Implementation of Predictive Algorithms for the Study of the Endarterectomy LOS
Abstract
1. Introduction
2. Materials and Methods
- Gender (male/female);
- Age;
- Hypertension (yes/no);
- Diabetes (yes/no);
- Previous heart attack (yes/no);
- Embolism (yes/no);
- Hyperlipidaemia (yes/no);
- Respiratory system disorders (yes/no);
- Obesity (yes/no);
- Kidney disorders (yes/no);
- Cardiomyopathy (yes/no);
- Rhythm abnormalities (yes/no);
- Anemia (yes/no);
- Personal history of allergies (yes/no);
- Pre-operative LOS;
- Type of endarterectomy (Indicates on which vessels the endarterectomy was performed: 1, vessels of the head and neck; 2, upper limb vessels; 3, aorta; and 4, lower limb vessels).
2.1. Regression and Machine Learning Algorithms
2.2. Parameter Optimization and Cross-Validation for Classification Algorithms
2.3. Voting Technique
3. Results
- Group 0: LOS ≤ 5;
- Group 1: 5 < LOS ≤ 7;
- Group 2: LOS > 7.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LOS | length of stay |
| ML | machine learning |
| MLR | multiple linear regression |
| DT | decision tree |
| RF | random forest |
| SVM | support vector machine |
| MLP | multilayer perception |
| NB | naive Bayes |
| VC | voting classifier |
References
- Italian Ministry of Economy and Finance, General Accounting Office. Rapporto N. 7: Il Monitoraggio della Spesa Sanitaria; Italian Ministry of Economy and Finance: Roma, Italy, 2020.
- Subak, L.L.; Caughey, A.B. measuring cost-effectiveness of surgical procedures. Clin. Obstet. Gynecol. 2000, 43, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Improta, G.; Scala, A.; Trunfio, T.A.; Guizzi, G. Application of Supply Chain Management at Drugs Flow in an Italian Hospital District. J. Phys. Conf. Ser. 2021, 1828, 012081. [Google Scholar] [CrossRef]
- Di Laura, D.; D’Angiolella, L.; Mantovani, L.; Squassabia, G.; Clemente, F.; Santalucia, I.; Improta, G.; Triassi, M. Efficiency measures of emergency departments: An Italian systematic literature review. BMJ Open Qual. 2021, 10, e001058. [Google Scholar] [CrossRef]
- Cesarelli, G.; Montella, E.; Scala, A.; Raiola, E.; Triassi, M.; Improta, G. DMAIC Approach for the Reduction of Healthcare-Associated Infections in the Neonatal Intensive Care Unit of the University Hospital of Naples ‘Federico II’. In European Medical and Biological Engineering Conference; Springer: Cham, Switzerland, 2020; pp. 414–423. [Google Scholar]
- Ferraro, A.; Centobelli, P.; Cerchione, R.; Cicco, M.V.D.; Montella, E.; Raiola, E.; Triassi, M.; Improta, G. Implementation of lean practices to reduce healthcare associated infections. Int. J. Healthc. Technol. Manag. 2020, 18, 51–72. [Google Scholar] [CrossRef]
- Stephen, A.; Berger, D. Shortened length of stay and hospital cost reduction with implementation of an accelerated clinical care pathway after elective colon resection. Surgery 2003, 133, 277–282. [Google Scholar] [CrossRef]
- Trunfio, T.A.; Maria Ponsiglione, A.; Ferrara, A.; Borrelli, A.; Gargiulo, P. A comparison of different regression and classification methods for predicting the length of hospital stay after cesarean sections. In Proceedings of the 2021 5th International Conference on Medical and Health Informatics, Kyoto, Japan, 14–16 May 2021. [Google Scholar]
- Morton, A.; Marzban, E.; Giannoulis, G.; Patel, A.; Aparasu, R.; Kakadiaris, I.A. A Comparison of Supervised Machine Learning Techniques for Predicting Short-Term In-Hospital Length of Stay among Diabetic Patients. In Proceedings of the 2014 13th International Conference on Machine Learning and Applications, Detroit, MI, USA, 3–6 December 2014; pp. 428–431. [Google Scholar] [CrossRef]
- Trunfio, T.A.; Borrelli, A.; Improta, G. Is It Possible to Predict the Length of Stay of Patients Undergoing Hip-Replacement Surgery? Int. J. Environ. Res. Public Health 2022, 19, 6219. [Google Scholar] [CrossRef] [PubMed]
- Trunfio, T.A.; Scala, A.; Giglio, C.; Rossi, G.; Borrelli, A.; Romano, M.; Improta, G. Multiple regression model to analyse the total LOS for patients undergoing laparoscopic appendectomy. BMC Med. Inform. Decis. Mak. 2022, 22, 141. [Google Scholar] [CrossRef] [PubMed]
- Trunfio, T.A.; Scala, A.; Borrelli, A.; Sparano, M.; Triassi, M.; Improta, G. Application of the Lean Six Sigma approach to the study of the LOS of patients who undergo laparoscopic cholecystectomy at the San Giovanni di Dio and Ruggi d’Aragona University Hospital. In 2021 5th International Conference on Medical and Health Informatics (ICMHI 2021); Association for Computing Machinery: New York, NY, USA, 2021; pp. 50–54. [Google Scholar] [CrossRef]
- Roederer, G.O.; Langlois, Y.E.; Jager, K.A.; Primozich, J.F.; Beach, K.W.; Phillips, D.J.; Strandness, D.E., Jr. The natural history of carotid arterial disease in asymptomatic patients with cervical bruits. Stroke 1984, 15, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Calligaro, K.D.; Dougherty, M.J.; Raviola, C.A.; Musser, D.J.; DeLaurentis, D.A. Impact of clinical pathways on hospital costs and early outcome after major vascular surgery. J. Vasc. Surg. 1995, 22, 649–660. [Google Scholar] [CrossRef][Green Version]
- Kadwa, A.M.; Robbs, J.V. Carotid endarterectomy in Durban-the first 10 years. S. Afr. Med. J. 1993, 83, 249–252. [Google Scholar]
- North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N. Engl. J. Med. 1991, 325, 445–453. [Google Scholar] [CrossRef]
- Seeger, J.M. Clinical Curriculum and Educational Objectives for Vascular Surgery. Available online: https://surgery.duke.edu/sites/default/files/2022-04/VascularSurgeryProgramHandbookandCurriculum2016-17.pdf (accessed on 5 July 2022).
- Biller, J.; Feinberg, W.M.; Castaldo, J.E.; Whittemore, A.D.; Harbaugh, R.E.; Dempsey, R.J.; Caplan, L.R.; Kresowik, T.F.; Matchar, D.B.; Toole, J.F.; et al. Guidelines for carotid endarterectomy: A statement for healthcare professionals from a special writing group of the stroke council, American Heart Association. Stroke 1998, 29, 554–562. [Google Scholar] [CrossRef]
- Ho, K.J.; Madenci, A.L.; McPhee, J.T.; Semel, M.E.; Bafford, R.A.; Nguyen, L.L.; Ozaki, C.K.; Belkin, M. Contemporary predictors of extended postoperative hospital length of stay after carotid endarterectomy. J. Vasc. Surg. 2014, 59, 1282–1290. [Google Scholar] [CrossRef]
- Back, M.R.; Harward, T.R.; Huber, T.S.; Carlton, L.M.; Flynn, T.C.; Seeger, J.M. Improving the cost-effectiveness of carotid endarterectomy. J. Vasc. Surg. 1997, 26, 456–462. [Google Scholar] [CrossRef][Green Version]
- Glaser, J.; Kuwayama, D.; Stone, D.; Schanzer, A.; Eldrup-Jorgensen, J.; Powell, R.; Stanley, A.; Nolan, B. Factors that determine the length of stay after carotid endarterectomy represent opportunities to avoid financial losses. J. Vasc. Surg. 2014, 60, 966–972. [Google Scholar] [CrossRef]
- Hernandez, N.; Salles-Cunha, S.X.; Daoud, Y.A.; Dosick, S.M.; Whalen, R.C.; Pigott, J.P.; Seiwert, A.J.; Russell, T.E.; Beebe, H.G. Factors related to short length of stay after carotid endarterectomy. Vasc. Endovasc. Surg. 2002, 36, 425–437. [Google Scholar] [CrossRef]
- Darling Iii, R.C.; Kreienberg, P.B.; Roddy, S.P.; Paty, P.S.; Chang, B.B.; Lloyd, W.E.; Shah, D.M. Analysis of the effect of asymptomatic carotid atherosclerosis study on the outcome and volume of carotid endarterectomy. Cardiovasc. Surg. 2000, 8, 436–440. [Google Scholar] [CrossRef]
- Roddy, S.P.; Estes, J.M.; Kwoun, M.O.; O’donnell, T.F.; Mackey, W.C. Factors predicting prolonged length of stay after carotid endarterectomy. J. Vasc. Surg. 2000, 32, 550–554. [Google Scholar] [CrossRef]
- Scala, A.; Trunfio, T.A.; Borrelli, A.; Ferrucci, G.; Triassi, M.; Improta, G. Modelling the hospital length of stay for patients undergoing laparoscopic cholecystectomy through a multiple regression model. In 2021 5th International Conference on Medical and Health Informatics (ICMHI 2021); Association for Computing Machinery: New York, NY, USA, 2021; pp. 68–72. [Google Scholar] [CrossRef]
- Ponsiglione, A.M.; Amato, F.; Romano, M. Multiparametric investigation of dynamics in fetal heart rate signals. Bioengineering 2021, 9, 8. [Google Scholar] [CrossRef]
- Ponsiglione, A.M.; Cosentino, C.; Cesarelli, G.; Amato, F.; Romano, M. A Comprehensive Review of Techniques for Processing and Analyzing Fetal Heart Rate Signals. Sensors 2021, 21, 6136. [Google Scholar] [CrossRef]
- Ponsiglione, A.M.; Cesarelli, G.; Amato, F.; Romano, M. Optimization of an artificial neural network to study accelerations of foetal heart rhythm. In Proceedings of the 2021 IEEE 6th International Forum on Research and Technology for Society and Industry (RTSI), Naples, Italy, 6–9 September 2021; pp. 159–164. [Google Scholar] [CrossRef]
- Cesarelli, M.; Romano, M.; Bifulco, P.; Improta, G.; D’Addio, G. An application of symbolic dynamics for FHRV assessment. Stud. Health Technol. Inform. 2012, 180, 123–127. [Google Scholar]
- Ponsiglione, A.M.; Romano, M.; Amato, F. A Finite-State Machine Approach to Study Patients Dropout from Medical Examinations. In Proceedings of the 2021 IEEE 6th International Forum on Research and Technology for Society and Industry (RTSI), Naples, Italy, 6–9 September 2021; pp. 289–294. [Google Scholar] [CrossRef]
- Sahoo, S.; Dash, M.; Behera, S.; Sabut, S. Machine Learning Approach to Detect Cardiac Arrhythmias in ECG Signals: A Survey. IRBM 2020, 41, 185–194. [Google Scholar] [CrossRef]
- Romano, M.; Bifulco, P.; Improta, G.; Faiella, G.; Cesarelli, M.; Clemente, F.; D’Addio, G. Symbolic dynamics in cardiotocographic monitoring. In Proceedings of the E-Health and Bioengineering Conference (EHB 2013), Iasi, Romania, 21–23 November 2013. [Google Scholar]
- Cesarelli, M.; Romano, M.; Bifulco, P.; Improta, G.; D’Addio, G. Prognostic decision support using symbolic dynamics in CTG monitoring. Stud. Health Technol. Inform. 2013, 186, 140–144. [Google Scholar]
- De Lauri, C.; Angela Trunfio, T.; Colella, Y.; Lombardi, A.; Borrelli, A.; Gargiulo, P. Investigating the impact of age, gender, and comorbid conditions on the prolonged length of stay after endarterectomy. In Proceedings of the 2021 International Symposium on Biomedical Engineering and Computational Biology, Nanchang, China, 13–15 August 2021. [Google Scholar]
- Moscato, V.; Picariello, A.; Sperlí, G. A benchmark of machine learning approaches for credit score prediction. Expert Syst. Appl. 2021, 165, 113986. [Google Scholar] [CrossRef]
- Bacchi, S.; Tan, Y.; Oakden-Rayner, L.; Jannes, J.; Kleinig, T.; Koblar, S. Machine learning in the prediction of medical inpatient length of stay. Intern. Med. J. 2022, 52, 176–185. [Google Scholar] [CrossRef]
- Scala, A.; Trunfio, T.A.; De Coppi, L.; Rossi, G.; Borrelli, A.; Triassi, M.; Improta, G. Regression models to study the total LOS related to valvuloplasty. Int. J. Environ. Res. Public Health 2022, 19, 3117. [Google Scholar] [CrossRef]
- Pollard, J.B.; Garnerin, P.H.; Dalman, R.L. Use of outpatient preoperative evaluation to decrease length of stay for vascular surgery. Anesth. Analg. 1997, 85, 1307–1311. [Google Scholar] [CrossRef]
- Sidawy, A.N.; Aidinian, G.; Johnson, O.N., III; White, P.W.; DeZee, K.J.; Henderson, W.G. Effect of chronic renal insufficiency on outcomes of carotid endarterectomy. J. Vasc. Surg. 2008, 48, 1423–1430. [Google Scholar] [CrossRef]
- Alsinglawi, B.; Alnajjar, F.; Mubin, O.; Novoa, M.; Alorjani, M.; Karajeh, O.; Darwish, O. Predicting length of stay for cardiovascular hospitalizations in the intensive care unit: Machine learning approach. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020. [Google Scholar]
- Daghistani, T.A.; Elshawi, R.; Sakr, S.; Ahmed, A.M.; Al-Thwayee, A.; Al-Mallah, M.H. Predictors of in-hospital length of stay among cardiac patients: A machine learning approach. Int. J. Cardiol. 2019, 288, 140–147. [Google Scholar] [CrossRef]
- van Gaal, S.; Alimohammadi, A.; Yu, A.Y.; Karim, M.E.; Zhang, W.; Sutherland, J.M. Accurate classification of carotid endarterectomy indication using physician claims and hospital discharge data. BMC Health Serv. Res. 2022, 22, 379. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Mukherjee, D.; Clair, D.G. Carotid artery stenting vs. endarterectomy. Eur. Heart J. 2009, 30, 2693–2704. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.D.; Lyrer, P.; Brown, M.M.; Bonati, L.H. Carotid artery stenting versus endarterectomy for treatment of carotid artery stenosis. Cochrane Database Syst. Rev. 2020. [Google Scholar] [CrossRef]
- Gahremanpour, A.; Perin, E.C.; Silva, G. Carotid artery stenting versus endarterectomy: A systematic review. Tex. Heart Inst. J. 2012, 39, 474. [Google Scholar]
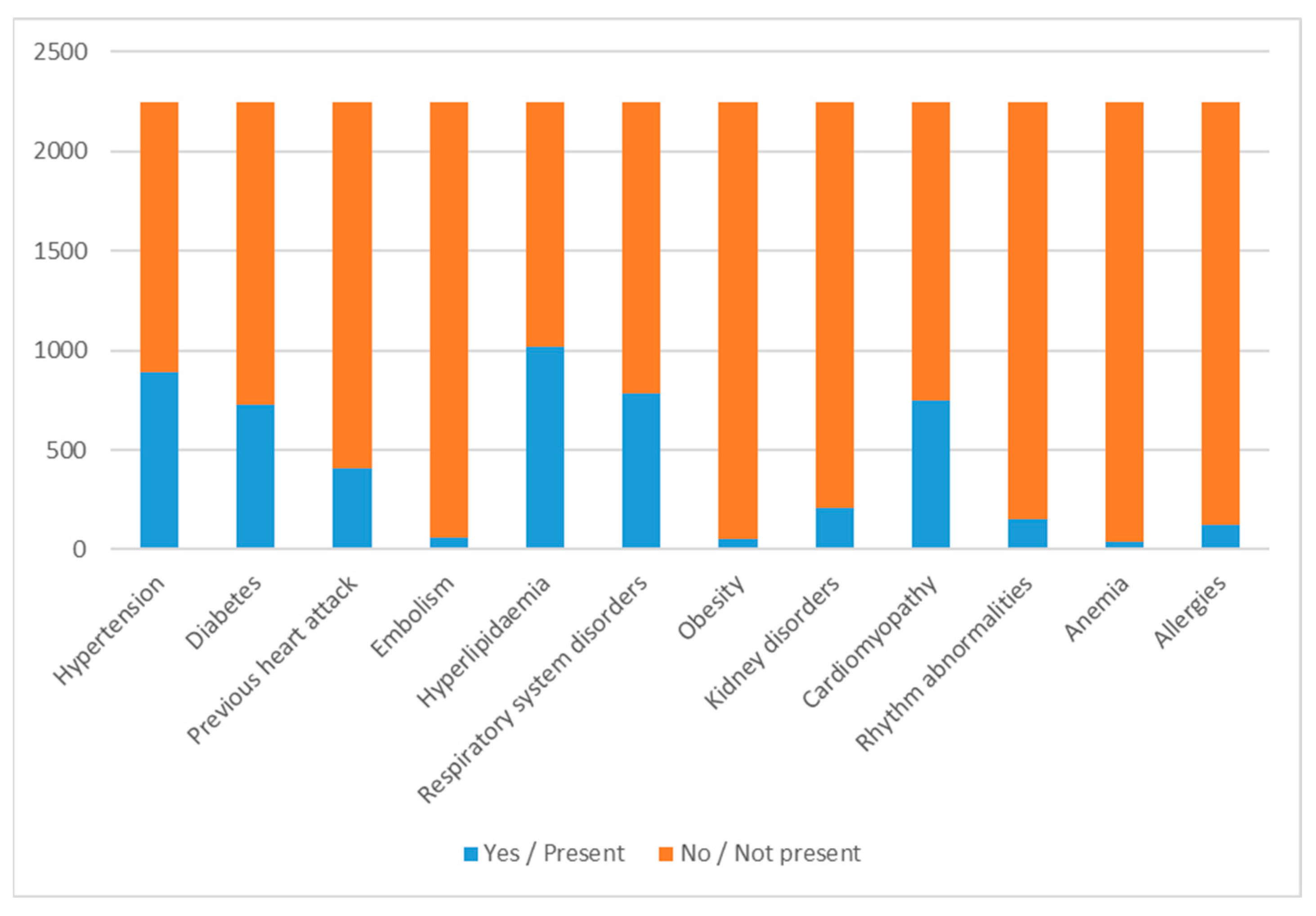

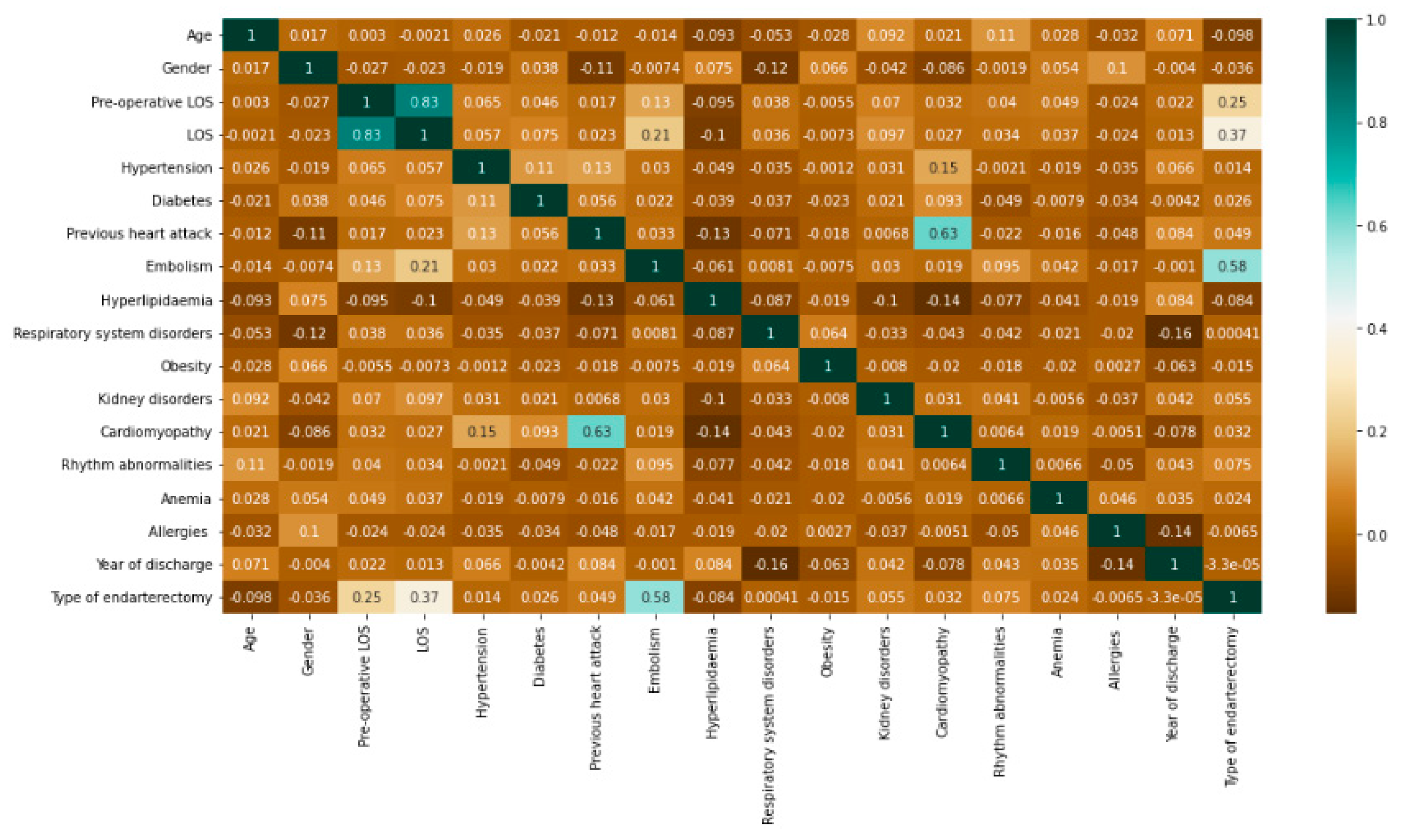
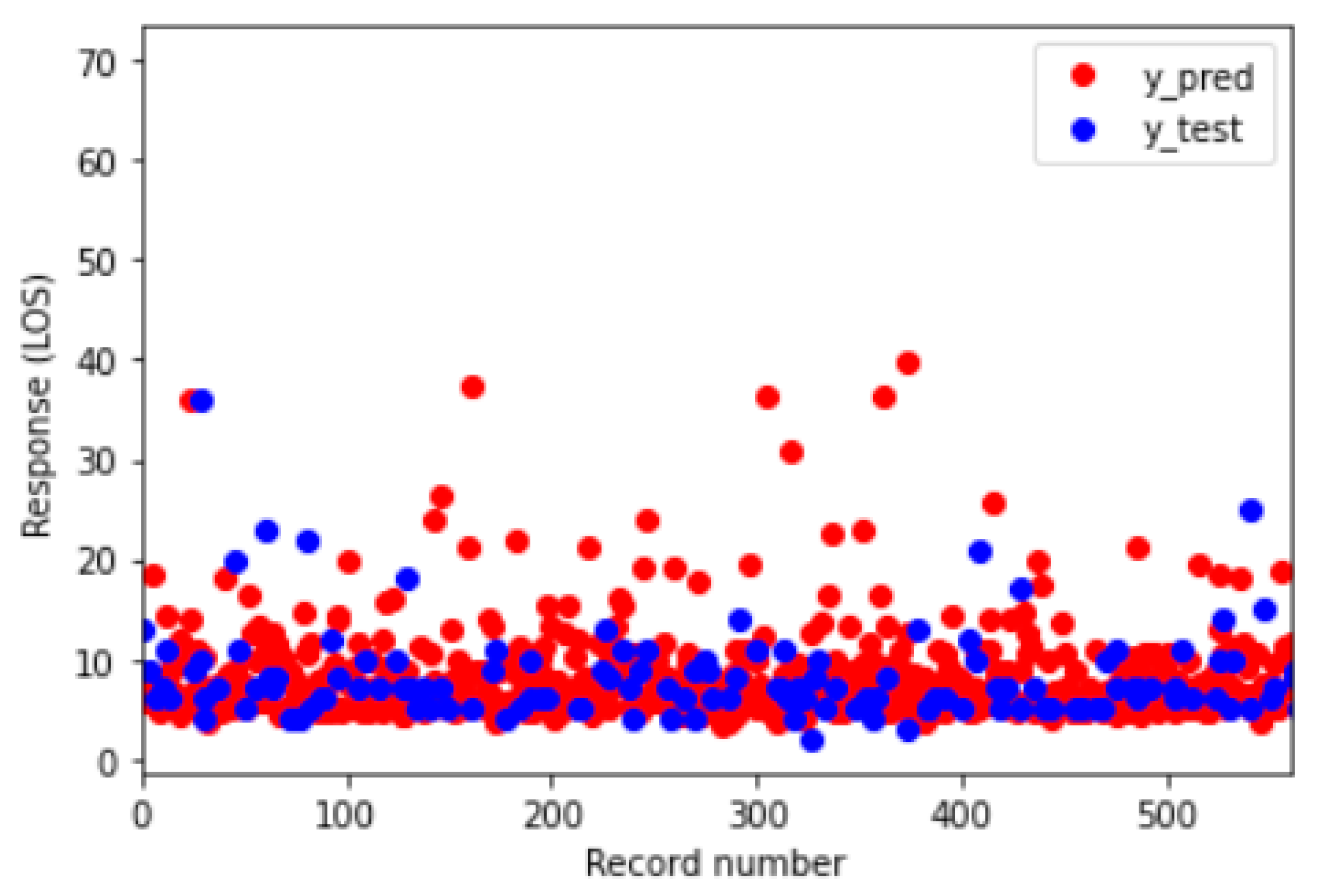


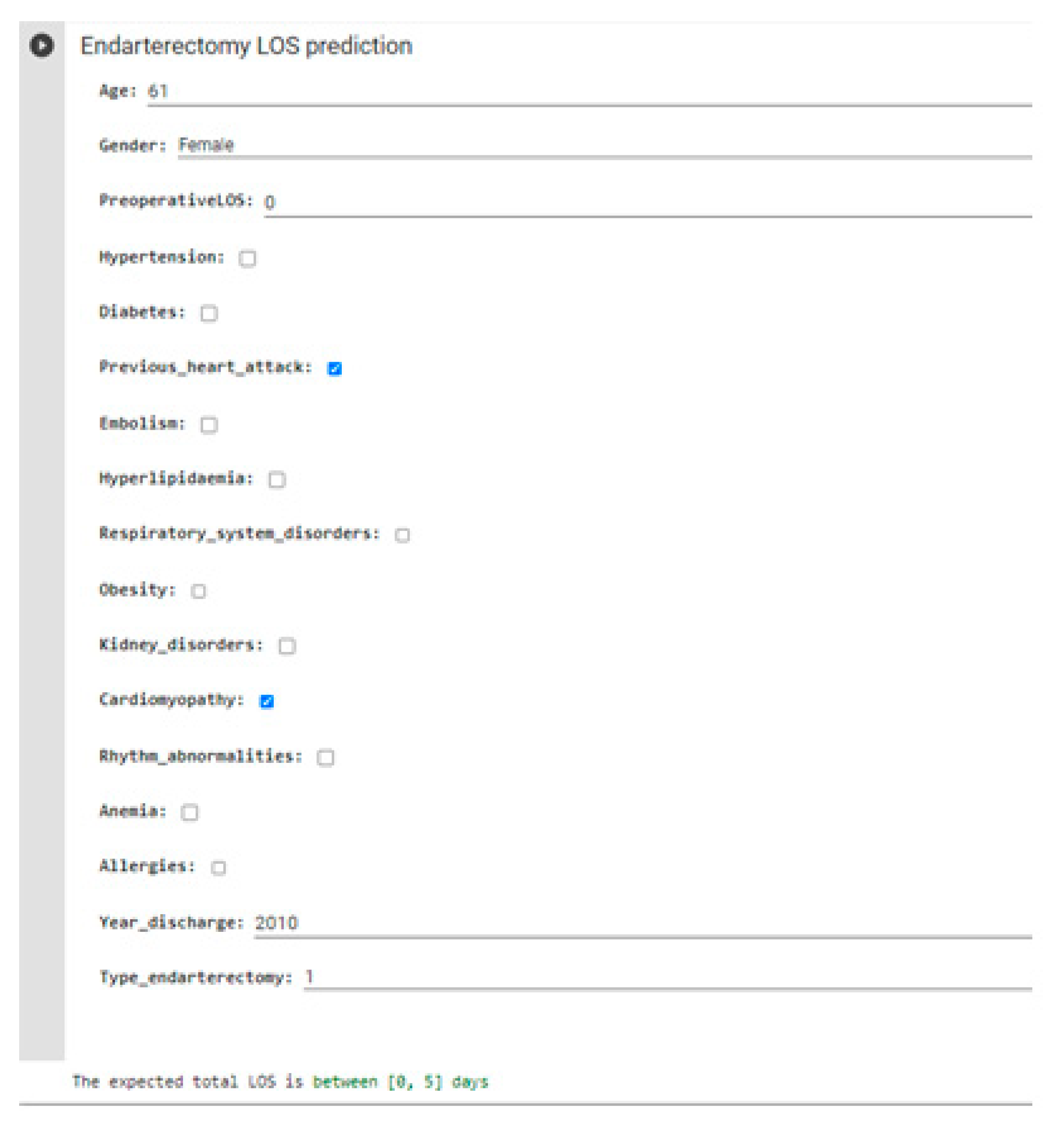
| Year of Discharge | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 |
| N° of discharges | 60 | 286 | 252 | 222 | 246 | 222 | 215 | 196 | 185 | 208 | 151 |
| Type of endarterectomy | 1 | 2 | 3 | 4 |
| N° of discharges | 2097 | 4 | 3 | 139 |
| Algorithms | Hyperparameters |
|---|---|
| SVM | ‘kernel’: (‘linear’, ‘rbf’), ‘C’: [1, 10, 100], cv = 10 |
| RF | ‘n_estimators’: [5, 10, 15, 20], ‘max_depth’: [2, 5, 7, 9], cv = 10 |
| DT | ‘max_depth’: range(3, 20), cv = 10 |
| MLP | ‘hidden_layer_sizes’: [(50, 50, 50), (50, 100, 50), (100,)], ‘activation’: [‘tanh’, ‘relu’], ‘solver’: [‘sgd’, ‘adam’], ‘alpha’: [0.0001, 0.05],’ learning_rate’: [‘constant’,’adaptive’], cv = 10 |
| NB | ‘var_smoothing’: np.logspace(0, −9, num = 100), cv = 10 |
| MLR | RF | DT | |
|---|---|---|---|
| R-squared | 0.845 | 0.782 | 0.584 |
| R-squared adjusted | 0.840 | 0.775 | 0.571 |
| RMSE | 2.217 | 2.628 | 3.630 |
| Unstandardized Coefficients | Standardized Coefficients | t | p−Value * | ||
|---|---|---|---|---|---|
| B | Std. Error | Beta | |||
| Intercept | 17.663 | 38,936 | − | 0.454 | 0.650 |
| Age | 0.007 | 0.007 | 0.012 | 1.030 | 0.303 |
| Gender | 0.063 | 0.116 | 0.006 | 0.539 | 0.590 |
| Pre−operative LOS | 1.013 | 0.015 | 0.781 | 66.633 | 0.000 |
| Hypertension | −0.003 | 0.113 | 0.000 | −0.029 | 0.977 |
| Diabetes | 0.348 | 0.117 | 0.034 | 2981 | 0.003 |
| Previous heart attack | 0.069 | 0.184 | 0.006 | 0.377 | 0.707 |
| Embolism | 0.214 | 0.407 | 0.007 | 0.527 | 0.598 |
| Hyperlipidaemia | −0.095 | 0.113 | −0.010 | −0.847 | 0.397 |
| Respiratory system disorders | 0.071 | 0.117 | 0.007 | 0.607 | 0.544 |
| Obesity | −0.023 | 0.364 | −0.001 | −0.062 | 0.950 |
| Kidney disorders | 0.515 | 0.188 | 0.031 | 2.745 | 0.006 |
| Cardiomyopathy | −0.119 | 0.151 | −0.012 | −0.789 | 0.430 |
| Rhythm abnormalities | −0.231 | 0.218 | −0.012 | −1.062 | 0.288 |
| Anemia | −0.189 | 0.426 | −0.005 | −0.444 | 0.657 |
| Allergies | −0.060 | 0.241 | −0.003 | −0.250 | 0.803 |
| Year of discharge | −0.008 | 0.019 | −0.005 | −0.403 | 0.687 |
| Type of endarterectomy | 1.146 | 0.094 | 0.174 | 12.152 | 0.000 |
| Variables | LOS | p-Value * | ||
|---|---|---|---|---|
| Group 0 N = 652 | Group 1 N = 805 | Group 2 N = 786 | ||
| Age | 71.8 ± 7.9 | 72.1 ± 8.0 | 71.8 ± 8.8 | 0.754 |
| Gender | ||||
| 0 | 414 | 523 | 536 | 0.152 |
| 1 | 238 | 282 | 250 | |
| Pre-operative LOS | 1.2 ± 0.6 | 2.8 ± 0.9 | 7.1 ± 0.2 | 0.000 |
| Hypertension | ||||
| 0 | 414 | 498 | 443 | 0.013 |
| 1 | 238 | 307 | 343 | |
| Diabetes | ||||
| 0 | 455 | 553 | 504 | 0.046 |
| 1 | 197 | 252 | 282 | |
| Previous heart attack | ||||
| 0 | 554 | 658 | 632 | 0.334 |
| 1 | 108 | 147 | 154 | |
| Embolism | ||||
| 0 | 645 | 797 | 739 | 0.000 |
| 1 | 7 | 8 | 47 | |
| Hyperlipidaemia | ||||
| 0 | 329 | 430 | 464 | 0.004 |
| 1 | 323 | 375 | 322 | |
| Respiratory system disorders | ||||
| 0 | 431 | 526 | 505 | 0.758 |
| 1 | 221 | 279 | 281 | |
| Obesity | ||||
| 0 | 631 | 789 | 772 | 0.151 |
| 1 | 21 | 16 | 14 | |
| Kidney disorders | ||||
| 0 | 602 | 731 | 700 | 0.103 |
| 1 | 50 | 74 | 86 | |
| Cardiomyopathy | ||||
| 0 | 440 | 545 | 506 | 0.302 |
| 1 | 212 | 260 | 280 | |
| Rhythm abnormalities | ||||
| 0 | 614 | 758 | 718 | 0.041 |
| 1 | 38 | 47 | 68 | |
| Anemia | ||||
| 0 | 638 | 792 | 776 | 0.429 |
| 1 | 14 | 13 | 10 | |
| Allergies | ||||
| 0 | 617 | 758 | 745 | 0.852 |
| 1 | 35 | 47 | 41 | |
| Year of discharge | ||||
| 2010 | 10 | 27 | 23 | 0.179 |
| 2011 | 87 | 103 | 96 | |
| 2012 | 72 | 106 | 74 | |
| 2013 | 59 | 83 | 81 | |
| 2014 | 60 | 84 | 102 | |
| 2015 | 74 | 72 | 76 | |
| 2016 | 59 | 78 | 78 | |
| 2017 | 63 | 67 | 66 | |
| 2018 | 55 | 59 | 71 | |
| 2019 | 58 | 81 | 69 | |
| 2020 | 55 | 46 | 50 | |
| Type of endarterectomy | ||||
| 1 | 643 | 789 | 665 | 0.000 |
| 2 | 3 | 1 | 0 | |
| 3 | 0 | 0 | 3 | |
| 4 | 6 | 15 | 118 | |
| Algorithms | Accuracy | Best Parameters |
|---|---|---|
| RF | 0.77 | ‘max_depth’: 9, n_estimators’: 15 |
| MLP | 0.78 | ‘activation’: ‘tanh’, ‘alpha’: 0.05, ‘hidden_layer_sizes’: (100), ‘learning_rate’: ‘constant’, ‘solver’: ‘adam’ |
| NB | 0.73 | ‘var_smoothing’: 0.001 |
| SVM | 0.79 | ‘C’: 10, ‘kernel’: ‘linear’ |
| DT | 0.80 | ‘max_depth’: 5 |
| VC | 0.79 | ‘voting technique’: hard, ‘weights’: None |
| Algorithms | Class | Precision | Recall | F1-Score |
|---|---|---|---|---|
| DT | 0 | 0.78 | 0.82 | 0.80 |
| 1 | 0.71 | 0.79 | 0.75 | |
| 2 | 0.95 | 0.78 | 0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trunfio, T.A.; Borrelli, A.; Improta, G. Implementation of Predictive Algorithms for the Study of the Endarterectomy LOS. Bioengineering 2022, 9, 546. https://doi.org/10.3390/bioengineering9100546
Trunfio TA, Borrelli A, Improta G. Implementation of Predictive Algorithms for the Study of the Endarterectomy LOS. Bioengineering. 2022; 9(10):546. https://doi.org/10.3390/bioengineering9100546
Chicago/Turabian StyleTrunfio, Teresa Angela, Anna Borrelli, and Giovanni Improta. 2022. "Implementation of Predictive Algorithms for the Study of the Endarterectomy LOS" Bioengineering 9, no. 10: 546. https://doi.org/10.3390/bioengineering9100546
APA StyleTrunfio, T. A., Borrelli, A., & Improta, G. (2022). Implementation of Predictive Algorithms for the Study of the Endarterectomy LOS. Bioengineering, 9(10), 546. https://doi.org/10.3390/bioengineering9100546







