The Role of the Innate Immune System in Wear Debris-Induced Inflammatory Peri-Implant Osteolysis in Total Joint Arthroplasty
Abstract
:1. Introduction
2. Innate Immune Response to Wear Debris Particles
2.1. Macrophages
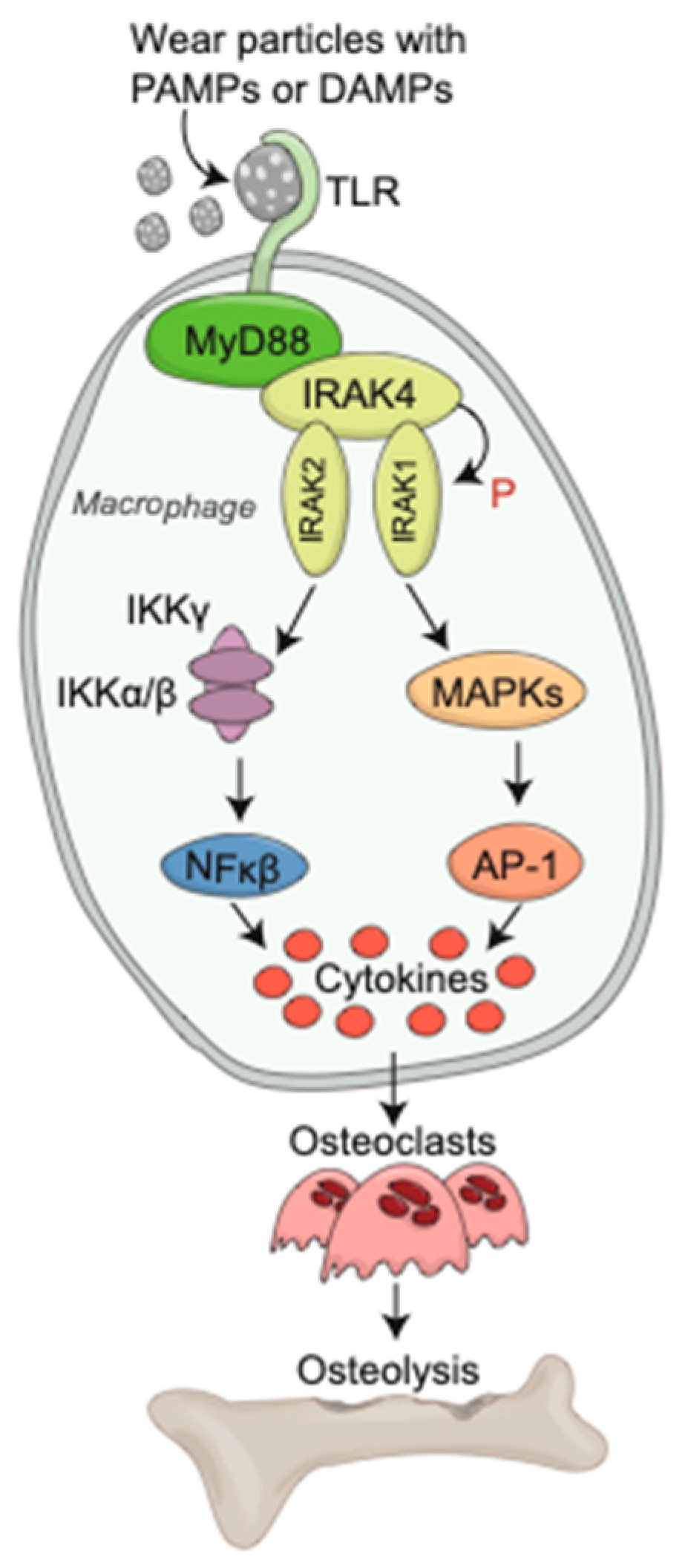
2.2. Toll-like Receptors
2.3. Chemokines and Chemokine Receptors
3. Bone and Soft Tissue Relationship
3.1. Osteoclasts
3.2. Osteoblasts
3.3. Fibroblasts
4. Therapeutic Targets of Aseptic Loosening through Modulation of the Innate Immune Response
5. Biomarkers
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, J.A. Epidemiology of Knee and Hip Arthroplasty: A Systematic Review. Open Orthop. J. 2011, 5, 80–85. [Google Scholar] [CrossRef] [Green Version]
- Man, K.; Jiang, L.-H.; Foster, R.; Yang, X.B. Immunological Responses to Total Hip Arthroplasty. J. Funct. Biomater. 2017, 8, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; van der Heide, E. In Silico Contact Pressure of Metal-on-Metal Total Hip Implant with Different Materials Subjected to Gait Loading. Metals 2022, 12, 1241. [Google Scholar] [CrossRef]
- Bozic, K.J.; Kurtz, S.M.; Lau, E.; Ong, K.; Chiu, V.; Vail, T.P.; Rubash, H.E.; Berry, D.J. The epidemiology of revision total knee arthroplasty in the United States. Clin. Orthop. Relat. Res. 2010, 468, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharkey, P.F.; Lichstein, P.M.; Shen, C.; Tokarski, A.T.; Parvizi, J. Why are total knee arthroplasties failing today—Has anything changed after 10 years? J. Arthroplast. 2014, 29, 1774–1778. [Google Scholar] [CrossRef]
- Ackerman, I.N.; Busija, L.; Lorimer, M.; de Steiger, R.; Graves, S.E. Lifetime Risk of Revision Hip Replacement Surgery in Australia Remains Low: A Population-Level Analysis Using National Registry Data. J. Bone Jt. Surg. Am. 2021, 103, 389–396. [Google Scholar] [CrossRef]
- Jorgensen, N.B.; McAuliffe, M.; Orschulok, T.; Lorimer, M.F.; De Steiger, R. Major Aseptic Revision Following Total Knee Replacement: A Study of 478,081 Total Knee Replacements from the Australian Orthopaedic Association National Joint Replacement Registry. J. Bone Jt. Surg. Am. 2019, 101, 302–310. [Google Scholar] [CrossRef]
- Fritz, J.; Lurie, B.; Miller, T.T. Imaging of hip arthroplasty. In Seminars in Musculoskeletal Radiology; Thieme Medical Publishers: New York, NY, USA, 2013; Volume 17, pp. 316–327. [Google Scholar]
- Robertsson, O.; Knutson, K.; Lewold, S.; Lidgren, L. The Swedish Knee Arthroplasty Register 1975–1997: An update with special emphasis on 41,223 knees operated on in 1988–1997. Acta Orthop. 2001, 72, 503–513. [Google Scholar] [CrossRef]
- Boehler, M.; Plenk, H.; Salzer, M. Alumina ceramic bearings for hip endoprostheses: The Austrian experiences. Clin. Orthop. Relat. Res. 2000, 379, 85–93. [Google Scholar] [CrossRef]
- Santavirta, S.; Takagi, M.; Gomez-Barrena, E.; Nevalainen, J.; Lassus, J.; Salo, J.; Konttinen, Y.T. Studies of host response to orthopedic implants and biomaterials. J. Long-Term Eff. Med. Implant. 1999, 9, 67–76. [Google Scholar]
- Willert, H.G.; Bertram, H.; Buchhorn, G.H. Osteolysis in alloarthroplasty of the hip. The role of ultra-high molecular weight polyethylene wear particles. Clin. Orthop. Relat. Res. 1990, 258, 95–107. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Prakoso, A.T.; Basri, H.; van der Heide, E. Computational Contact Pressure Prediction of CoCrMo, SS 316L and Ti6Al4V Femoral Head against UHMWPE Acetabular Cup under Gait Cycle. J. Funct. Biomater. 2022, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Toh, S.M.S.; Ashkanfar, A.; English, R.; Rothwell, G. The relation between body weight and wear in Total Hip Prosthesis: A finite element study. Comput. Methods Programs Biomed. Updat. 2022, 2, 100060. [Google Scholar] [CrossRef]
- Landgraeber, S.; Jäger, M.; Jacobs, J.J.; Hallab, N.J. The pathology of orthopedic implant failure is mediated by innate immune system cytokines. Mediat. Inflamm. 2014, 2014, 185150. [Google Scholar] [CrossRef]
- Schwarz, E.M.; Lu, A.P.; Goater, J.J.; Benz, E.B.; Kollias, G.; Rosier, R.N.; Puzas, J.E.; O’Keefe, R.J. Tumor necrosis factor-α/nuclear transcription factor-?B signaling in periprosthetic osteolysis. J. Orthop. Res. 2000, 18, 472–480. [Google Scholar] [CrossRef]
- Sandell, L.J.; Silva, M.J. Specialty update: What’s new in orthopaedic research. J. Bone Jt. Surg. 2001, 83, 1117–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.-Y.; Qiu, G.-X.; Weng, X.-S.; Yu, B.; Wang, Y. What is the optimum fusion technique for adult spondylolisthesis—PLIF or PLF or PLIF plus PLF? A meta-analysis from 17 comparative studies. Spine 2014, 39, 1887–1898. [Google Scholar] [CrossRef]
- Jiang, J.; Jia, T.; Gong, W.; Ning, B.; Wooley, P.H.; Yang, S.-Y. Macrophage Polarization in IL-10 Treatment of Particle-Induced Inflammation and Osteolysis. Am. J. Pathol. 2015, 186, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.J.; Gibon, E.; Ma, T.; Yao, Z.; Smith, R.L.; Goodman, S.B. Revision joint replacement, wear particles, and macrophage polarization. Acta Biomater. 2012, 8, 2815–2823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadoya, Y.; Kobayashi, A.; Ohashi, H. Wear and osteolysis in total joint replacements. Acta Orthop. 1998, 69 (Suppl. S278), i-16. [Google Scholar] [CrossRef] [Green Version]
- Shanbhag, A.S.; Jacobs, J.J.; Black, J.; Galante, J.O.; Glant, T.T. Macrophage/particle interactions: Effect of size, composition and surface area. J. Biomed. Mater. Res. 1994, 28, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Revell, P.A. Joint Replacement Technology; Woodhead Publishing: Southen, UK, 2008. [Google Scholar] [CrossRef]
- Gu, Q.; Shi, Q.; Yang, H. The Role of TLR and Chemokine in Wear Particle-Induced Aseptic Loosening. J. Biomed. Biotechnol. 2012, 2012, 596870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takagi, M. Toll-like Receptor. J. Clin. Exp. Hematop. 2011, 51, 77–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearl, J.I.; Ma, T.; Irani, A.R.; Huang, Z.; Robinson, W.H.; Smith, R.L.; Goodman, S.B. Role of the Toll-like receptor pathway in the recognition of orthopedic implant wear-debris particles. Biomaterials 2011, 32, 5535–5542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenfield, E.M.; Beidelschies, M.A.; Tatro, J.M.; Goldberg, V.M.; Hise, A. Bacterial Pathogen-associated Molecular Patterns Stimulate Biological Activity of Orthopaedic Wear Particles by Activating Cognate Toll-like Receptors. J. Biol. Chem. 2010, 285, 32378–32384. [Google Scholar] [CrossRef] [Green Version]
- Ohashi, K.; Burkart, V.; Flohé, S.; Kolb, H. Cutting Edge: Heat Shock Protein 60 Is a Putative Endogenous Ligand of the Toll-Like Receptor-4 Complex. J. Immunol. 2000, 164, 558–561. [Google Scholar] [CrossRef] [Green Version]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Takagi, M.; Tamaki, Y.; Hasegawa, H.; Takakubo, Y.; Konttinen, L.; Tiainen, V.-M.; Lappalainen, R.; Konttinen, Y.T.; Salo, J. Toll-like receptors in the interface membrane around loosening total hip replacement implants. J. Biomed. Mater. Res. Part A 2007, 81A, 1017–1026. [Google Scholar] [CrossRef]
- Bi, Y.; Seabold, J.M.; Kaar, S.G.; Ragab, A.A.; Goldberg, V.M.; Anderson, J.M.; Greenfield, E.M. Adherent Endotoxin on Orthopedic Wear Particles Stimulates Cytokine Production and Osteoclast Differentiation. J. Bone Miner. Res. 2001, 16, 2082–2091. [Google Scholar] [CrossRef]
- Hao, H.-N.; Zheng, B.; Nasser, S.; Ren, W.; Latteier, M.; Wooley, P.; Morawa, L. The roles of monocytic heat shock protein 60 and Toll-like receptors in the regional inflammation response to wear debris particles. J. Biomed. Mater. Res. Part A 2009, 92A, 1373–1381. [Google Scholar] [CrossRef]
- Mao, X.; Pan, X.; Peng, X.; Cheng, T.; Zhang, X. Inhibition of Titanium Particle-Induced Inflammation by the Proteasome Inhibitor Bortezomib in Murine Macrophage-Like RAW 264.7 Cells. Inflammation 2012, 35, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Maitra, R.; Clement, C.C.; Scharf, B.; Crisi, G.M.; Chitta, S.; Paget, D.; Purdue, P.E.; Cobelli, N.; Santambrogio, L. Endosomal damage and TLR2 mediated inflammasome activation by alkane particles in the generation of aseptic osteolysis. Mol. Immunol. 2009, 47, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Rollins, B.J. Chemokines. Blood 1997, 90, 909–928. [Google Scholar] [CrossRef]
- Fernandez, E.J.; Lolis, E. Structure, function, and inhibition of chemokines. Annu. Rev. Pharm. Toxicol. 2002, 42, 469–499. [Google Scholar] [CrossRef]
- Zlotnik, A.; Yoshie, O. Chemokines: A New Classification System and Their Role in Immunity. Immunity 2000, 12, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, S.J.; Crown, S.E.; Handel, T.M. Chemokine: Receptor Structure, Interactions, and Antagonism. Annu. Rev. Immunol. 2007, 25, 787–820. [Google Scholar] [CrossRef] [PubMed]
- Lind, M.; Trindade, M.; Schurman, D.; Goodman, S.; Smith, R. Monocyte migration inhibitory factor synthesis and gene expression in particle-activated macrophages. Cytokine 2000, 12, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, Y.; Sun, D.H.; Trindade, M.C.D.; Chun, L.E.; Song, Y.; Goodman, S.B.; Schurman, D.J.; Maloney, W.J.; Smith, R.L. Induction of macrophage C-C chemokine expression by titanium alloy and bone cement particles. J. Bone Jt. Surg. 1999, 81, 155–162. [Google Scholar] [CrossRef]
- Gibon, E.; Ma, T.; Ren, P.-G.; Fritton, K.; Biswal, S.; Yao, Z.; Smith, L.; Goodman, S.B. Selective inhibition of the MCP-1-CCR2 ligand-receptor axis decreases systemic trafficking of macrophages in the presence of UHMWPE particles. J. Orthop. Res. 2011, 30, 547–553. [Google Scholar] [CrossRef] [Green Version]
- Ren, P.-G.; Huang, Z.; Ma, T.; Biswal, S.; Smith, R.L.; Goodman, S.B. Surveillance of systemic trafficking of macrophages induced by UHMWPE particles in nude mice by noninvasive imaging. J. Biomed. Mater. Res. Part A 2010, 9999A, 706–711. [Google Scholar] [CrossRef]
- Ren, P.-G.; Irani, A.; Huang, Z.; Ma, T.; Biswal, S.; Goodman, S.B. Continuous Infusion of UHMWPE Particles Induces Increased Bone Macrophages and Osteolysis. Clin. Orthop. Relat. Res. 2010, 469, 113–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Amer, Y.; Darwech, I.; Clohisy, J.C. Aseptic loosening of total joint replacements: Mechanisms underlying osteolysis and potential therapies. Arthritis Res. Ther. 2007, 9, S6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufman, A.M.; Alabre, C.I.; Rubash, H.E.; Shanbhag, A.S. Human macrophage response to UHMWPE, TiAlV, CoCr, and alumina particles: Analysis of multiple cytokines using protein arrays. J. Biomed. Mater. Res. Part A 2007, 84A, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Haleem-Smith, H.; Argintar, E.; Bush, C.; Hampton, D.; Postma, W.F.; Chen, F.H.; Rimington, T.; Lamb, J.; Tuan, R.S. Biological responses of human mesenchymal stem cells to titanium wear debris particles. J. Orthop. Res. 2011, 30, 853–863. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Ma, T.; Ren, P.-G.; Smith, R.L.; Goodman, S.B. Effects of orthopedic polymer particles on chemotaxis of macrophages and mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2010, 94A, 1264–1269. [Google Scholar] [CrossRef] [Green Version]
- Cadosch, D.; Gautschi, O.P.; Chan, E.; Simmen, H.P.; Filgueira, L. Titanium induced production of chemokines CCL17/TARC and CCL22/MDC in human osteoclasts and osteoblasts. J. Biomed. Mater. Res.—Part A 2010, 92, 475–483. [Google Scholar] [CrossRef]
- Leibbrandt, A.; Penninger, J.M. RANK(L) as a Key Target for Controlling Bone Loss. In Therapeutic Targets of the TNF Superfamily; Springer: New York, NY, USA, 2009; Volume 647, pp. 130–145. [Google Scholar] [CrossRef]
- Kadoya, Y.; Al-Saffar, N.; Kobayashi, A.; Revell, P. The expression of osteoclast markers on foreign body giant cells. Bone Miner. 1994, 27, 85–96. [Google Scholar] [CrossRef]
- Athanasou, N.; Quinn, J.; Bulstrode, C. Resorption of bone by inflammatory cells derived from the joint capsule of hip arthroplasties. J. Bone Jt. Surg. 1992, 74, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Ferguson, D.J.P.; Quinn, J.M.W.; Simpson, A.H.R.W.; Athanasou, N.A. Osteoclasts are capable of particle phagocytosis and bone resorption. J. Pathol. 1997, 182, 92–98. [Google Scholar] [CrossRef]
- Wang, W.; Ferguson, D.J.; Quinn, J.M.; Simpson, A.H.R.; Athanasou, N.A. Biomaterial Particle Phagocytosis by Bone-Resorbing Osteoclasts. J. Bone Jt. Surg. 1997, 79, 849–856. [Google Scholar] [CrossRef]
- Vermes, C.; Glant, T.T.; Hallab, N.J.; Fritz, E.A.; Roebuck, K.A.; Jacobs, J.J. The potential role of the osteoblast in the development of periprosthetic osteolysis: Review of in vitro osteoblast responses to wear debris, corrosion products, and cytokines and growth factors. J. Arthroplast. 2001, 16, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Zreiqat, H.; Crotti, T.; Howlett, C.; Capone, M.; Markovic, B.; Haynes, D. Prosthetic particles modify the expression of bone-related proteins by human osteoblastic cells in vitro. Biomaterials 2002, 24, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Cs-Szabo, G.; Jacobs, J.J.; Kuettner, K.E.; Glant, T.T. Suppression of osteoblast function by titanium particles. J. Bone Jt. Surg.—Ser. A 1997, 79, 107–112. [Google Scholar] [CrossRef]
- Dean, D.D.; Lohmann, C.; Sylvia, V.L.; Köster, G.; Liu, Y.; Schwartz, Z.; Boyan, B.D. Effect of polymer molecular weight and addition of calcium stearate on response of MG63 osteoblast-like cells to UHMWPE particles. J. Orthop. Res. 2001, 19, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Pioletti, D.P.; Takei, H.; Kwon, S.Y.; Wood, D.; Sung, K.L.P. The cytotoxic effect of titanium particles phagocytosed by osteoblasts. J. Biomed. Mater. Res. 1999, 46, 399–407. [Google Scholar] [CrossRef]
- Pioletti, D.P.; Takei, H.; Lin, T.; Van Landuyt, P.; Ma, Q.J.; Kwon, S.Y.; Sung, K.-L.P. The effects of calcium phosphate cement particles on osteoblast functions. Biomaterials 2000, 21, 1103–1114. [Google Scholar] [CrossRef] [Green Version]
- Pioletti, D.P.; Kottelat, A. The influence of wear particles in the expression of osteoclastogenesis factors by osteoblasts. Biomaterials 2004, 25, 5803–5808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Saffar, N.; Revell, P.A. Pathology of the bone-implant interfaces. J. Long-Term Eff. Med. Implant. 1999, 9, 319–347. [Google Scholar]
- Koreny, T.; Tunyogi-Csapó, M.; Gál, I.; Vermes, C.; Jacobs, J.J.; Glant, T.T. The role of fibroblasts and fibroblast-derived factors in periprosthetic osteolysis. Arthritis Care Res. 2006, 54, 3221–3232. [Google Scholar] [CrossRef]
- McDonald, C.L.; Lemme, N.J.; Testa, E.J.; Aaron, R.; Hartnett, D.A.; Cohen, E.M. Bisphosphonates in Total Joint Arthroplasty: A Review of Their Use and Complications. Arthroplast. Today 2022, 14, 133–139. [Google Scholar] [CrossRef]
- Hall, A. Rho GTPases and the Actin Cytoskeleton. Science 1998, 279, 509–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavanagh, K.L.; Guo, K.; Dunford, J.E.; Wu, X.; Knapp, S.; Ebetino, F.H.; Rogers, M.J.; Russell, R.G.G.; Oppermann, U. The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs. Proc. Natl. Acad. Sci. USA 2006, 103, 7829–7834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunford, J.E.; Thompson, K.; Coxon, F.P.; Luckman, S.P.; Hahn, F.M.; Poulter, C.D.; Ebetino, F.H.; Rogers, M.J. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J. Pharmacol. Exp. Ther. 2001, 296, 235–242. [Google Scholar] [PubMed]
- Bhandari, M.; Bajammal, S.S.; Guyatt, G.H.; Griffith, L.; Busse, J.; Schunemann, H.J.; Einhorn, T. Effect of Bisphosphonates on Periprosthetic Bone Mineral Density After Total Joint Arthroplasty. J. Bone Jt. Surg. 2005, 87, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-J.; Wang, J.-W.; Weng, L.-H.; Hsu, C.-C.; Huang, C.-C.; Chen, H.-S. The Effect of Alendronate on Bone Mineral Density in the Distal Part of the Femur And Proximal Part of the Tibia After Total Knee Arthroplasty. J. Bone Jt. Surg. 2003, 85, 2121–2126. [Google Scholar] [CrossRef]
- Namba, R.S.; Inacio, M.C.; Cheetham, T.C.; Dell, R.M.; Paxton, E.W.; Khatod, M.X. Lower Total Knee Arthroplasty Revision Risk Associated With Bisphosphonate Use, Even in Patients With Normal Bone Density. J. Arthroplast. 2016, 31, 537–541. [Google Scholar] [CrossRef]
- Prieto-Alhambra, D.; Javaid, M.K.; Judge, A.; Murray, D.; Carr, A.; Cooper, C.; Arden, N.K. Association between bisphosphonate use and implant survival after primary total arthroplasty of the knee or hip: Population based retrospective cohort study. BMJ 2011, 343, d7222. [Google Scholar] [CrossRef] [Green Version]
- Ro, D.H.; Jin, H.; Park, J.-Y.; Lee, M.C.; Won, S.; Han, H.-S. The use of bisphosphonates after joint arthroplasty is associated with lower implant revision rate. Knee Surg. Sport. Traumatol. Arthrosc. 2018, 27, 2082. [Google Scholar] [CrossRef]
- von Knoch, F.; Eckhardt, C.; Alabre, C.I.; Schneider, E.; Rubash, H.E.; Shanbhag, A.S. Anabolic effects of bisphosphonates on peri-implant bone stock. Biomaterials 2007, 28, 3549–3559. [Google Scholar] [CrossRef]
- Rubash, H.E.; Dorr, L.D.; Jacobs, J.; Maloney, W.; Saag, K.; Malbecq, W.; Leung, A. Does alendronate inhibit the progression of periprosthetic osteolysis? Trans ORS 2004, 29, 1492. [Google Scholar]
- Spaan, I.; Raymakers, R.A.; Van De Stolpe, A.; Peperzak, V. Wnt signaling in multiple myeloma: A central player in disease with therapeutic potential. J. Hematol. Oncol. 2018, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Schwarz, E.M. Are Biologic Treatments a Potential Approach to Wear- and Corrosion-related Problems? Clin. Orthop. Relat. Res. 2014, 472, 3740–3746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Mahatma, M.M.; Jayasuriya, R.L.; Hughes, D.; Hoggard, N.; Buckley, S.C.; Gordon, A.; Hamer, A.J.; Tomouk, M.W.; Kerry, R.M.; Eastell, R.; et al. Effect of denosumab on osteolytic lesion activity after total hip arthroplasty: A single-centre, randomised, double-blind, placebo-controlled, proof of concept trial. Lancet Rheumatol. 2021, 3, e195–e203. [Google Scholar] [CrossRef]
- Sköldenberg, O.; Rysinska, A.; Eisler, T.; Salemyr, M.; Bodén, H.; Muren, O. Denosumab for treating periprosthetic osteolysis; study protocol for a randomized, double-blind, placebo-controlled trial. BMC Musculoskelet. Disord. 2016, 17, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, I.R.; Billington, E.O. Drug therapy for osteoporosis in older adults. Lancet 2022, 399, 1080–1092. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Kang, H.; Liu, W.; Liu, P.; Zhang, J.; Harris, S.E.; Wu, D. Sclerostin Binds to LRP5/6 and Antagonizes Canonical Wnt Signaling. J. Biol. Chem. 2005, 280, 19883–19887. [Google Scholar] [CrossRef] [Green Version]
- Krause, C.; Korchynskyi, O.; de Rooij, K.; Weidauer, S.E.; de Gorter, D.J.; van Bezooijen, R.L.; Hatsell, S.; Economides, A.; Mueller, T.D.; Löwik, C.W.; et al. Distinct Modes of Inhibition by Sclerostin on Bone Morphogenetic Protein and Wnt Signaling Pathways. J. Biol. Chem. 2010, 285, 41614–41626. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Virdi, A.S.; Sena, K.; Sumner, D.R. Sclerostin antibody prevents particle-induced implant loosening by stimulating bone formation and inhibiting bone resorption in a rat model. Arthr. Care Res. 2012, 64, 4012–4020. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Guo, Y.; Shi, S.; Mao, X.; Pan, X.; Cheng, T. MMP-9 inhibition suppresses wear debris-induced inflammatory osteolysis through downregulation of RANK/RANKL in a murine osteolysis model. Int. J. Mol. Med. 2012, 30, 1417–1423. [Google Scholar] [CrossRef] [Green Version]
- Jagga, B.; Edwards, M.; Pagin, M.; Wagstaff, K.M.; Aragão, D.; Roman, N.; Nanson, J.D.; Raidal, S.R.; Dominado, N.; Stewart, M.; et al. Structural basis for nuclear import selectivity of pioneer transcription factor SOX2. Nat. Commun. 2021, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Lu, J.; Zhu, X.; Mao, H.; Yang, H.; Geng, D.; Xu, Y. Effects of a Cannabinoid Receptor 2 Selective Antagonist on the Inflammatory Reaction to Titanium Particles In Vivo and In Vitro. J. Int. Med. Res. 2010, 38, 2023–2032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.-B.; Ding, Y.; Huang, D.-S.; Liang, A.-J.; Zeng, W.-K.; Zeng, Z.-P.; Qin, C.-Q.; Barden, B. Inhibition of the PI3K/AKT Pathway Reduces Tumor Necrosis Factor-Alpha Production in the Cellular Response to Wear Particles In Vitro. Artif. Organs 2013, 37, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Aspenberg, P.; Agholme, F.; Magnusson, P.; Fahlgren, A. Targeting RANKL for reduction of bone loss around unstable implants: OPG-Fc compared to alendronate in a model for mechanically induced loosening. Bone 2011, 48, 225–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, R.D.; Deng, Y.; Fang, R.; Frisch, N.B.; Jacobs, J.J.; Sumner, D.R. Discovery of biomarkers to identify peri-implant osteolysis before radiographic diagnosis. J. Orthop. Res. 2018, 36, 2754–2761. [Google Scholar] [CrossRef] [Green Version]
- Li, M.G.; Thorsen, K.; Nilsson, K.G. Increased bone turnover as reflected by biochemical markers in patients with potentially unstable fixation of the tibial component. Arch. Orthop. Trauma Surg. 2004, 124, 404–409. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Connors, J.P.; Stelzer, J.W.; Garvin, P.M.; Wellington, I.J.; Solovyova, O. The Role of the Innate Immune System in Wear Debris-Induced Inflammatory Peri-Implant Osteolysis in Total Joint Arthroplasty. Bioengineering 2022, 9, 764. https://doi.org/10.3390/bioengineering9120764
Connors JP, Stelzer JW, Garvin PM, Wellington IJ, Solovyova O. The Role of the Innate Immune System in Wear Debris-Induced Inflammatory Peri-Implant Osteolysis in Total Joint Arthroplasty. Bioengineering. 2022; 9(12):764. https://doi.org/10.3390/bioengineering9120764
Chicago/Turabian StyleConnors, John Patrick, John W. Stelzer, Patrick M. Garvin, Ian J. Wellington, and Olga Solovyova. 2022. "The Role of the Innate Immune System in Wear Debris-Induced Inflammatory Peri-Implant Osteolysis in Total Joint Arthroplasty" Bioengineering 9, no. 12: 764. https://doi.org/10.3390/bioengineering9120764
APA StyleConnors, J. P., Stelzer, J. W., Garvin, P. M., Wellington, I. J., & Solovyova, O. (2022). The Role of the Innate Immune System in Wear Debris-Induced Inflammatory Peri-Implant Osteolysis in Total Joint Arthroplasty. Bioengineering, 9(12), 764. https://doi.org/10.3390/bioengineering9120764





