
| cre-miR1157* | 1.25 | Up | Cytochrome P450 CYP4/CYP19/CYP26 subfamilies | 0.76 | Down | 0.284 |
| cre-miR1163.1 | 16.71 | Up | Fatty acid desaturase | 0.28 | Down | 4.921 × 10−2 |
| Sterol C5 desaturase | 0.86 | Down | 0.611 | |||
| START domain-containing proteins | 1.25 | Up | 1.55 × 10−3 | |||
| cre-miR1169 | 2.98 | Up | Lysophospholipase | 0.60 | Down | 0.135 |
| cre-miR910 | 15.55 | Up | acyl-CoA oxidase | 1.34 | Up | 4.70 × 10−2 |
| cre-miR-new5 | 6.61 | Up | Putative phosphoinositide phosphatase | 0.35 | Down | 0.146 |
| cre-miR-new14 | 0.30 | Down | 3-Methylcrotonyl-CoA carboxylase | 3.66 | Up | 0 |
| cre-miR-new16 | 2.19 | Up | Long-chain acyl-CoA transporter | 0.46 | Down | 4.66 × 10−3 |
| cre-miR-new19 | 0.36 | Down | Acyl-CoA synthetase | 4.13 | Up | 3.24 × 10−12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Shi, J.; Yuan, C.; Liu, X.; Du, G.; Fan, R.; Zhang, B. MicroRNA Expression Profile Analysis of Chlamydomonas reinhardtii during Lipid Accumulation Process under Nitrogen Deprivation Stresses. Bioengineering 2022, 9, 6. https://doi.org/10.3390/bioengineering9010006
Zhang J, Shi J, Yuan C, Liu X, Du G, Fan R, Zhang B. MicroRNA Expression Profile Analysis of Chlamydomonas reinhardtii during Lipid Accumulation Process under Nitrogen Deprivation Stresses. Bioengineering. 2022; 9(1):6. https://doi.org/10.3390/bioengineering9010006
Chicago/Turabian StyleZhang, Jingxian, Jiping Shi, Chenyang Yuan, Xiangcen Liu, Guilin Du, Ruimei Fan, and Baoguo Zhang. 2022. "MicroRNA Expression Profile Analysis of Chlamydomonas reinhardtii during Lipid Accumulation Process under Nitrogen Deprivation Stresses" Bioengineering 9, no. 1: 6. https://doi.org/10.3390/bioengineering9010006








