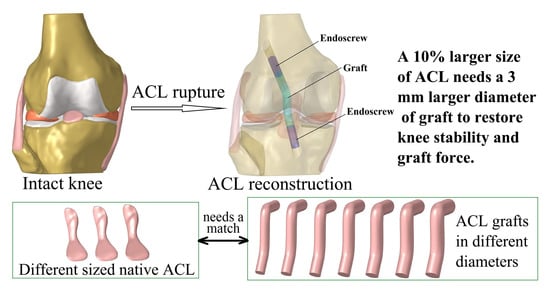
Graft Diameter Should Reflect the Size of the Native Anterior Cruciate Ligament (ACL) to Improve the Outcome of ACL Reconstruction: A Finite Element Analysis
Abstract
:1. Introduction
2. Materials and Methods
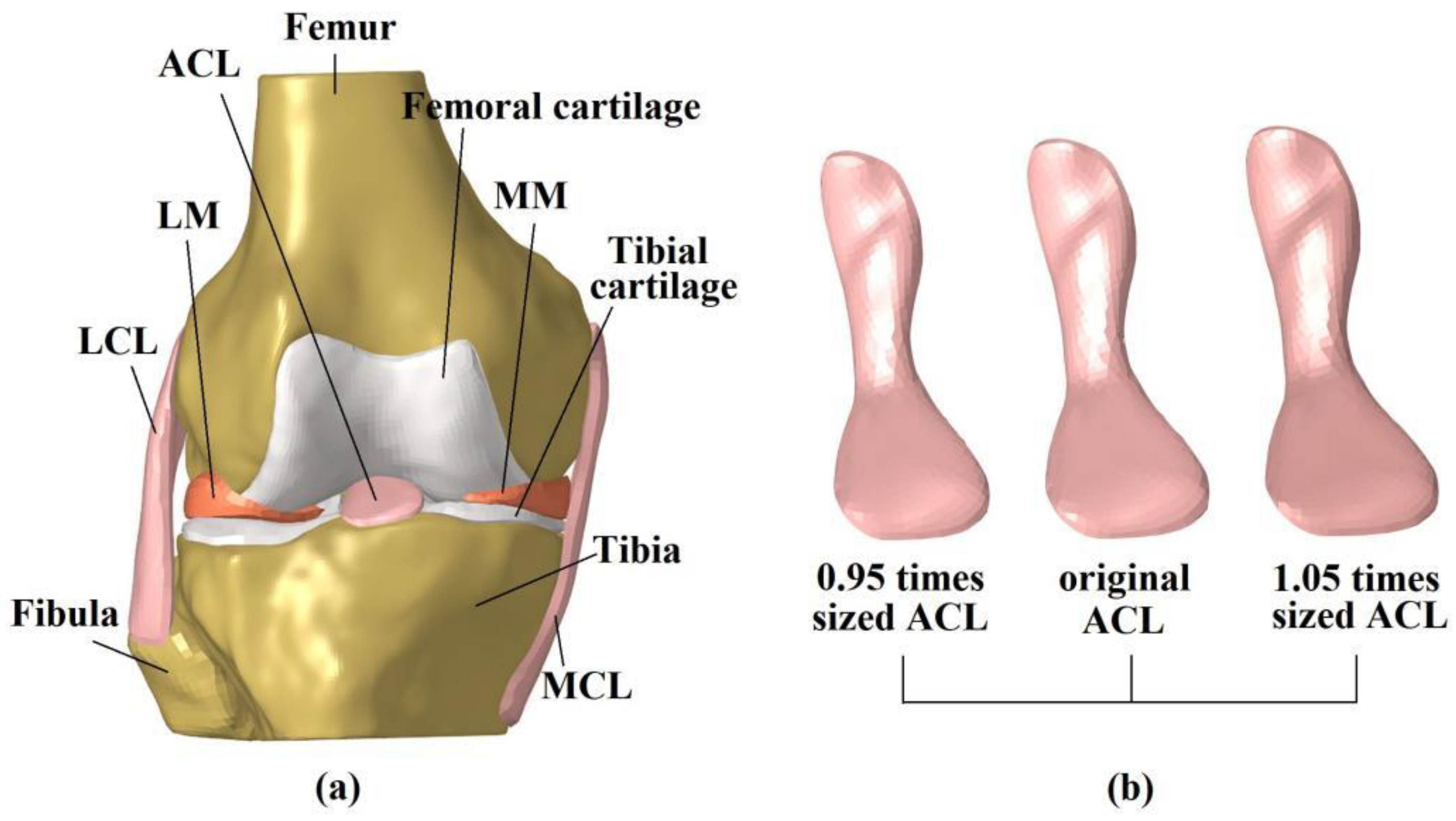
2.1. Finite Element Model of the Intact Knee
2.2. Simulation of Intact Knee Using Different-Sized ACLs
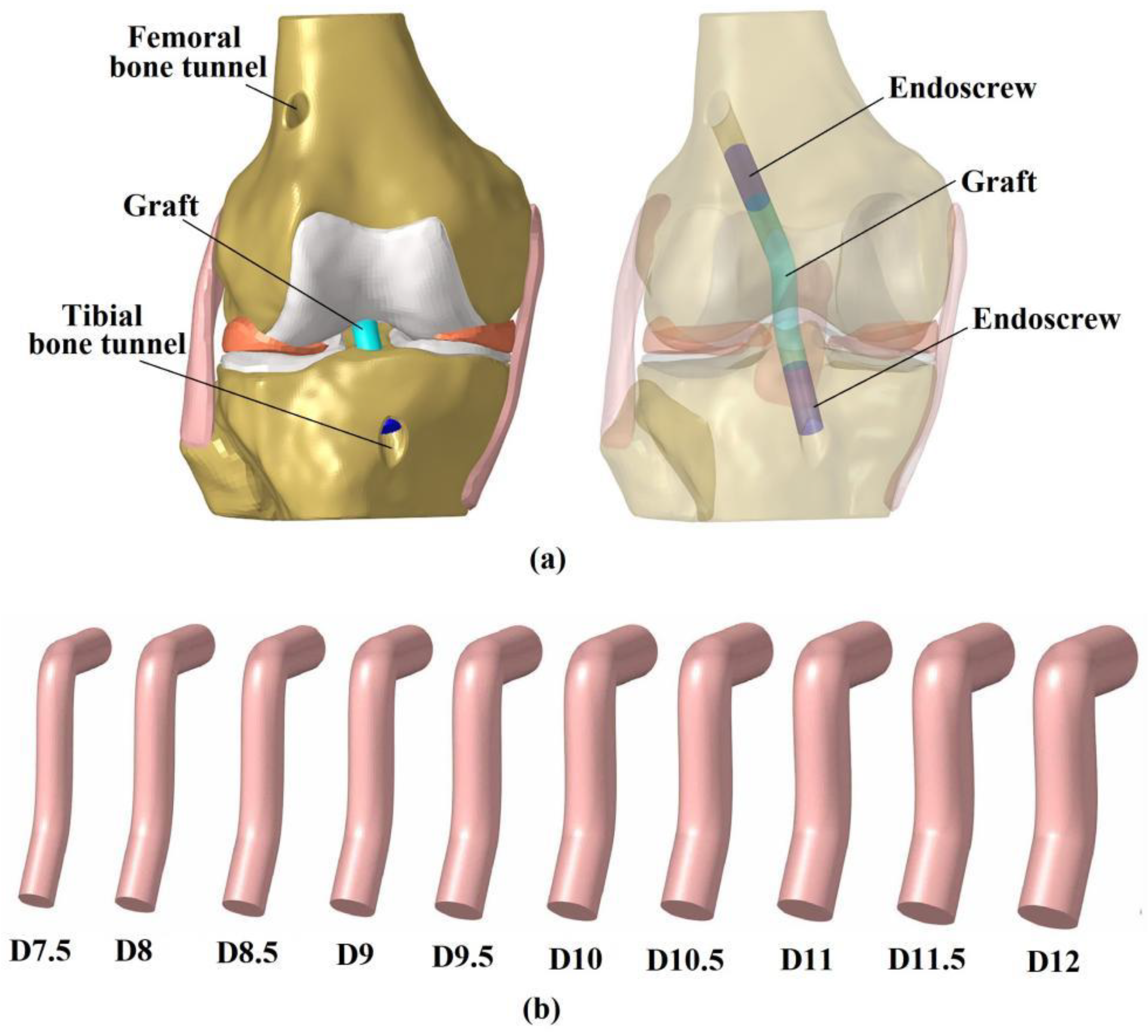
2.3. Simulation of ACLR Using Different Graft Diameters
2.4. Boundary and Loading Conditions and Simulation Outputs
3. Results
3.1. ACLR vs. Intact Knee with Original-Sized ACL
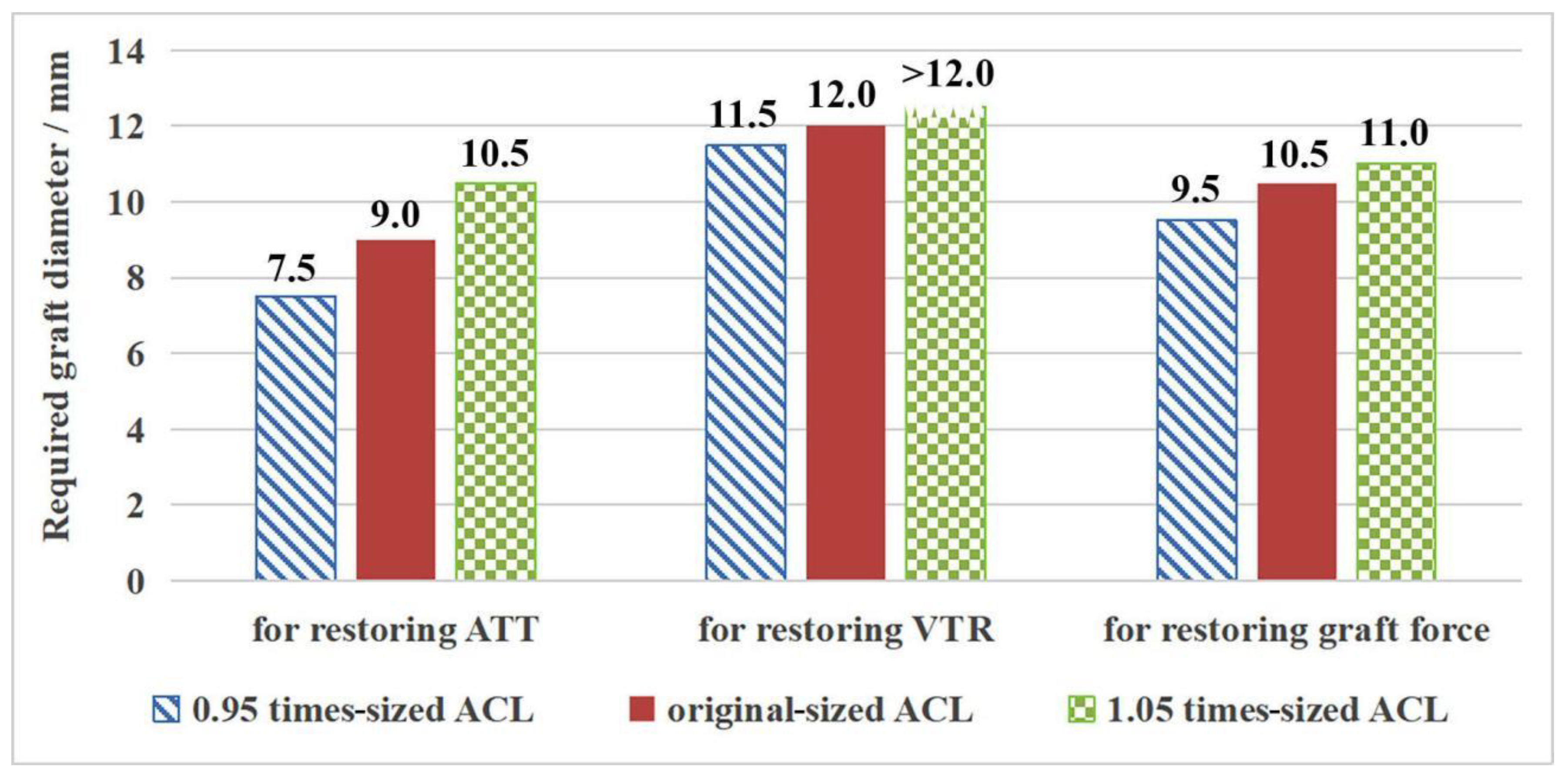
3.2. Outcome for the Intact Knee Groups with Different Sized ACL
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fukuda, Y.; Woo SL, Y.; Loh, J.C.; Tsuda, E.; Tang, P.; McMahon, P.J.; Debski, R.E. A quantitative analysis of valgus torque on the ACL: A human cadaveric study. J. Orthop. Res. 2003, 21, 1107–1112. [Google Scholar] [CrossRef]
- Kanamori, A.; Zeminski, J.; Rudy, T.W.; Li, G.; Fu, F.H.; Woo, S.L.Y. The effect of axial tibial torque on the function of the anterior cruciate ligament: A biomechanical study of a simulated pivot shift test. Arthroscopy 2002, 18, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Sakane, M.; Fox, R.J.; Woo, S.L.Y.; Livesay, G.A.; Li, G.; Fu, F.H. In situ forces in the anterior cruciate ligament and its bundles in response to anterior tibial loads. J. Orthop. Res. 1997, 15, 285–293. [Google Scholar] [CrossRef]
- Shimokochi, Y.; Shultz, S.J. Mechanisms of noncontact anterior cruciate ligament injury. J. Athl. Train. 2008, 43, 396–408. [Google Scholar] [CrossRef]
- Musahl, V.; Nazzal, E.M.; Lucidi, G.A.; Serrano, R.; Hughes, J.D.; Margheritini, F.; Zaffagnini, S.; Fu, F.H.; Karlsson, J. Current trends in the anterior cruciate ligament part 1: Biology and biomechanics. Knee Surg. Sport. Traumatol. Arthrosc. 2022, 30, 20–33. [Google Scholar] [CrossRef]
- Lai, C.H.; Ardern, C.L.; Feller, J.A.; Webster, K.E. Eighty-three per cent of elite athletes return to preinjury sport after anterior cruciate ligament reconstruction: A systematic review with meta-analysis of return to sport rates, graft rupture rates and performance outcomes. Br. J. Sport. Med. 2018, 52, 128–138. [Google Scholar] [CrossRef]
- Barenius, B.; Ponzer, S.; Shalabi, A.; Bujak, R.; Norlen, L.; Eriksson, K. Increased Risk of Osteoarthritis After Anterior Cruciate Ligament Reconstruction A 14-Year Follow-up Study of a Randomized Controlled Trial. Am. J. Sport. Med. 2014, 42, 1049–1057. [Google Scholar] [CrossRef]
- Snaebjörnsson, T.; Hamrin Senorski, E.; Ayeni, O.R.; Alentorn-Geli, E.; Krupic, F.; Norberg, F.; Karlsson, J.; Samuelsson, K. Graft Diameter as a Predictor for Revision Anterior Cruciate Ligament Reconstruction and KOOS and EQ-5D Values A Cohort Study from the Swedish National Knee Ligament Register Based on 2240 Patients. Am. J. Sport. Med. 2017, 45, 2092–2097. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Cheng, C.K. Changing the Diameter of the Bone Tunnel Is More Effective Than Changing the Tunnel Shape for Restoring Joint Functionality After ACL Reconstruction. Front. Bioeng. Biotechnol. 2020, 8, 173. [Google Scholar] [CrossRef]
- Park, S.Y.; Oh, H.; Park, S.; Lee, J.H.; Lee, S.H.; Yoon, K.H. Factors predicting hamstring tendon autograft diameters and resulting failure rates after anterior cruciate ligament reconstruction. Knee Surg. Sport. Traumatol. Arthrosc. 2013, 21, 1111–1118. [Google Scholar] [CrossRef]
- Fujimaki, Y.; Thorhauer, E.; Sasaki, Y.; Smolinski, P.; Tashman, S.; Fu, F.H. Quantitative In Situ Analysis of the Anterior Cruciate Ligament: Length, Midsubstance Cross-sectional Area, and Insertion Site Areas. Am. J. Sport. Med. 2016, 44, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Pujol, N.; Queinnec, S.; Boisrenoult, P.; Maqdes, A.; Beaufils, P. Anatomy of the anterior cruciate ligament related to hamstring tendon grafts. A cadaveric study. Knee 2013, 20, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, M.; Cheng, C.K. A novel protection liner to improve graft-tunnel interaction following anterior cruciate ligament reconstruction: A finite element analysis. J. Orthop. Surg. Res. 2020, 15, 232. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-C.; Chen, W.-C.; Yang, C.-T.; Cheng, C.-K.; Chen, C.-H.; Lai, Y.-S. Interference screw versus Endoscrew fixation for anterior cruciate ligament reconstruction: A biomechanical comparative study in sawbones and porcine knees. J. Orthop. Transl. 2014, 2, 82–90. [Google Scholar] [CrossRef]
- Wilson, T.W.; Zafuta, M.P.; Zobitz, M. A biomechanical analysis of matched bone-patellar tendon-bone and double-looped semitendinosus and gracilis tendon grafts. Am. J. Sport. Med. 1999, 27, 202–207. [Google Scholar] [CrossRef]
- Kutzner, I.; Heinlein, B.; Graichen, F.; Bender, A.; Rohlmann, A.; Halder, A.; Beier, A.; Bergmann, G. Loading of the knee joint during activities of daily living measured in vivo in five subjects. J. Biomech. 2010, 43, 2164–2173. [Google Scholar] [CrossRef]
- Sasaki, Y.; Fujii, M.; Araki, D.; Marshall, B.D.; Linde, M.A.; Smolinski, P.; Fu, F.H. Effect of Percentage of Femoral Anterior Cruciate Ligament Insertion Site Reconstructed with Hamstring Tendon on Knee Kinematics and Graft Force. Am. J. Sport. Med. 2021, 49, 1279–1285. [Google Scholar] [CrossRef]
- Fu, F.H. Pearls: Individualized Approach to ACL Reconstruction—One Size Does Not Fit All. Clin. Orthop. Relat. Res. 2020, 478, 1735–1737. [Google Scholar] [CrossRef]
- Hashemi, J.; Mansouri, H.; Chandrashekar, N.; Slauterbeck, J.R.; Hardy, D.M.; Beynnon, B.D. Age, Sex, Body Anthropometry, and ACL Size Predict the Structural Properties of the Human Anterior Cruciate Ligament. J. Orthop. Res. 2011, 29, 993–1001. [Google Scholar] [CrossRef]
- Harner, C.D.; Baek, G.H.; Vogrin, T.M.; Carlin, G.J.; Kashiwaguchi, S.; Woo, S.L.Y. Quantitative analysis of human cruciate ligament insertions. Arthroscopy 1999, 15, 741–749. [Google Scholar] [CrossRef]
- Siebold, R.; Schuhmacher, P.; Fernandez, F.; Śmigielski, R.; Fink, C.; Brehmer, A.; Kirsch, J. Flat midsubstance of the anterior cruciate ligament with tibial “C”-shaped insertion site. Knee Surg. Sport. Traumatol. Arthrosc. 2015, 23, 3136–3142. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Hu, X.Q.; Zhang, X.; Jiang, Y.F.; Wang, J.Q.; Ao, Y.F. Clinical study of anatomical ACL reconstruction with adjustable oval shaped bone tunnels: A CT evaluation. Am. J. Transl. Res. 2018, 10, 3357–3369. [Google Scholar] [PubMed]
- Beveridge, J.E.; Proffen, B.L.; Karamchedu, N.P.; Chin, K.E.; Sieker, J.T.; Badger, G.J.; Kiapour, A.M.; Murray, M.M.; Fleming, B.C. Cartilage Damage Is Related to ACL Stiffness in a Porcine Model of ACL Repair. J. Orthop. Res. 2019, 37, 2249–2257. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Z.Y.; Qu, Y.; Shi, Q.Y.; Ai, S.T.; Cheng, C.K. Correlation between ACL size and dimensions of bony structures in the knee joint. Anat. Anz. 2022, 241, 151906. [Google Scholar] [CrossRef]

| Femoral Insertion | Isthmus | Tibial Insertion | ||||
|---|---|---|---|---|---|---|
| D (mm) | S (mm2) | D (mm) | S (mm2) | D (mm) | S (mm2) | |
| 0.95 times sized ACL | 11.8 | 109.5 | 6.4 | 31.9 | 14.6 | 167.5 |
| Original ACL | 12.4 | 121.3 | 6.7 | 35.3 | 15.4 | 185.6 |
| 1.05 times sized ACL | 13.0 | 133.7 | 7.0 | 38.9 | 16.2 | 204.6 |
| ATT (mm) | ITR (°) | VTR (°) | ACL/Graft Force (N) | ||
|---|---|---|---|---|---|
| Native ACL | 4.2 | 12.1 | 0.9 | 128 | |
| Diameter of the graft in ACLR (mm) | 7.5 | 4.4 | 11.8 | 1.3 | 115 |
| 8 | 4.3 | 11.6 | 1.2 | 116 | |
| 8.5 | 4.3 | 11.5 | 1.2 | 120 | |
| 9 | 4.2 | 11.4 | 1.2 | 122 | |
| 9.5 | 4.2 | 11.3 | 1.2 | 124 | |
| 10 | 4.2 | 11.2 | 1.1 | 127 | |
| 10.5 | 4.1 | 11.0 | 1.1 | 129 | |
| 11 | 4.1 | 10.9 | 1.1 | 134 | |
| 11.5 | 4.0 | 10.7 | 1.0 | 139 | |
| 12 | 3.8 | 10.4 | 0.9 | 147 | |
| ATT (mm) | ITR (°) | VTR (°) | ACL/Graft Force (N) | |
|---|---|---|---|---|
| 0.95 times-sized ACL | 4.4 | 12.1 | 1.0 | 124 |
| Native ACL | 4.2 | 12.1 | 0.9 | 128 |
| 1.05 times-sized ACL | 4.1 | 11.9 | 0.8 | 134 |
| 0.95 times-sized ACL | 11.5 | 10.9 |
| Original-sized ACL | 12.0 | 11.5 |
| 1.05 times-sized ACL | 12.0 | 12.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Tao, M.; Shi, Q.; He, K.; Cheng, C.-K. Graft Diameter Should Reflect the Size of the Native Anterior Cruciate Ligament (ACL) to Improve the Outcome of ACL Reconstruction: A Finite Element Analysis. Bioengineering 2022, 9, 507. https://doi.org/10.3390/bioengineering9100507
Wang H, Tao M, Shi Q, He K, Cheng C-K. Graft Diameter Should Reflect the Size of the Native Anterior Cruciate Ligament (ACL) to Improve the Outcome of ACL Reconstruction: A Finite Element Analysis. Bioengineering. 2022; 9(10):507. https://doi.org/10.3390/bioengineering9100507
Chicago/Turabian StyleWang, Huizhi, Mingzhu Tao, Qinyi Shi, Kaixin He, and Cheng-Kung Cheng. 2022. "Graft Diameter Should Reflect the Size of the Native Anterior Cruciate Ligament (ACL) to Improve the Outcome of ACL Reconstruction: A Finite Element Analysis" Bioengineering 9, no. 10: 507. https://doi.org/10.3390/bioengineering9100507