Accuracy of a Three-Dimensional (3D)-Printed Patient-Specific (PS) Femoral Osteotomy Guide: A Computed Tomography (CT) Study
Abstract
:1. Introduction
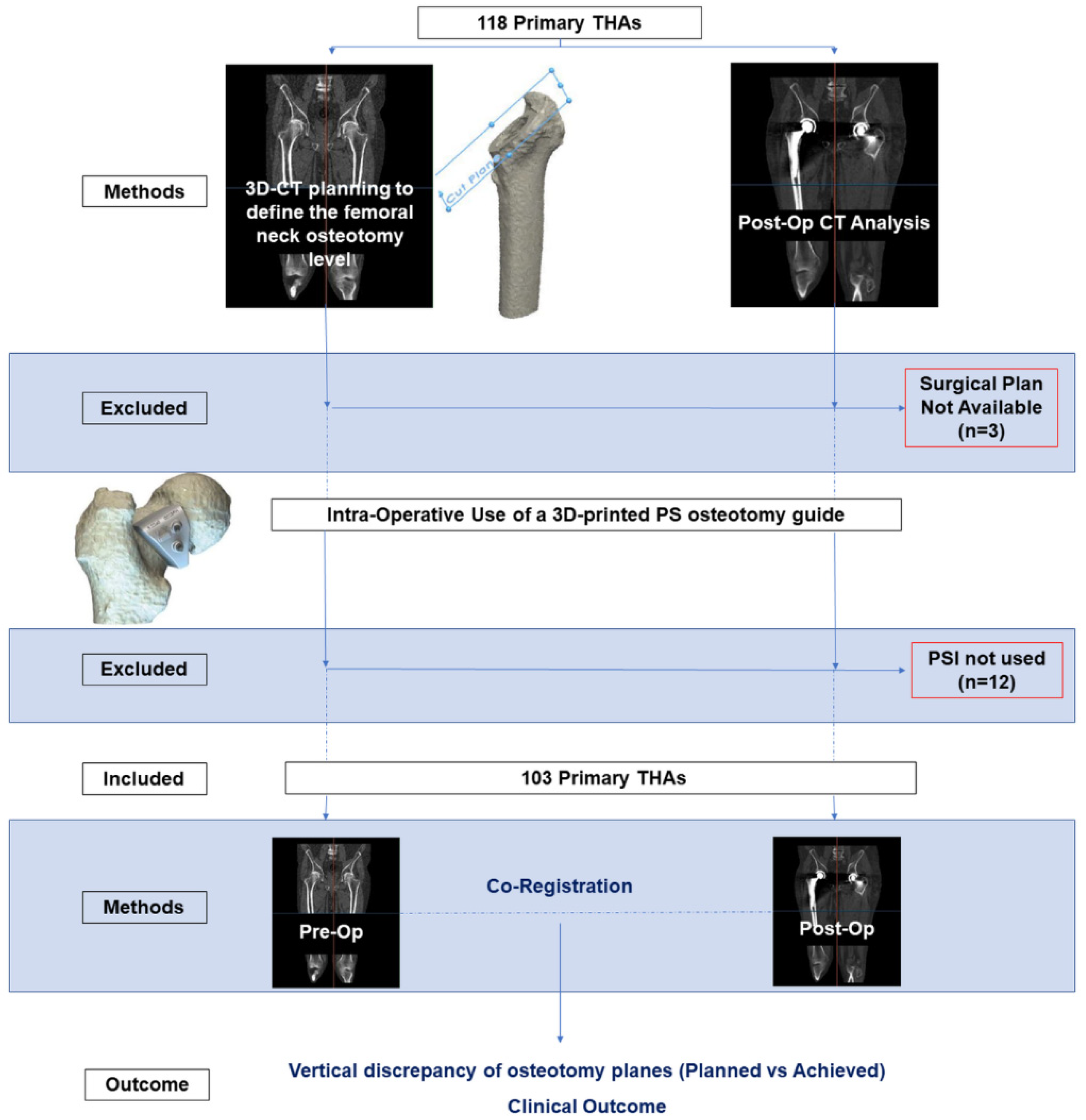
2. Materials and Methods
2.1. Study Design
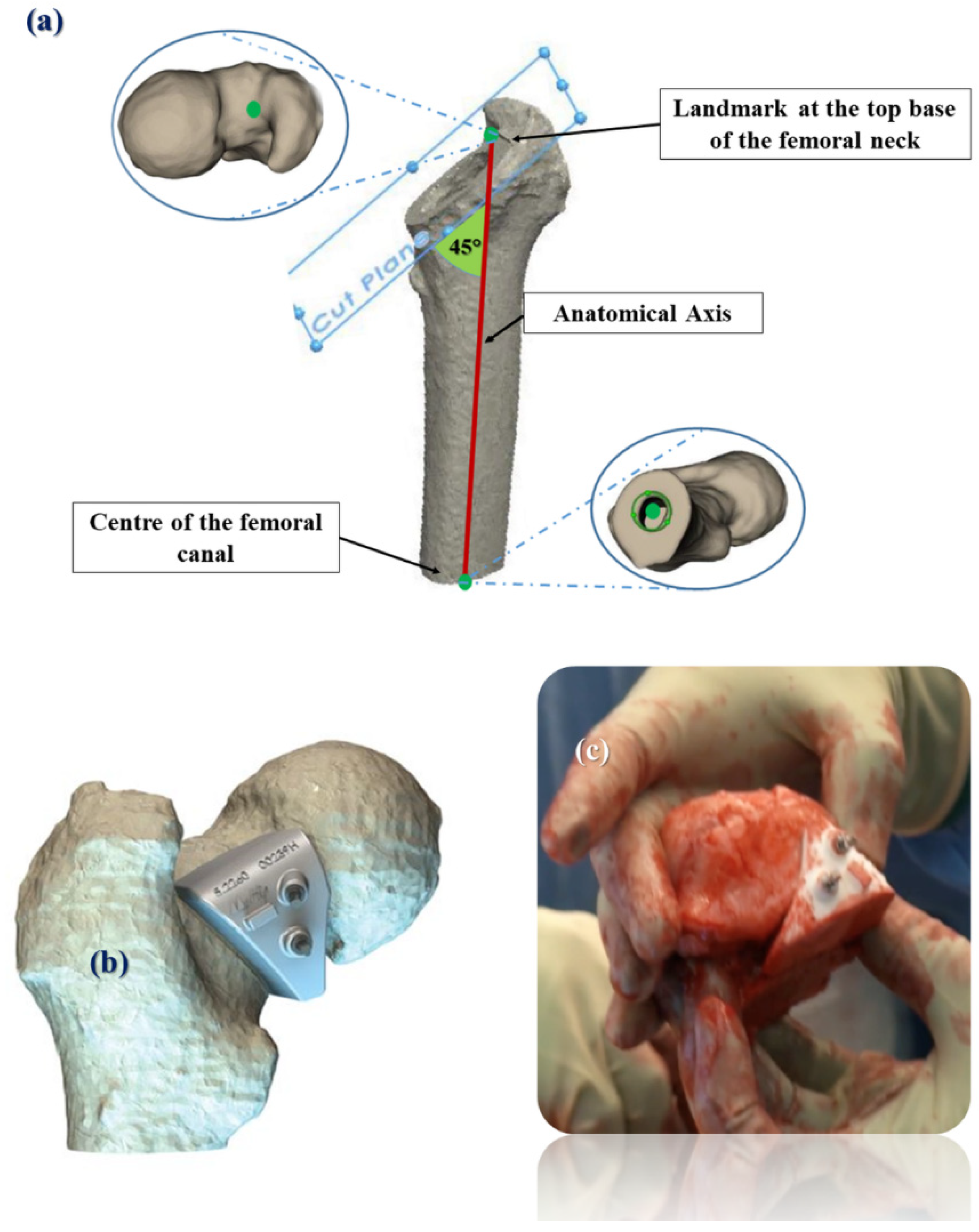
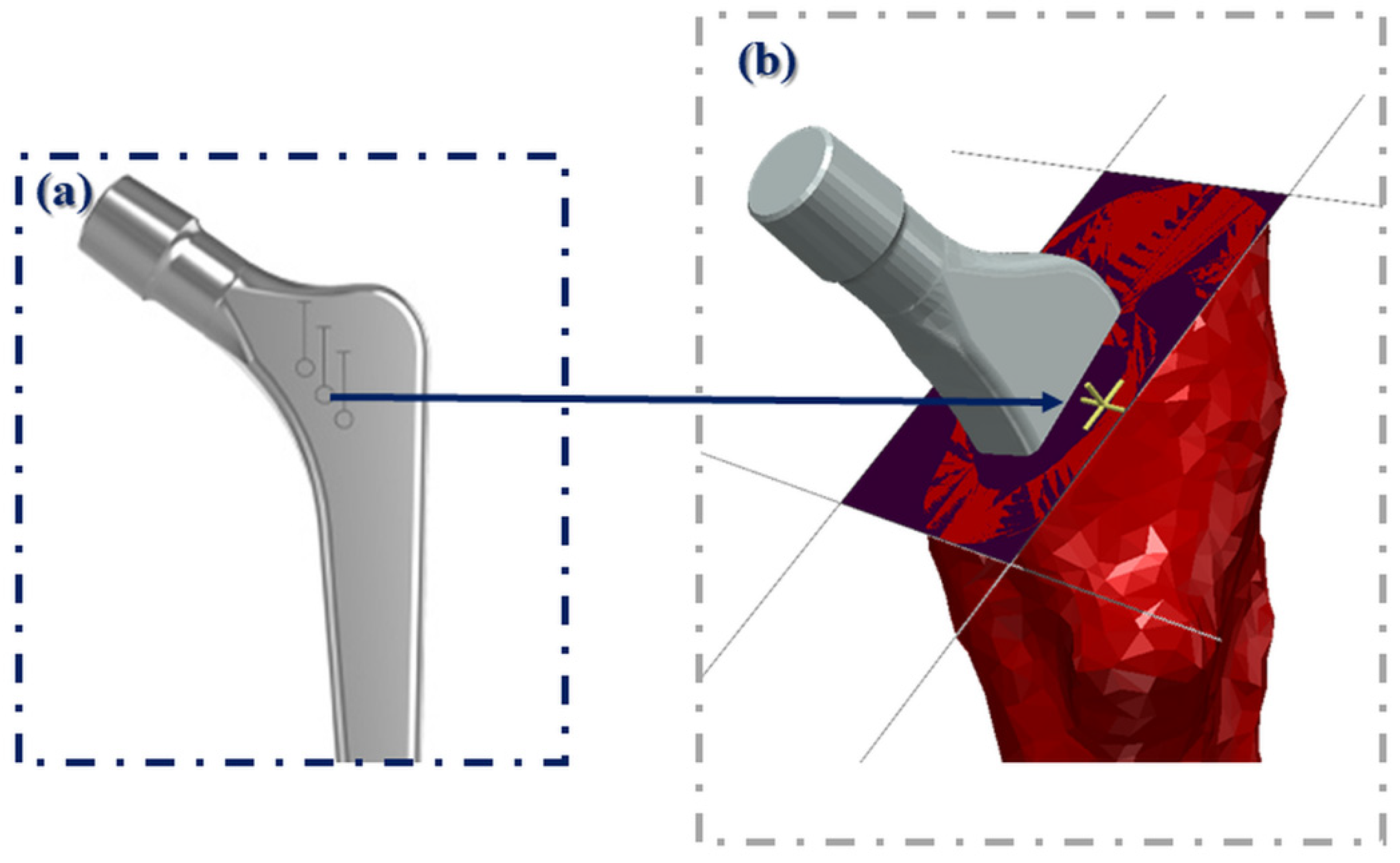
2.2. Pre-Operative Planning and Surgery
2.3. Radiological Evaluation
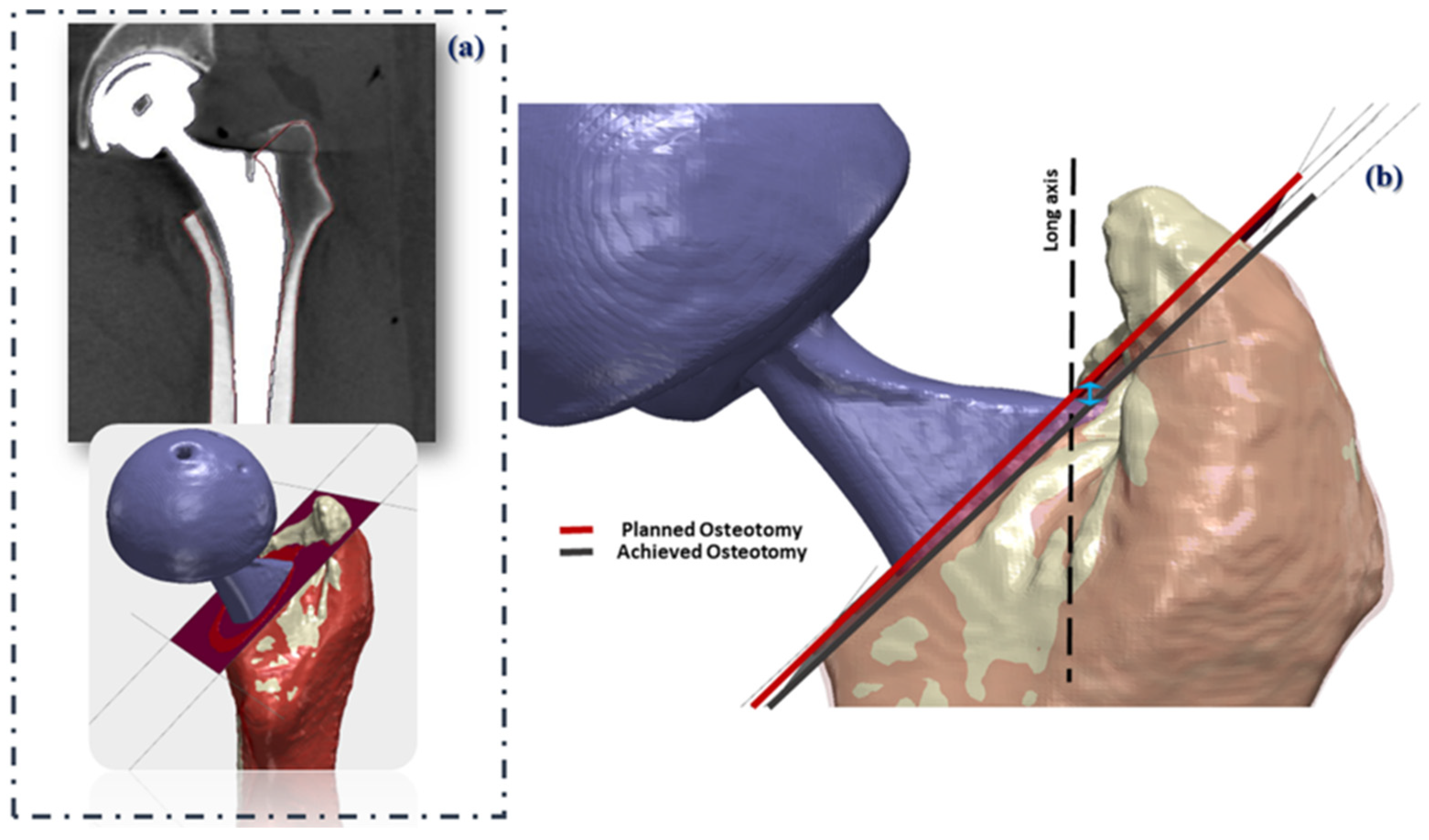
2.4. Pre- and Post-Operative CT Alignment
2.5. Clinical Evaluation
2.6. Statistics
3. Results
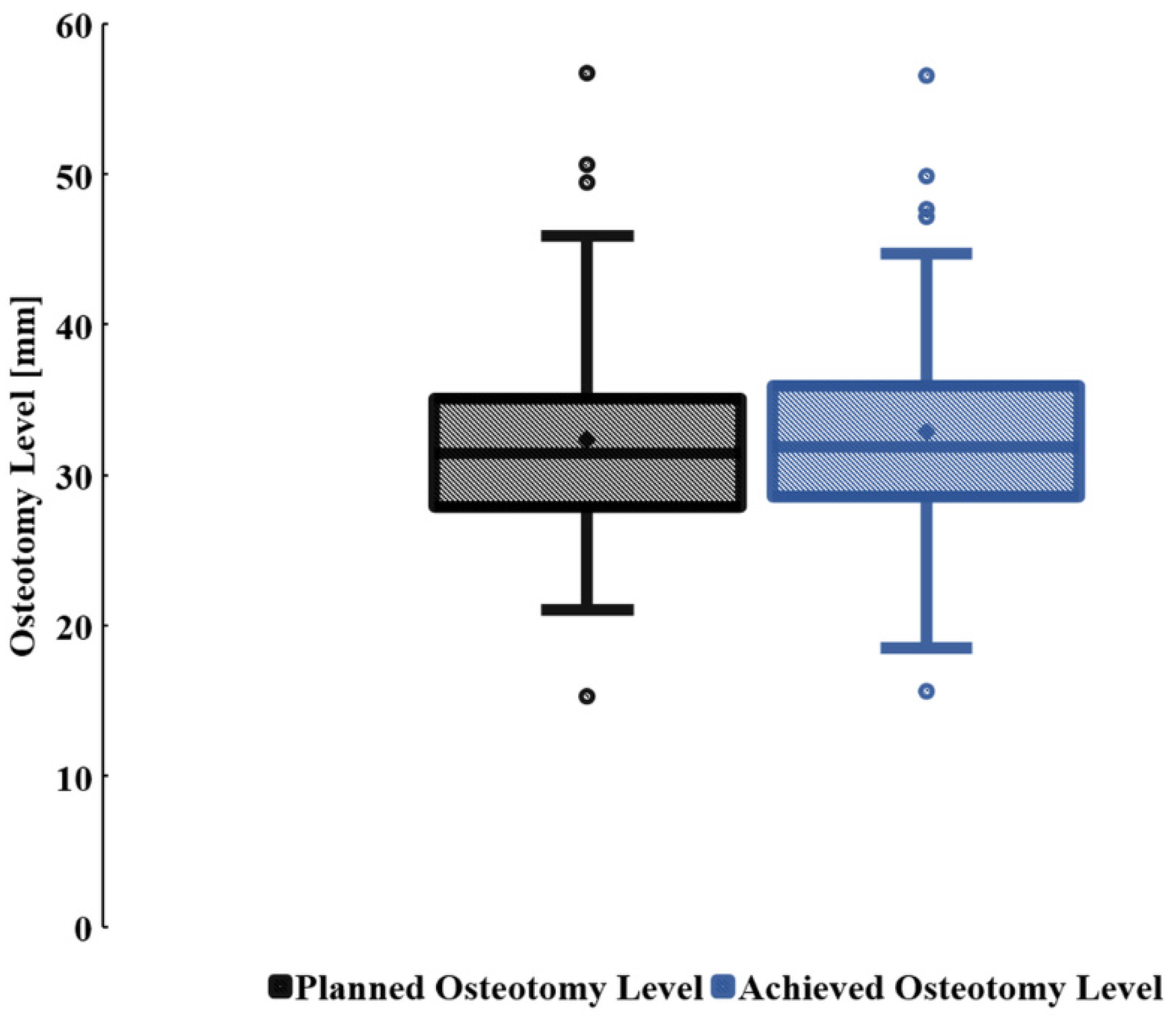
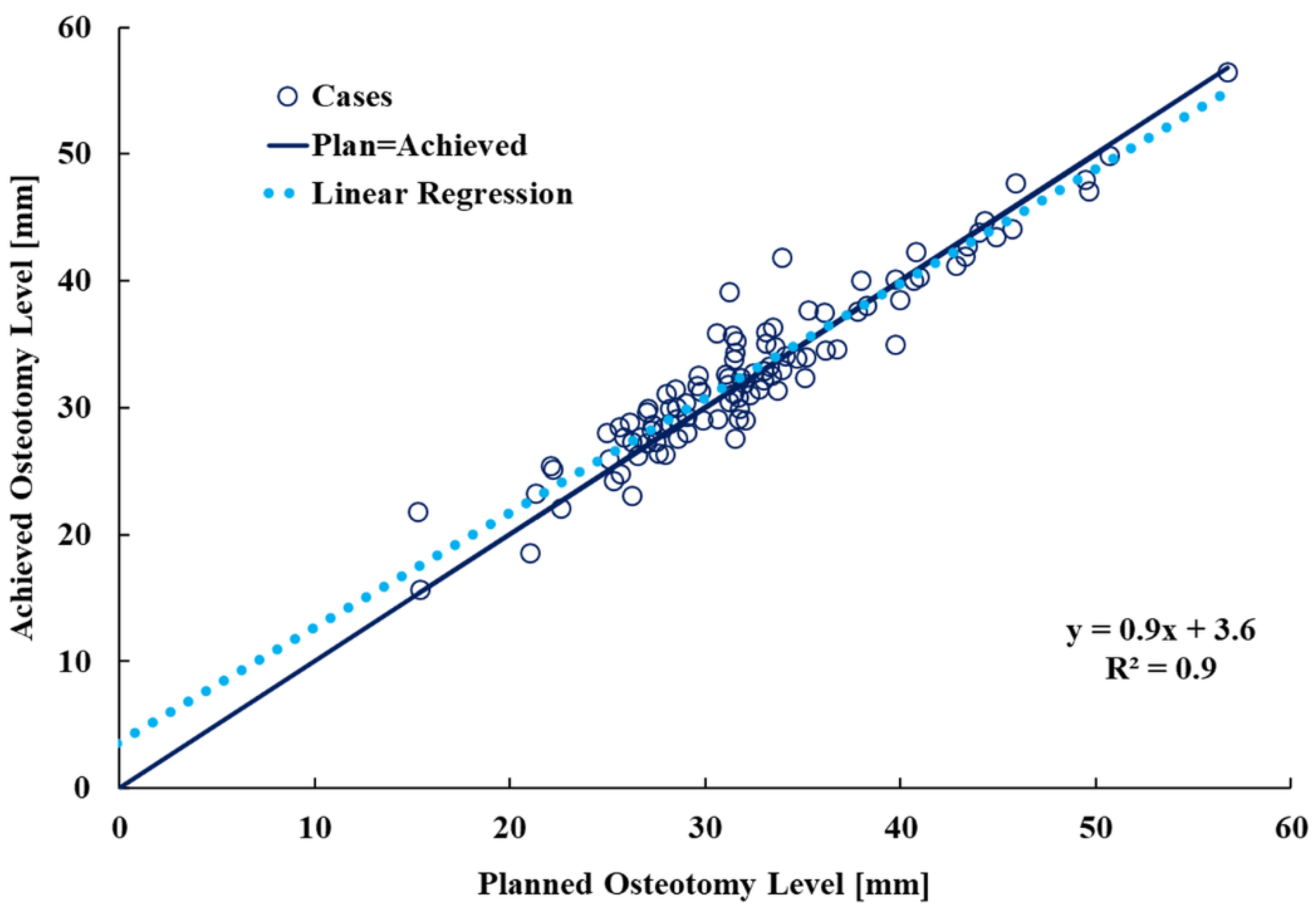
3.1. Planned and Achieved Femoral Neck Osteotomy Levels
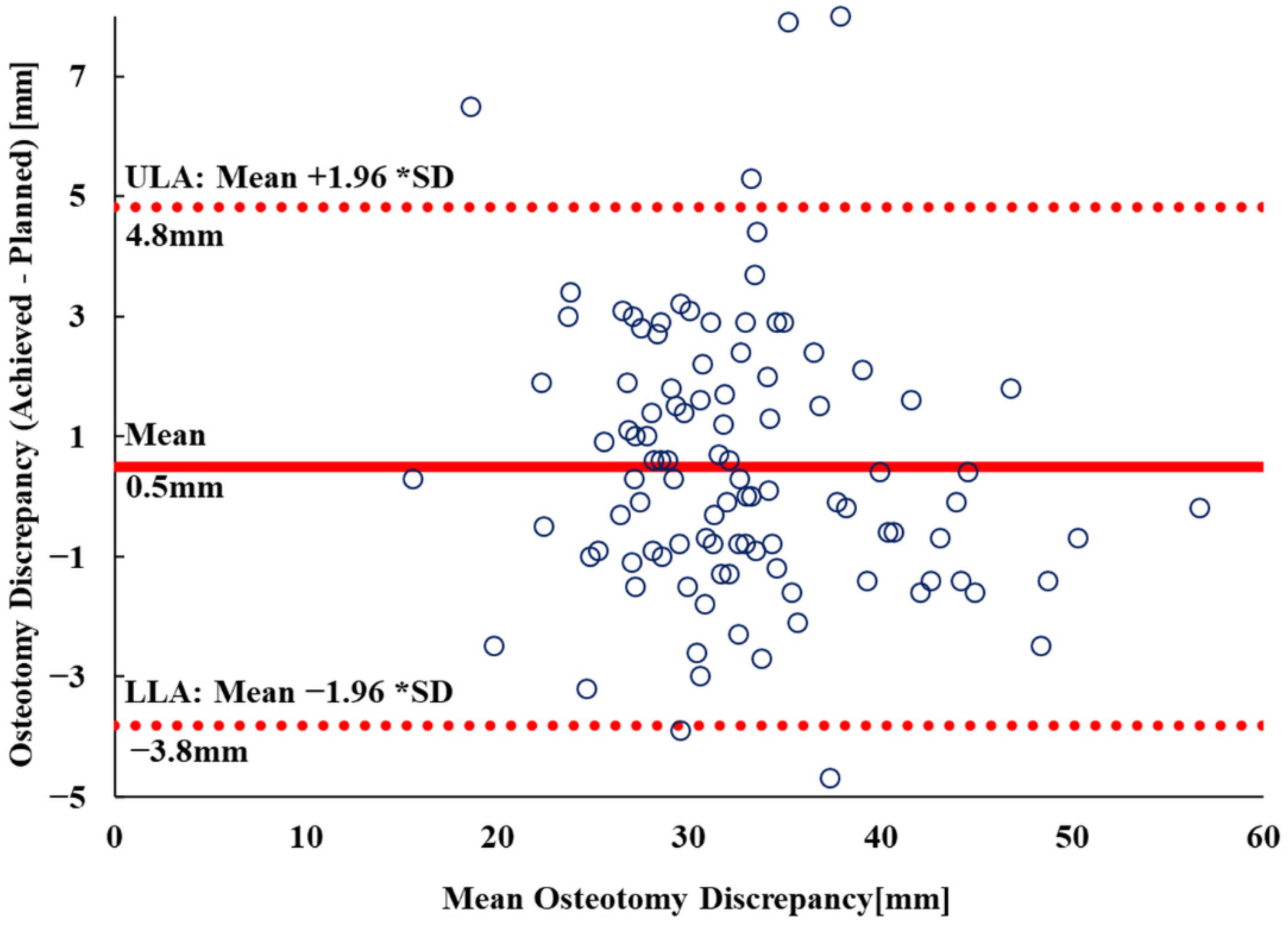
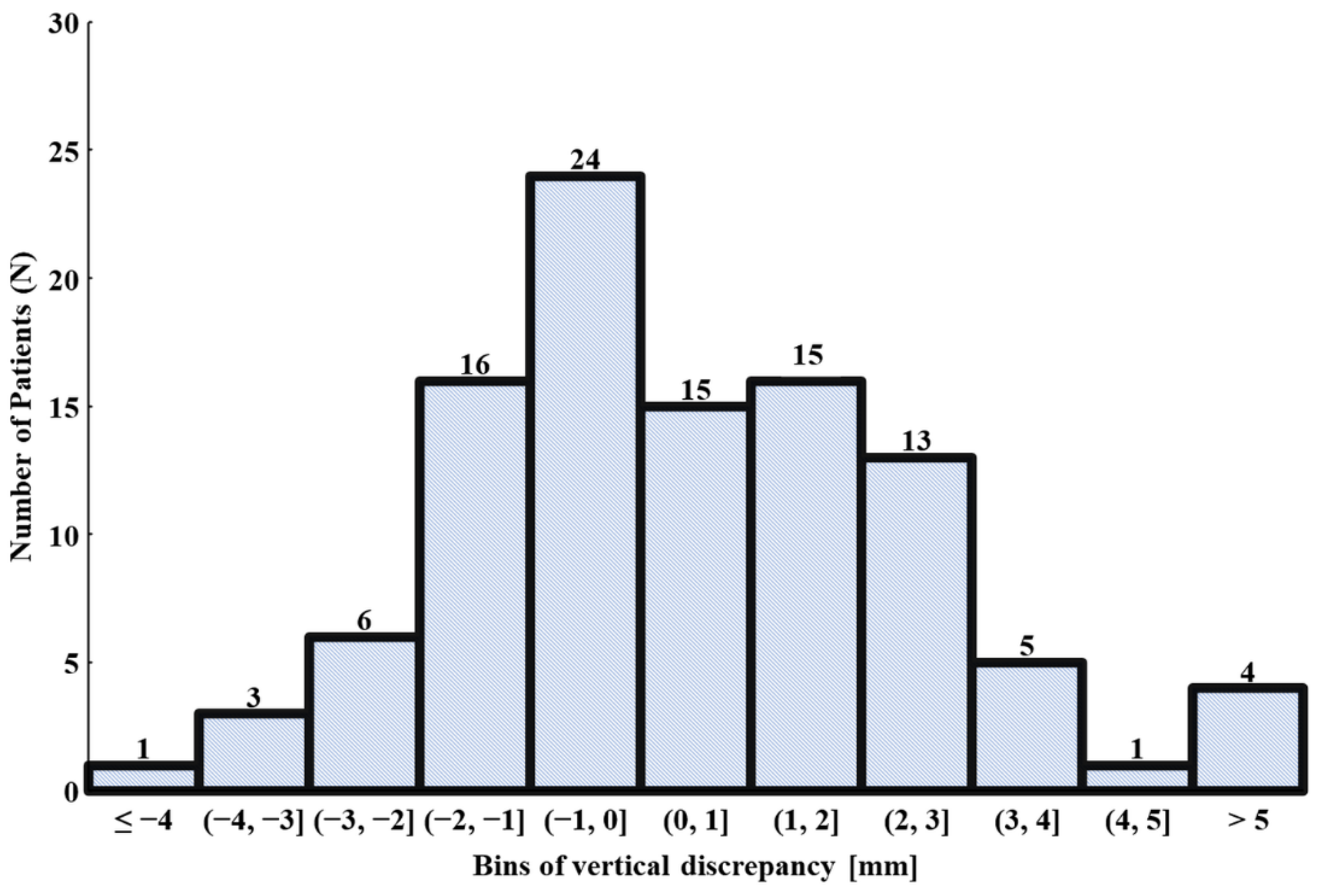
3.2. Vertical Osteotomy Discrepancy
3.3. Clinical Evaluation
4. Discussion
4.1. Novelty/Aim/Summarizing Results
4.2. Comparison with Existing Literature
4.3. Limitations
4.4. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsai, T.-Y.; Dimitriou, D.; Li, G.; Kwon, Y.-M. Does Total Hip Arthroplasty Restore Native Hip Anatomy? Three-Dimensional Reconstruction Analysis. Int. Orthop. 2014, 38, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.; Howell, S.M.; Hull, M.L. Errors in Femoral Anteversion, Femoral Offset, and Vertical Offset Following ROBOT-ASSISTED Total Hip Arthroplasty. Int. J. Med. Robot. Comput Assist. Surg. 2020, 16, 1577–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Classen, T.; Zaps, D.; Landgraeber, S.; Li, X.; Jäger, M. Assessment and Management of Chronic Pain in Patients with Stable Total Hip Arthroplasty. Int. Orthop. 2013, 7, e2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimitriou, D.; Tsai, T.-Y.; Kwon, Y.-M. The Effect of Femoral Neck Osteotomy on Femoral Component Position of a Primary Cementless Total Hip Arthroplasty. Int. Orthop. 2015, 39, 2315–2321. [Google Scholar] [CrossRef]
- Hirata, M.; Nakashima, Y.; Itokawa, T.; Ohishi, M.; Sato, T.; Akiyama, M.; Hara, D.; Iwamoto, Y. Influencing Factors for the Increased Stem Version Compared to the Native Femur in Cementless Total Hip Arthroplasty. Int. Orthop. 2014, 38, 1341–1346. [Google Scholar] [CrossRef] [Green Version]
- Sendtner, E.; Tibor, S.; Winkler, R.; Wörner, M.; Grifka, J.; Renkawitz, T. Stem Torsion in Total Hip Replacement: CT Measurements in 60 Patients. Acta Orthop. 2010, 81, 579–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujishiro, T.; Hayashi, S.; Kanzaki, N.; Hashimoto, S.; Kurosaka, M.; Kanno, T.; Masuda, T. Computed Tomographic Measurement of Acetabular and Femoral Component Version in Total Hip Arthroplasty. Int. Orthop. 2014, 38, 941–946. [Google Scholar] [CrossRef] [Green Version]
- Sarangi, P.P.; Bannister, G.C. Leg Length Discrepancy after Total Hip Replacement. HIP Int. 1997, 7, 121–124. [Google Scholar] [CrossRef]
- Lecoanet, P.; Vargas, M.; Pallaro, J.; Thelen, T.; Ribes, C.; Fabre, T. Leg Length Discrepancy after Total Hip Arthroplasty: Can Leg Length Be Satisfactorily Controlled via Anterior Approach without a Traction Table? Evaluation in 56 Patients with EOS 3D. Orthop. Traumatol. Surg. Res. 2018, 104, 1143–1148. [Google Scholar] [CrossRef]
- Konyves, A.; Bannister, G.C. The Importance of Leg Length Discrepancy after Total Hip Arthroplasty. J. Bone Jt. Surg. Br. Vol. 2005, 87, 3. [Google Scholar] [CrossRef]
- Rösler, J.; Perka, C. The Effect of Anatomical Positional Relationships on Kinetic Parameters after Total Hip Replacement. Int. Orthop. 2000, 24, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Kiernan, S.; Hermann, K.L.; Wagner, P.; Ryd, L.; Flivik, G. The Importance of Adequate Stem Anteversion for Rotational Stability in Cemented Total Hip Replacement: A Radiostereometric Study with Ten-Year Follow-Up. Bone Jt. J. 2013, 95-B, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Fujishiro, T.; Hiranaka, T.; Hashimoto, S.; Hayashi, S.; Kurosaka, M.; Kanno, T.; Masuda, T. The Effect of Acetabular and Femoral Component Version on Dislocation in Primary Total Hip Arthroplasty. Int. Orthop. 2016, 40, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, K.A.; Noticewala, M.S.; Macaulay, W.; Lee, J.H.; Geller, J.A. Effect of Femoral Offset on Pain and Function After Total Hip Arthroplasty. J. Arthroplast. 2012, 27, 1863–1869. [Google Scholar] [CrossRef] [PubMed]
- Röder, C.; Vogel, R.; Burri, L.; Dietrich, D.; Staub, L.P. Total Hip Arthroplasty: Leg Length Inequality Impairs Functional Outcomes and Patient Satisfaction. BMC Musculoskelet. Disord. 2012, 13, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renkawitz, T.; Weber, T.; Dullien, S.; Woerner, M.; Dendorfer, S.; Grifka, J.; Weber, M. Leg Length and Offset Differences above 5 Mm after Total Hip Arthroplasty Are Associated with Altered Gait Kinematics. Gait Posture 2016, 49, 196–201. [Google Scholar] [CrossRef]
- Floerkemeier, T.; Gronewold, J.; Berner, S.; Olender, G.; Hurschler, C.; Windhagen, H.; von Lewinski, G. The Influence of Resection Height on Proximal Femoral Strain Patterns after Metha Short Stem Hip Arthroplasty: An Experimental Study on Composite Femora. Int. Orthop. 2013, 37, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Zheng, Z.; Chen, W.; Wang, J.; Zhang, Y. Femoral Neck Osteotomy Guide for Total Hip Arthroplasty. BMC Surg. 2015, 15, 29. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Cai, X.; Wang, W.; Wu, H.; Wu, L.; Ye, Z.; Yan, S. MAKO Robotic Assisted Total Hip Replacement (THR) for Patients with Fused Hips. Robot. Comput. Surg. 2022, 18, e2369. [Google Scholar] [CrossRef]
- Henckel, J.; Holme, T.J.; Radford, W.; Skinner, J.A.; Hart, A.J. 3D-Printed Patient-Specific Guides for Hip Arthroplasty. J. Am. Acad. Orthop. Surg. 2018, 26, e342–e348. [Google Scholar] [CrossRef]
- Schneider, A.K.; Pierrepont, J.W.; Hawdon, G.; McMahon, S. Clinical Accuracy of a Patient-Specific Femoral Osteotomy Guide in Minimally-Invasive Posterior Hip Arthroplasty. HIP Int. 2018, 28, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Spencer, B.A.; Mont, M.A.; McGrath, M.S.; Boyd, B.; Mitrick, M.F. Initial Experience with Custom-Fit Total Knee Replacement: Intra-Operative Events and Long-Leg Coronal Alignment. Int. Orthop. 2009, 33, 1571–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, V.Y.; DeClaire, J.H.; Berend, K.R.; Gulick, B.C.; Lombardi, A.V. Improved Accuracy of Alignment With Patient-Specific Positioning Guides Compared With Manual Instrumentation in TKA. Clin. Orthop. Relat. Res. 2012, 470, 99–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunley, R.M.; Ellison, B.S.; Zhu, J.; Ruh, E.L.; Howell, S.M.; Barrack, R.L. Do Patient-Specific Guides Improve Coronal Alignment in Total Knee Arthroplasty? Clin. Orthop. Relat. Res. 2012, 470, 895–902. [Google Scholar] [CrossRef] [Green Version]
- Conteduca, F.; Iorio, R.; Mazza, D.; Caperna, L.; Bolle, G.; Argento, G.; Ferretti, A. Are MRI-Based, Patient Matched Cutting Jigs as Accurate as the Tibial Guides? Int. Orthop. 2012, 36, 1589–1593. [Google Scholar] [CrossRef] [Green Version]
- Daniilidis, K.; Tibesku, C.O. Frontal Plane Alignment after Total Knee Arthroplasty Using Patient-Specific Instruments. Int. Orthop. 2013, 37, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Chareancholvanich, K.; Narkbunnam, R.; Pornrattanamaneewong, C. A Prospective Randomised Controlled Study of Patient-Specific Cutting Guides Compared with Conventional Instrumentation in Total Knee Replacement. Bone Jt. J. 2013, 95-B, 354–359. [Google Scholar] [CrossRef]
- Noble, J.W.; Moore, C.A.; Liu, N. The Value of Patient-Matched Instrumentation in Total Knee Arthroplasty. J. Arthroplast. 2012, 27, 153–155. [Google Scholar] [CrossRef]
- Bali, K.; Walker, P.; Bruce, W. Custom-Fit Total Knee Arthroplasty: Our Initial Experience in 32 Knees. J. Arthroplast. 2012, 27, 1149–1154. [Google Scholar] [CrossRef]
- Lustig, S.; Scholes, C.J.; Oussedik, S.I.; Kinzel, V.; Coolican, M.R.J.; Parker, D.A. Unsatisfactory Accuracy as Determined by Computer Navigation of VISIONAIRE Patient-Specific Instrumentation for Total Knee Arthroplasty. J. Arthroplast. 2013, 28, 469–473. [Google Scholar] [CrossRef]
- Chotanaphuti, T.; Wangwittayakul, V.; Khuangsirikul, S.; Foojareonyos, T. The Accuracy of Component Alignment in Custom Cutting Blocks Compared with Conventional Total Knee Arthroplasty Instrumentation: Prospective Control Trial. Knee 2014, 21, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Yeo, S.J.; Yew, A.K.S.; Tay, D.K.J.; Chia, S.-L.; Lo, N.N.; Chin, P.L. The Radiological Outcomes of Patient-Specific Instrumentation versus Conventional Total Knee Arthroplasty. Knee Surg. Sport. Traumatol. Arthrosc. 2014, 22, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Pourgiezis, N.; Reddy, S.P.; Nankivell, M.; Morrison, G.; VanEssen, J. Alignment and Component Position after Patient-Matched Instrumentation versus Conventional Total Knee Arthroplasty. J. Orthop. Surg. 2016, 24, 170–174. [Google Scholar] [CrossRef] [Green Version]
- Levengood, G.; Dupee, J. Accuracy of Coronal Plane Mechanical Alignment in a Customized, Individually Made Total Knee Replacement with Patient-Specific Instrumentation. J. Knee Surg. 2018, 31, 792–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauzenberger, L.; Munz, M.; Brandl, G.; Frank, J.K.; Heuberer, P.R.; Laky, B.; Schwameis, E.; Anderl, W. Patient-Specific Instrumentation Improved Three-Dimensional Accuracy in Total Knee Arthroplasty: A Comparative Radiographic Analysis of 1257 Total Knee Arthroplasties. J. Orthop. Surg. Res 2019, 14, 437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turgeon, T.R.; Cameron, B.; Burnell, C.D.; Hedden, D.R.; Bohm, E.R. A Double-Blind Randomized Controlled Trial of Total Knee Replacement Using Patient-Specific Cutting Block Instrumentation versus Standard Instrumentation. CJS 2019, 62, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Sariali, E.; Mauprivez, R.; Khiami, F.; Pascal-Mousselard, H.; Catonné, Y. Accuracy of the Preoperative Planning for Cementless Total Hip Arthroplasty. A Randomised Comparison between Three-Dimensional Computerised Planning and Conventional Templating. Orthop. Traumatol. Surg. Res. 2012, 98, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Hassani, H.; Cherix, S.; Ek, E.T.; Rüdiger, H.A. Comparisons of Preoperative Three-Dimensional Planning and Surgical Reconstruction in Primary Cementless Total Hip Arthroplasty. J. Arthroplast. 2014, 29, 1273–1277. [Google Scholar] [CrossRef] [Green Version]
- Di Laura, A.; Henckel, J.; Hothi, H.; Hart, A. Can 3D Surgical Planning and Patient Specific Instrumentation Reduce Hip Implant Inventory? A Prospective Study. 3D Print. Med. 2020, 6, 25. [Google Scholar] [CrossRef]
- Belzunce, M.A.; Henckel, J.; Di Laura, A.; Hart, A. Uncemented Femoral Stem Orientation and Position in Total Hip Arthroplasty: A CT Study. J. Orthop. Res. 2020, 38, 1486–1496. [Google Scholar] [CrossRef]
- Meyer, E.; Raupach, R.; Lell, M.; Schmidt, B.; Kachelrieß, M. Normalized Metal Artifact Reduction (NMAR) in Computed Tomography. Med. Phys. 2010, 37, 13. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.P.M.; Tavares, J.M.R.S. Medical Image Registration: A Review. Comput. Methods Biomech. Biomed. Eng. 2014, 17, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Eggli, S.; Pisan, M.; Muller, M.E. The Value of Preoperative Planning for Total Hip Arthroplasty. J. Bone Jt. Surg. Br. Vol. 1998, 80, 9. [Google Scholar] [CrossRef]
- Sakai, T.; Hamada, H.; Takao, M.; Murase, T.; Yoshikawa, H.; Sugano, N. Validation of Patient-specific Surgical Guides for Femoral Neck Cutting in Total Hip Arthroplasty through the Anterolateral Approach. Int. J. Med. Robot. 2017, 13, e1830. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.F.; Keat, N. Artifacts in CT: Recognition and Avoidance. RadioGraphics 2004, 24, 1679–1691. [Google Scholar] [CrossRef]
| Study Group (n = 103 Hips) | |
|---|---|
| Gender (Females) (%) | 53 (51) |
| Age (Years) (Median, Range) | 62 (39–89) |
| Treatment Side (Right) (%) | 56 (54) |
| Follow-Up (Median, Range) | 37 (8–66) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moralidou, M.; Di Laura, A.; Henckel, J.; Hothi, H.; Hart, A.J. Accuracy of a Three-Dimensional (3D)-Printed Patient-Specific (PS) Femoral Osteotomy Guide: A Computed Tomography (CT) Study. Bioengineering 2022, 9, 667. https://doi.org/10.3390/bioengineering9110667
Moralidou M, Di Laura A, Henckel J, Hothi H, Hart AJ. Accuracy of a Three-Dimensional (3D)-Printed Patient-Specific (PS) Femoral Osteotomy Guide: A Computed Tomography (CT) Study. Bioengineering. 2022; 9(11):667. https://doi.org/10.3390/bioengineering9110667
Chicago/Turabian StyleMoralidou, Maria, Anna Di Laura, Johann Henckel, Harry Hothi, and Alister James Hart. 2022. "Accuracy of a Three-Dimensional (3D)-Printed Patient-Specific (PS) Femoral Osteotomy Guide: A Computed Tomography (CT) Study" Bioengineering 9, no. 11: 667. https://doi.org/10.3390/bioengineering9110667
APA StyleMoralidou, M., Di Laura, A., Henckel, J., Hothi, H., & Hart, A. J. (2022). Accuracy of a Three-Dimensional (3D)-Printed Patient-Specific (PS) Femoral Osteotomy Guide: A Computed Tomography (CT) Study. Bioengineering, 9(11), 667. https://doi.org/10.3390/bioengineering9110667








