Bioprocessing by Decellularized Scaffold Biomaterials in Cultured Meat: A Review
Abstract
1. Introduction
2. Review Methodology
3. Challenges of Cell-Cultured Meat Bioprocessing
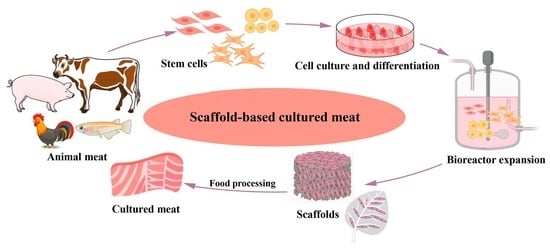
3.1. Current Research Progress on Scaffolds
3.2. Applied Types of Meat Scaffolds in Cell Culture
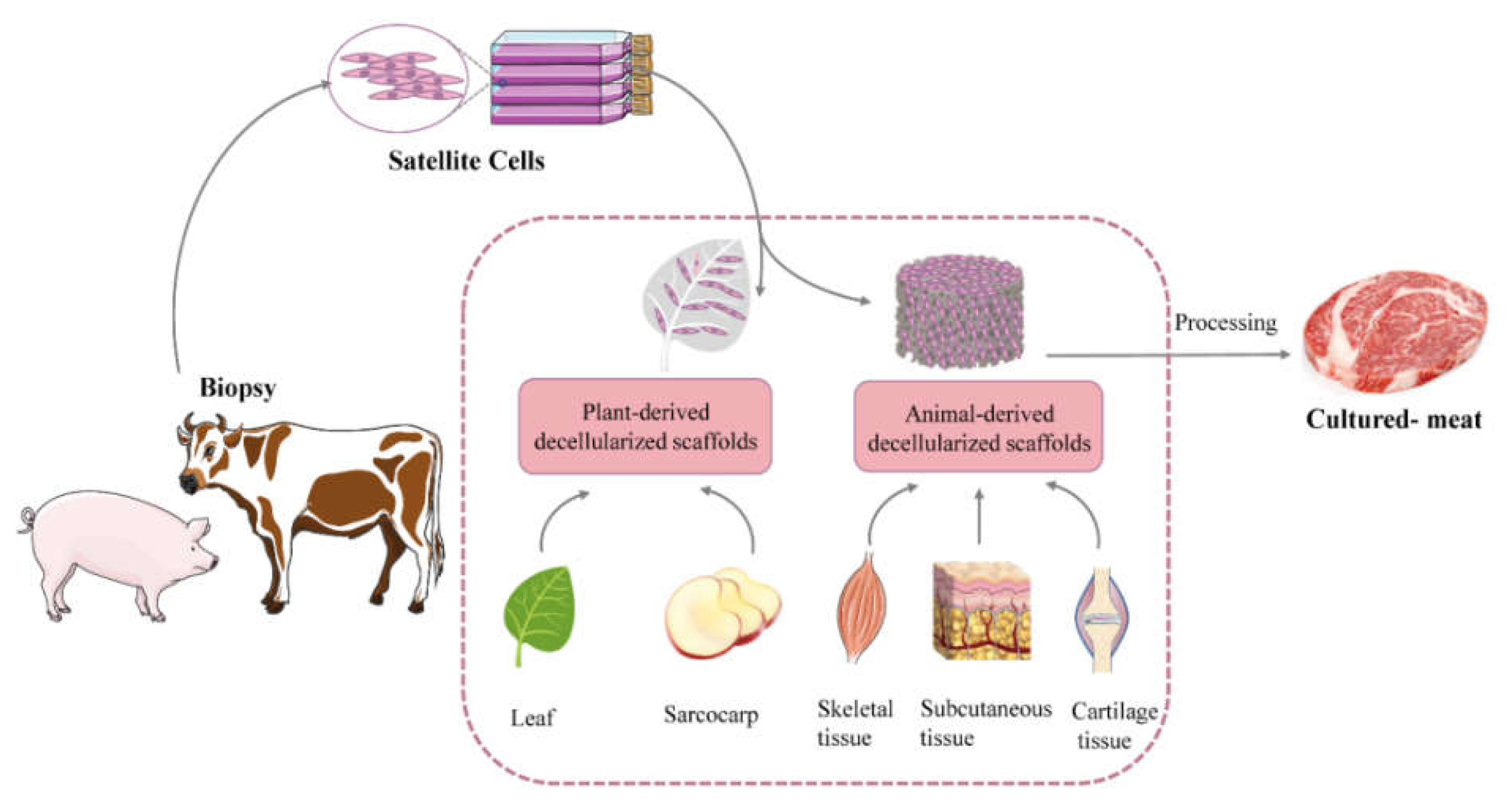
4. The Principles, Methods, and Application of Animal-Derived Decellularized Scaffolds
4.1. Application Principles of Animal-Derived Decellularized Scaffolds
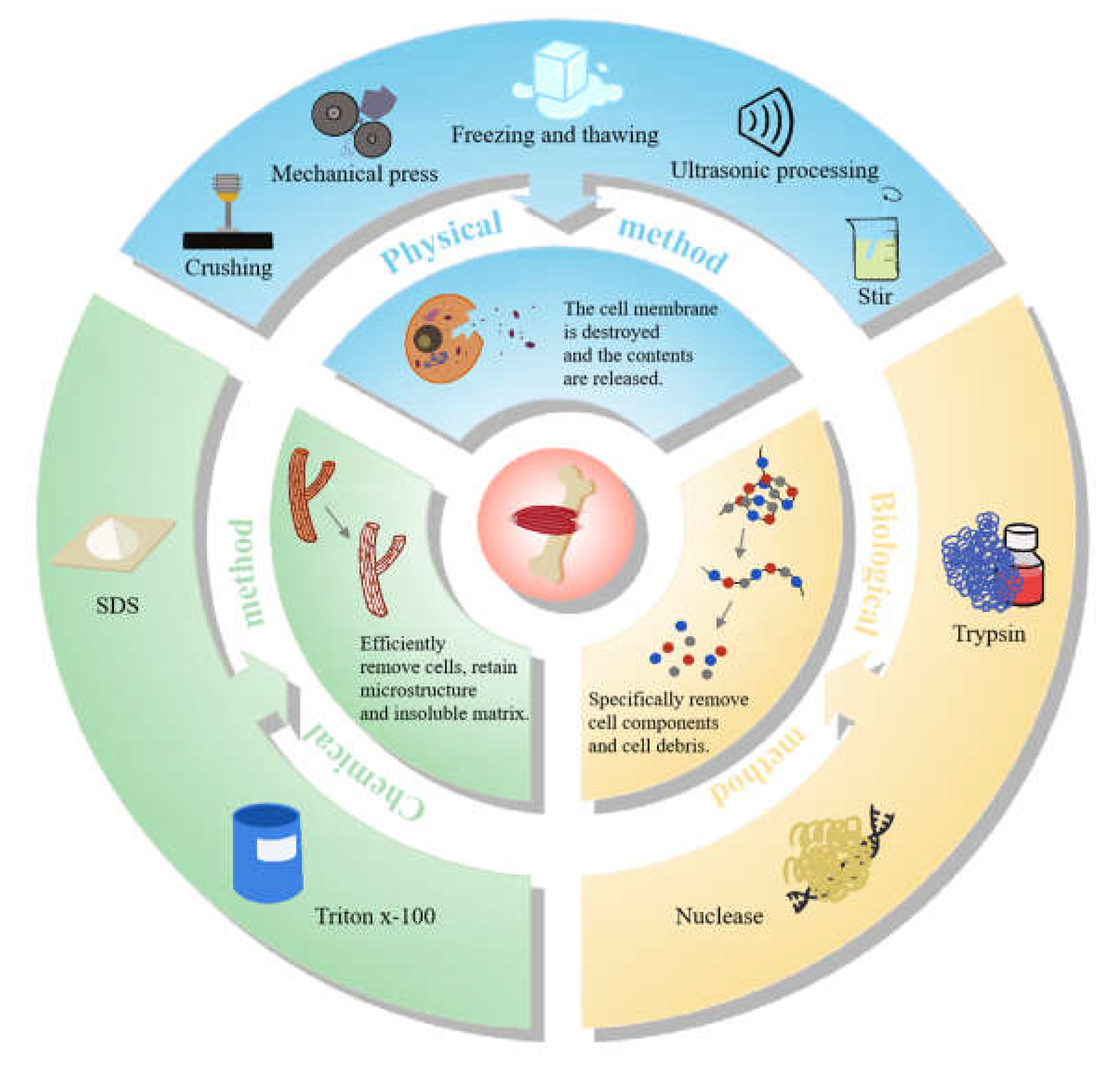
4.2. Preparation Methods of Animal-Derived Decellularized Scaffolds
4.3. The Application of Animal-Derived Decellularized Scaffolds of Cultured Meat
5. The Methods and Application of Plant-Derived Decellularized Scaffolds
5.1. Principles of Plant-Derived Decellularized Scaffolds
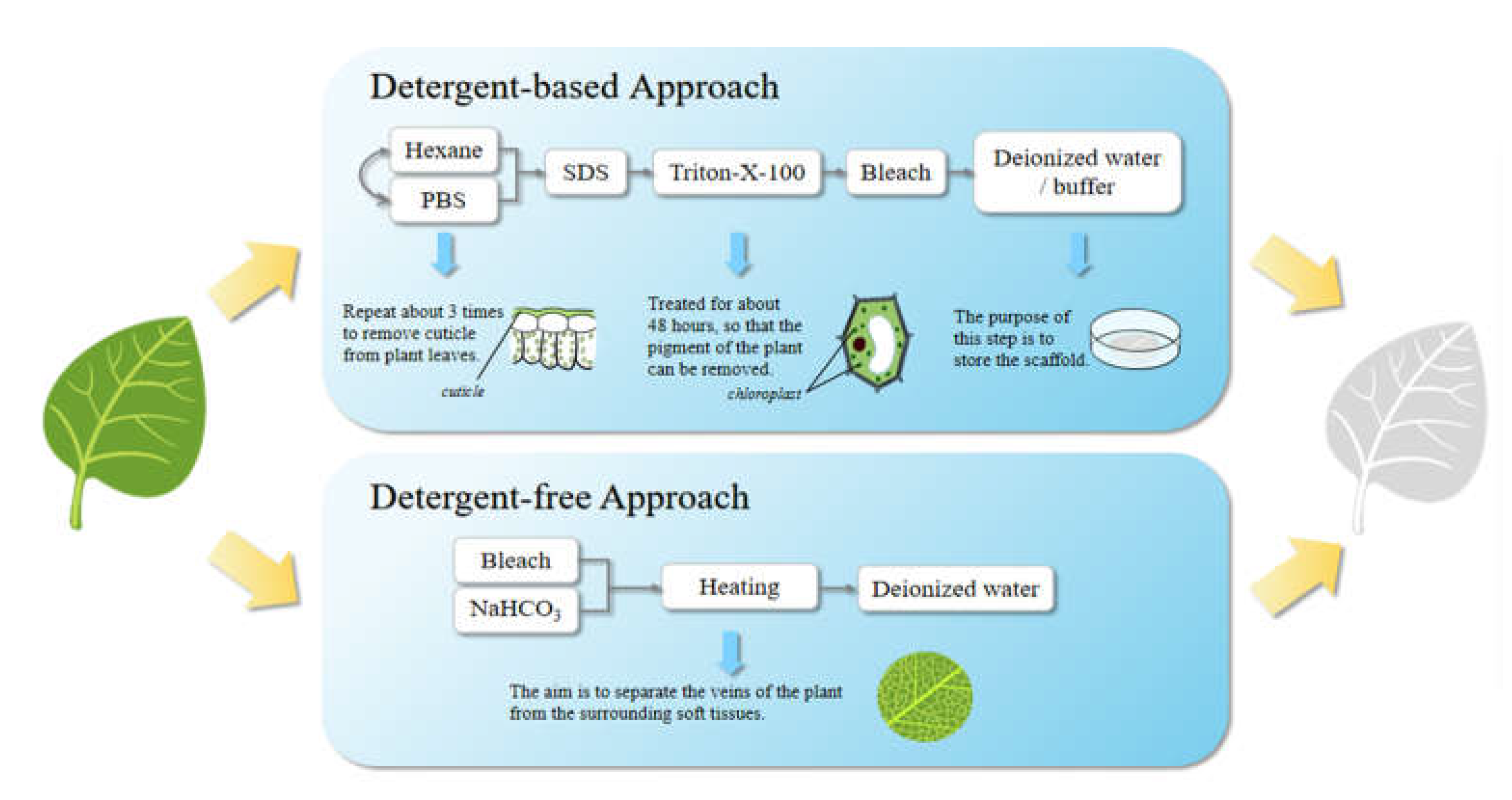
5.2. Preparation Strategy for Plant-Derived Decellularized Scaffolds
5.3. Application in Cultured Meat Process
6. Extensive Application Prospects and Current Shortcomings of Decellularized Scaffolds
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Post, M.J.; Levenberg, S.; Kaplan, D.L.; Genovese, N.; Fu, J.A.; Bryant, C.J.; Negowetti, N.; Verzijden, K.; Moutsatsou, P. Scientific, sustainability and regulatory challenges of cultured meat. Nat. Food 2020, 1, 403–415. [Google Scholar] [CrossRef]
- Ben-Arye, T.; Levenberg, S. Tissue Engineering for Clean Meat Production. Front. Sustain. Food Syst. 2019, 3. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.H.; Joo, S.T. Meat analog as future food: A review. J. Anim. Sci. Technol. 2020, 62, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Rubio, N.R.; Xiang, N.; Kaplan, D.L. Plant-based and cell-based approaches to meat production. Nat. Commun. 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.D. Review: Analysis of the process and drivers for cellular meat production. Animal 2019, 13, 3041–3058. [Google Scholar] [CrossRef]
- Rischer, H.; Szilvay, G.R.; Oksman-Caldentey, K.M. Cellular agriculture-industrial biotechnology for food and materials. Curr. Opin. Biotechnol. 2020, 61, 128–134. [Google Scholar] [CrossRef]
- Djisalov, M.; Knezic, T.; Podunavac, I.; Zivojevic, K.; Radonic, V.; Knezevic, N.Z.; Bobrinetskiy, I.; Gadjanski, I. Cultivating Multidisciplinarity: Manufacturing and Sensing Challenges in Cultured Meat Production. Biology 2021, 10, 204. [Google Scholar] [CrossRef]
- Bomkamp, C.; Skaalure, S.C.; Fernando, G.F.; Ben-Arye, T.; Swartz, E.W.; Specht, E.A. Scaffolding Biomaterials for 3D Cultivated Meat: Prospects and Challenges. Adv. Sci. 2022, 9, 1–40. [Google Scholar] [CrossRef]
- Bodiou, V.; Moutsatsou, P.; Post, M.J. Microcarriers for Upscaling Cultured Meat Production. Front. Nutr. 2020, 7. [Google Scholar] [CrossRef]
- Seah, J.S.H.; Singh, S.; Tan, L.P.; Choudhury, D. Scaffolds for the manufacture of cultured meat. Crit. Rev. Biotechnol. 2022, 42, 311–323. [Google Scholar] [CrossRef]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Handral, H.K.; Tay, S.H.; Chan, W.W.; Choudhury, D. 3D Printing of cultured meat products. Crit. Rev. Food Sci. Nutr. 2022, 62, 272–281. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Zhao, X.R.; Li, X.L.; Du, G.C.; Zhou, J.W.; Chen, J. Challenges and possibilities for bio-manufacturing cultured meat. Trends Food Sci. Technol. 2020, 97, 443–450. [Google Scholar] [CrossRef]
- Post, M.J. Cultured meat from stem cells: Challenges and prospects. Meat Sci. 2012, 92, 297–301. [Google Scholar] [CrossRef]
- Moritz, M.S.M.; Verbruggen, S.E.L.; Post, M.J. Alternatives for large-scale production of cultured beef: A review. J. Integr. Agric. 2015, 14, 208–216. [Google Scholar] [CrossRef]
- Sharma, S.; Thind, S.S.; Kaur, A. In vitro meat production system: Why and how? J. Food Sci. Technol. Mysore 2015, 52, 7599–7607. [Google Scholar] [CrossRef]
- Wolfson, W. Raising the steaks—A taste of what’s to come. Food Sci. Technol. 2019, 33, 50–52. [Google Scholar] [CrossRef]
- Lee, J.; Jung, H.; Park, N.; Park, S.H.; Ju, J.H. Induced Osteogenesis in Plants Decellularized Scaffolds. Sci. Rep. 2019, 9, 1. [Google Scholar] [CrossRef]
- Peng, W.J.; Datta, P.; Wu, Y.; Dey, M.; Ayan, B.; Dababneh, A.; Ozbolat, I.T. Challenges in Bio-fabrication of Organoid Cultures. In Cell Biology and Translational Medicine; Turksen, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Ahmad, K.; Lim, J.H.; Lee, E.J.; Chun, H.J.; Ali, S.; Ahmad, S.S.; Shaikh, S.; Choi, I. Extracellular Matrix and the Production of Cultured Meat. Foods 2021, 10, 3116. [Google Scholar] [CrossRef]
- MacQueen, L.A.; Alver, C.G.; Chantre, C.O.; Ahn, S.; Cera, L.; Gonzalez, G.M.; O’Connor, B.B.; Drennan, D.J.; Peters, M.M.; Motta, S.E.; et al. Muscle tissue engineering in fibrous gelatin: Implications for meat analogs. Npj Sci. Food 2019, 3, 1–2. [Google Scholar] [CrossRef]
- Campuzano, S.; Pelling, A.E. Scaffolds for 3D Cell Culture and Cellular Agriculture Applications Derived From Non-animal Sources. Front. Sustain. Food Syst. 2019, 3, 1–2. [Google Scholar] [CrossRef]
- Lin, C.W.; Wu, P.T.; Liu, K.T.; Fan, Y.J.; Yu, J.S. An Environmental Friendly Tapioca Starch-Alginate Cultured Scaffold as Biomimetic Muscle Tissue. Polymers 2021, 13, 882. [Google Scholar] [CrossRef] [PubMed]
- Enrione, J.; Blaker, J.J.; Brown, D.I.; Weinstein-Oppenheimer, C.R.; Pepczynska, M.; Olguin, Y.; Sanchez, E.; Acevedo, C.A. Edible Scaffolds Based on Non-Mammalian Biopolymers for Myoblast Growth. Materials 2017, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Orellana, N.; Sanchez, E.; Benavente, D.; Prieto, P.; Enrione, J.; Acevedo, C.A. A New Edible Film to Produce In Vitro Meat. Foods 2020, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, C.A.; Orellana, N.; Avarias, K.; Ortiz, R.; Benavente, D.; Prieto, P. Micropatterning Technology to Design an Edible Film for In Vitro Meat Production. Food Bioprocess Technol. 2018, 11, 1267–1273. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, N.; Sharma, S.; Mehta, N.; Verma, A.K.; Chemmalar, S.; Sazili, A.Q. In-vitro meat: A promising solution for sustainability of meat sector. J. Anim. Sci. Technol. 2021, 63, 693–724. [Google Scholar] [CrossRef]
- Brovold, M.; Almeida, J.I.; Pla-Palacin, I.; Sainz-Arnal, P.; Sanchez-Romero, N.; Rivas, J.J.; Almeida, H.; Dachary, P.R.; Serrano-Aullo, T.; Soker, S.; et al. Naturally-Derived Biomaterials for Tissue Engineering Applications. In Novel Biomaterials for Regenerative Medicine; Chun, H.J., Park, K., Kim, C.H., Khang, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Ong, S.J.; Loo, L.; Pang, M.; Tan, R.; Teng, Y.; Lou, X.M.; Chin, S.K.; Naik, M.Y.; Yu, H. Decompartmentalisation as a simple color manipulation of plant-based marbling meat alternatives. Biomaterials 2021, 277, 1015–10125. [Google Scholar] [CrossRef]
- Urciuolo, A.; De Coppi, P. Decellularized Tissue for Muscle Regeneration. Int. J. Mol. Sci. 2018, 19, 2392. [Google Scholar] [CrossRef]
- Holmes, J.T.; Jaberansari, Z.; Collins, W.; Latour, M.L.; Modulevsky, D.J.; Pelling, A.E. Homemade bread: Repurposing an ancient technology for in vitro tissue engineering. Biomaterials 2022, 280, 121267. [Google Scholar] [CrossRef]
- Hanai, H.; Jacob, G.; Nakagawa, S.; Tuan, R.S.; Nakamura, N.; Shimomura, K. Potential of Soluble Decellularized Extracellular Matrix for Musculoskeletal Tissue Engineering-Comparison of Various Mesenchymal Tissues. Front. Cell Dev. Biol. 2020, 8, 581972. [Google Scholar] [CrossRef]
- Lin, M.H.; Ge, J.B.; Wang, X.C.; Dong, Z.Q.; Xing, M.; Lu, F.; He, Y.F. Biochemical and biomechanical comparisions of decellularized scaffolds derived from porcine subcutaneous and visceral adipose tissue. J. Tissue Eng. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Ibsirlioglu, T.; Elçin, A.E.; Elçin, Y.M. Decellularized biological scaffold and stem cells from autologous human adipose tissue for cartilage tissue engineering. Methods 2020, 171, 97–107. [Google Scholar] [CrossRef]
- Kara, A.; Tamburaci, S.; Tihminlioglu, F.; Havitcioglu, H. Bioactive fish scale incorporated chitosan biocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019, 130, 266–279. [Google Scholar] [CrossRef]
- Sun, Y.; Yan, L.; Chen, S.; Pei, M. Functionality of decellularized matrix in cartilage regeneration: A comparison of tissue versus cell sources. Acta Biomater. 2018, 74, 56–73. [Google Scholar] [CrossRef]
- Khajavi, M.; Hajimoradloo, A.; Zandi, M.; Pezeshki-Modaress, M.; Bonakdar, S.; Zamani, A. Fish cartilage: A promising source of biomaterial for biological scaffold fabrication in cartilage tissue engineering. J. Biomed. Mater. Res. A. 2021, 109, 1737–1750. [Google Scholar] [CrossRef]
- Su, M.Z.; Zhang, Q.; Zhu, Y.W.; Wang, S.Y.; Lv, J.W.; Sun, J.A.; Qiu, P.C.; Fan, S.W.; Jin, K.K.; Chen, L.; et al. Preparation of Decellularized Triphasic Hierarchical Bone-Fibrocartilage-Tendon Composite Extracellular Matrix for Enthesis Regeneration. Adv. Healthc. Mater. 2019, 8, 1900831. [Google Scholar] [CrossRef]
- Stone, R.N.; Frahs, S.M.; Hardy, M.J.; Fujimoto, A.; Pu, X.Z.; Keller-Peck, C.; Oxford, J.T. Decellularized Porcine Cartilage Scaffold; Validation of Decellularization and Evaluation of Biomarkers of Chondrogenesis. Int. J. Mol. Sci. 2021, 22, 6241. [Google Scholar] [CrossRef]
- Lim, T.; Tang, Q.; Zhu, Z.Z.; Feng, Y.; Zhan, S.; Wei, X.J.; Zhang, C.Q. A decellularized scaffold derived from squid cranial cartilage for use in cartilage tissue engineering. J. Mater. Chem. B 2020, 8, 4516–4526. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Song, L.; Chen, J.; Ma, Y.; Chen, Y.; Fan, S.; Su, M.; Lin, X. Decellularized tendon as a prospective scaffold for tendon repair. Mater. Sci. Eng. C 2017, 77, 1290–1301. [Google Scholar] [CrossRef]
- Bottagisio, M.; D’Arrigo, D.; Talo, G.; Bongio, M.; Ferroni, M.; Boschetti, F.; Moretti, M.; Lovati, A.B. Achilles Tendon Repair by Decellularized and Engineered Xenografts in a Rabbit Model. Stem Cells Int. 2019, 2019. [Google Scholar] [CrossRef]
- Iyer, H.; Lanier, S.; Dolivo, D.; Arenas, G.A.; Hong, S.J.; Mustoe, T.A.; Galiano, R.D. Allogeneic Decellularized Muscle Scaffold Is Less Fibrogenic and Inflammatory than Acellular Dermal Matrices in a Rat Model of Skeletal Muscle Regeneration. Plast. Reconstr. Surg. 2020, 146, 43E–53E. [Google Scholar] [CrossRef]
- Choi, Y.J.; Park, S.J.; Yi, H.G.; Lee, H.; Kim, D.S.; Cho, D.W. Muscle-derived extracellular matrix on sinusoidal wavy surfaces synergistically promotes myogenic differentiation and maturation. J. Mater. Chem. B 2018, 6, 5530–5539. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, C.; Fallas, M.E.A.; Maghin, E.; Franzin, C.; Pavan, P.; Caccin, P.; Chiavegato, A.; Carraro, E.; Boso, D.; Boldrin, F.; et al. Generation of a Functioning and Self-Renewing Diaphragmatic Muscle Construct. Stem Cells Transl. Med. 2019, 8, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.; Mobarhan, M.A.; Mohammadi, J.; Shahsavarani, H.; Shokrgozar, M.A.; Alipour, A. Efficient mineralization and osteogenic gene overexpression of mesenchymal stem cells on decellularized spinach leaf scaffold. Gene 2020, 757, 144852. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Rebello, A.S.; Gaudette, G.R. Decellularized spinach: An edible scaffold for laboratory-grown meat. Food Biosci. 2021, 41, 100986. [Google Scholar] [CrossRef]
- Salehi, A.; Mobarhan, M.A.; Mohammadi, J.; Shahsavarani, H.; Shokrgozar, M.A.; Alipour, A. Natural cellulose-based scaffold for improvement of stem cell osteogenic differentiation. J. Drug Deliv. Sci. Technol. 2021, 63, 106–112. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Shiwarski, D.J.; Ball, R.L.; Whitehead, K.A.; Feinberg, A.W. Engineering Aligned Skeletal Muscle Tissue Using Decellularized Plant-Derived Scaffolds. Acs Biomater. Sci. Eng. 2020, 6, 3046–3054. [Google Scholar] [CrossRef]
- Negrini, N.C.; Toffoletto, N.; Fare, S.; Altomare, L. Plant Tissues as 3D Natural Scaffolds for Adipose, Bone and Tendon Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 723. [Google Scholar] [CrossRef]
- Thyden, R.; Perreault, L.R.; Jones, J.D.; Notman, H.; Varieur, B.M.; Patmanidis, A.A.; Dominko, T.; Gaudette, G.R. An Edible, Decellularized Plant Derived Cell Carrier for Lab Grown Meat. Appl. Sci. 2022, 12, 5155. [Google Scholar] [CrossRef]
- Aswathy, S.H.; Mohan, C.C.; Unnikrishnan, P.S.; Krishnan, A.G.; Nair, M.B. Decellularization and oxidation process of bamboo stem enhance biodegradation and osteogenic differentiation. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 119, 11500. [Google Scholar] [CrossRef]
- Allan, S.J.; Ellis, M.J.; De Bank, P.A. Decellularized grass as a sustainable scaffold for skeletal muscle tissue engineering. J. Biomed. Mater. Res. Part A 2021, 109, 2471–2482. [Google Scholar] [CrossRef]
- Balasundari, R.; Bishi, D.K.; Mathapati, S.; Naser, S.B.; Cherian, K.M.; Guhathakurta, S. Nanocoated Botanical Scaffold in Salvage for Human Tissue Regeneration. J. Biomater. Tissue Eng. 2012, 2, 330–335. [Google Scholar] [CrossRef]
- Wei, Y.R.X.; Zhang, Y.J. Decellularized Liver Scaffold for Liver Regeneration. Methods Mol. Biol. 2018, 1577, 11–23. [Google Scholar]
- Stephens, N.; Di Silvio, L.; Dunsford, I.; Ellis, M.; Glencross, A.; Sexton, A. Bringing cultured meat to market: Technical, socio-political, and regulatory challenges in cellular agriculture. Trends Food Sci. Technol. 2018, 78, 155–166. [Google Scholar] [CrossRef]
- Predeina, A.L.; Dukhinova, M.S.; Vinogradov, V.V. Bioreactivity of decellularized animal, plant, and fungal scaffolds: Perspectives for medical applications. J. Mater. Chem. B 2020, 8, 10010–10022. [Google Scholar] [CrossRef]
- Rajab, T.K.; O’Malley, T.J.; Tchantchaleishvili, V. Decellularized scaffolds for tissue engineering: Current status and future perspective. Artif. Organs 2020, 44, 1031–1043. [Google Scholar] [CrossRef]
- Saleh, T.M.; Ahmed, E.A.; Yu, L.; Kwak, H.H.; Hussein, K.H.; Park, K.M.; Kang, B.J.; Choi, K.Y.; Kang, K.S.; Woo, H.M. Incorporation of nanoparticles into transplantable decellularized matrices: Applications and challenges. Int. J. Organs 2018, 41, 421–430. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Xia, C.; Mei, S.; Gu, C.; Zheng, L.; Fang, C.; Shi, Y.; Wu, K.; Lu, T.; Jin, Y.; Lin, X.; et al. Decellularized cartilage as a prospective scaffold for cartilage repair. Mater. Sci. Eng. C 2019, 101, 588–595. [Google Scholar] [CrossRef]
- Chen, G.; Lv, Y.M.i.m.b. Decellularized Bone Matrix Scaffold for Bone Regeneration. Methods Mol Biol. 2017, 1577, 239–254. [Google Scholar]
- Liao, J.; Xu, B.; Zhang, R.H.; Fan, Y.B.; Xie, H.Q.; Li, X.M. Applications of decellularized materials in tissue engineering: Advantages, drawbacks and current improvements, and future perspectives. J. Mater. Chem. B 2020, 8, 10023–10049. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Stocco, E.; Barbon, S.; Grandi, F.; Macchi, V.; De Caro, R. Tissue-Engineered Grafts from Human Decellularized Extracellular Matrices: A Systematic Review and Future Perspectives. Int. J. Mol. Sci. 2018, 19, 4117. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.S.; Hassanbhai, A.; Wen, F.; Wang, D.; Chanchareonsook, N.; Goh, B.T.; Yu, N.; Teoh, S.-H. Evaluation of decellularized tilapia skin as a tissue engineering scaffold. J. Tissue Eng. Regen. Med. 2019, 13, 1779–1791. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.K.Y.; Leung, V.Y.L.; Tam, V.; Lu, W.W.; Sze, K.Y.; Cheung, K.M.C. Decellularized bovine intervertebral disc as a natural scaffold for xenogenic cell studies. Acta Biomater. 2013, 9, 5262–5272. [Google Scholar] [CrossRef] [PubMed]
- Hussein, K.H.; Park, K.-M.; Kang, K.-S.; Woo, H.-M. Biocompatibility evaluation of tissue-engineered decellularized scaffolds for biomedical application. Mater. Sci. Eng. C 2016, 67, 766–778. [Google Scholar] [CrossRef]
- Modulevsky, D.J.; Lefebvre, C.; Haase, K.; Al-Rekabi, Z.; Pelling, A.E. Apple Derived Cellulose Scaffolds for 3D Mammalian Cell Culture. PLoS ONE 2014, 9, e97835. [Google Scholar] [CrossRef]
- Fontana, G.; Gershlak, J.; Adamski, M.; Lee, J.S.; Matsumoto, S.; Le, H.D.; Binder, B.; Wirth, J.; Gaudette, G.; Murphy, W.L. Biofunctionalized Plants as Diverse Biomaterials for Human Cell Culture. Adv. Healthc. Mater. 2017, 6, 1601225. [Google Scholar] [CrossRef]
- Adamski, M.; Fontana, G.; Gershlak, J.R.; Gaudette, G.R.; Le, H.D.; Murphy, W.L. Two Methods for Decellularization of Plant Tissues for Tissue Engineering Applications. Jove-J. Vis. Exp. 2018, 135, 57586. [Google Scholar] [CrossRef]
- Walawalkar, S.; Almelkar, S. Fabricating a pre-vascularized large-sized metabolically-supportive scaffold using Brassica oleracea leaf. J. Biomater. Appl. 2021, 36, 165–178. [Google Scholar] [CrossRef]
- Gershlak, J.R.; Hernandez, S.; Fontana, G.; Perreault, L.R.; Hansen, K.J.; Larson, S.A.; Binder, B.Y.K.; Dolivo, D.M.; Yang, T.; Dominko, T.; et al. Crossing kingdoms: Using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials 2017, 125, 13–22. [Google Scholar] [CrossRef]
- Harris, A.F.; Lacombe, J.; Liyanage, S.; Han, M.Y.; Wallace, E.; Karsunky, S.; Abidi, N.; Zenhausern, F. Supercritical carbon dioxide decellularization of plant material to generate 3D biocompatible scaffolds. Sci. Rep. 2021, 11, 1–3. [Google Scholar] [CrossRef]
- Phan, N.V.; Wright, T.; Rahman, M.M.; Xu, J.F.; Coburn, J.M. In Vitro Biocompatibility of Decellularized Cultured Plant Cell-Derived Matrices. Acs Biomater. Sci. Eng. 2020, 6, 822–832. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Zhang, Q.; Wang, S.Y.; Zhang, J.F.; Fan, S.W.; Lin, X.F. Current Advances in the Development of Decellularized Plant Extracellular Matrix. Front. Bioeng. Biotechnol. 2021, 9, 650. [Google Scholar] [CrossRef]
- Jansen, K.; Evangelopoulou, M.; Casellas, C.P.; Abrishamcar, S.; Jansen, J.; Vermonden, T.; Masereeuw, R. Spinach and Chive for Kidney Tubule Engineering: The Limitations of Decellularized Plant Scaffolds and Vasculature. Aaps 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Lacombe, J.; Harris, A.F.; Zenhausern, R.; Karsunsky, S.; Zenhausern, F. Plant-Based Scaffolds Modify Cellular Response to Drug and Radiation Exposure Compared to Standard Cell Culture Models. Front. Bioeng. Biotechnol. 2020, 8, 932. [Google Scholar] [CrossRef]
- Bai, H.L.; Xie, B.A.; Wang, Z.W.; Li, M.X.; Sun, P.; Wei, S.B.; Wang, W.; Wu, H.L.; Bai, L.; Li, J.G. Application of the Tissue-Engineered Plant Scaffold as a Vascular Patch. Acs Omega 2021, 6, 11595–11601. [Google Scholar] [CrossRef]
- Nowacki, M.; Nowacka, K.; Kloskowski, T.; Pokrywczynska, M.; Tyloch, D.; Rasmus, M.; Warda, K.; Drewa, T. Are agricultural and natural sources of bio-products important for modern regenerative medicine? A review. Ann. Agric. Environ. Med. 2017, 24, 207–212. [Google Scholar] [CrossRef]
- Ng, S.; Kurisawa, M. Integrating biomaterials and food biopolymers for cultured meat production. Acta Biomater. 2021, 124, 108–129. [Google Scholar] [CrossRef]
- Wysokowski, M.; Behm, T.; Born, R.; Bazhenov, V.V.; Meissner, H.; Richter, G.; Szwarc-Rzepka, K.; Makarova, A.; Vyalikh, D.; Schupp, P.; et al. Preparation of chitin-silica composites by in vitro silicification of two-dimensional Ianthella basta demosponge chitinous scaffolds under modified Stober conditions. Mater. Sci. Eng. C-Mater. Biol. Appl. 2013, 33, 3935–3941. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A. Electrospun Nanofibers as Carriers of Microorganisms, Stem Cells, Proteins, and Nucleic Acids in Therapeutic and Other Applications. Front. Bioeng. Biotechnol. 2020, 8, 130. [Google Scholar] [CrossRef]
| Decellularized Tissue | Decellularization Protocols | Application | Classification | Cell Type | References |
|---|---|---|---|---|---|
| Animal-derived Decellularized Scaffolds | |||||
| Porcine ligament | 1% Triton X-100 and 200 U/mL DNase and 50 U/ml RNase | Skeletal tissues differentiation | Connective tissue | Human synovium derived mesenchymal stem cells (hSMSCs) | [32] |
| Porcine synovium | 1% Triton X-100 and 200 U/mL DNase and 50 U/ml RNase | Skeletal tissues differentiation | Connective tissue | hSMSCs | [32] |
| Porcine subcutaneous | Digested with 0.05% trypsin for 16 h, underwent 48 h in 99.9% isopropanol, incubated with Benzonase digestion solution for 16 h and 99.9% isopropanol for 8 h, and disinfected with 0.1% peracetic acid in 4% ethanol | Promote morphological changes and cell differentiation | Fat | Human adipose stem cells (hASCs) | [33] |
| Human dermis fat | Digested in collagenase type I for 45 min at 37 °C | Promote cell adhesion, proliferation and differentiation | Connective tissue | hASCs | [34] |
| Porcine fat/adipose | 1% Triton X-100 and 200 U/mL DNase and 50 U/mL RNase | Skeletal tissues differentiation | Connective tissue | hSMSCs | [32] |
| Porcine fat pad | 1% Triton X-100 and 200 U/mL DNase and 50 U/mL RNase | Skeletal tissues differentiation | Connective tissue | hSMSCs | [32] |
| Fish scale | Incubated in 10 mM Tris-HCl buffer and 0.1% EDTA at 4 °C for 24 h and 0.1% SDS in Tris-HCl buffer at 4 °C for 3 days, and rinsed with 70% ethanol | Promote cell proliferation, increase osteogenic activity | Connective tissue | Human osteosarcoma cells (SaOS-2) | [35] |
| Cartilage | Sodium dodecyl sulfate, Triton X-100, ethylenediaminetetraacetic acid and Tris-Hydrochloride | Promote chondrogenic differentiation and proliferation | Connective tissue | Chondrocytes/stem cells | [36] |
| Porcine cartilage | 1% Triton X-100 and 200 U/mL DNase and 50 U/ml RNase | Skeletal tissues differentiation | Connective tissue | hSMSCs | [32] |
| Sturgeon fish cartilage | Incubated in 1% SDS/PBS at 4 °C, and exposed to 0.1% EDTA/PBS, and digested in 1 U/mL DNase-I solution for 24 h, respectively | Induce matrix synthesis | Connective tissue | hASCs | [37] |
| Cartilage | Soaked in 1% Triton X-100 for 1d and 1% SDS | Induce the osteogenic differentiation of MSCs | Connective tissue | Mesenchymal stem cells (MSCs) | [38] |
| Porcine auricular cartilage | Washed in 10 mM Tris–HCl, 2 mM EDTA, 5 mM MgCl2, 100 mM DTT, 1% SDS, and 1% Triton-X100, incubated in PBS with 21 U/mL of hyaluronidase, and treated with DNase and RNase | Support cell attachment and growth | Connective tissue | Chondrocyte cells | [39] |
| Squid cranial cartilage | 0.02% EDTA and 0.05 μg mL−1 trypsin solution | Promote chondrocyte migration, maintain its viability and spreading morphology | Connective tissue | Chondrocyte cells | [40] |
| Porcine meniscus | 1% Triton X-100 and 200 U/mL DNase and 50 U/mL RNase | Skeletal tissues differentiation | Connective tissue | hSMSCs | [32] |
| Porcine bone | 1% Triton X-100 and 200 U/mL DNase and 50 U/mL RNase | Skeletal tissues differentiation | Connective tissue | hSMSCs | [32] |
| Tendon | SDS, Triton X-100, trypsin, and freezing-thawing | Similar to natural tendons in bioactive components, collagen arrangement and biomechanical characteristics; recellularization and repair ability | Skeletal muscle | Tendon cells | [41] |
| Porcine tendon | 1% Triton X-100 and 200 U/mL DNase and 50 U/mL RNase | Skeletal tissues differentiation | Connective tissue | hSMSCs | [32] |
| Equine-derived tendon | 1% TBP followed by 1% PAA,1% TBP followed by 3% PAA, 1% TBP followed by 5% PAA, and 1% TBP | Promote osteogenic differentiation and tendon reconstruction | Skeletal muscle | Bone marrow mesenchymal stem cells (BMSCs) | [42] |
| Porcine muscle | 1% Triton X-100 and 200 U/mL DNase and 50 U/mL RNase | Skeletal tissues differentiation | Connective tissue | hSMSCs | [32] |
| Rat muscle and dermis | Treated with 0.25% SDS and DNase I | Promote myocyte differentiation, reduce inflammation and fibrosis of the muscle | Skeletal muscle | Muscle spectrum cells | [43] |
| Porcine tibialis anterior skeletal muscle | Incubated in 1% SDS for 3d, washed with 50 U mL−1 DNase and 1 U mL−1 RNase in 10 mM Tris–HCl, treated with 0.5% Triton X-100 and Isopropanol for 1d, respectively | Enhance myogenic differentiation and maturation | Skeletal muscle | Myogenic cells | [44] |
| Mouse diaphragmatic extracellular matrix | Processed with three 4% sodium deoxycholate-2000 kU DNase-I treatment (DET) cycles | Promote proliferation and differentiation capability of muscle precursors, generate functional 3D skeletal muscle tissue constructs | Skeletal muscle | Muscle precursor cells | [45] |
| Plant-derived Decellularized Scaffolds | |||||
| Spinach | Rinsed in a normal hexane (98%) for 5 min, incubated in 10X SDS for 5 days at 25 °C, washed with 0.1% Triton-X100 in 10% sodium hypochlorite for 2 days | Promote osteogenic differentiation of stem cells in bone tissue engineering | Connective tissue | Bone marrow derived mesenchymal stem cells | [46] |
| Spinach | Rinsed in a normal hexane (98%) for 3 min, incubated in 1% SDS for 5 days, washed with 0.1% Triton-X100 and 10% concentrated bleach for 2 days | Provide an edible substrate for the growth of bovine satellite cells | Skeletal muscle | Primary bovine satellite cells | [47] |
| Onion | Rinsed in a normal hexane (98%) for 5 min, incubated in 10% SDS for 5 days at 25 °C, washed with 0.1% Triton-X100 in 10% sodium hypochlorite for 2 days | Promote the osteogenic differentiation of bone mesenchymal stem cells | Connective tissue | MSCs | [48] |
| Carrot | Submerged in 1% SDS and shaken at 70 rpm at 25 °C for 3 weeks, with the 1% SDS solution refreshed weekly | Promote the arrangement and differentiation of human and mouse skeletal muscle cells | Skeletal muscle | Mouse and human muscle cells | [49] |
| Carrot | Immersed 0.1% SDS for 48 h and washed in 100 mM CaCl2 for 24 h | Promote osteoclast adhesion, proliferation, and differentiation | Connective tissue | 3T3-L1 preadipocytes, MC3T3-E1 Pre-osteoblasts and L929 cells | [50] |
| Broccoli | Submerged in 1% SDS and shaken at 70 rpm at 25 °C for 3 weeks, with the 1% SDS solution refreshed weekly | Promote the arrangement and differentiation of human and mouse skeletal muscle cells | Skeletal muscle | Mouse and human muscle cells | [49] |
| Cucumber | Submerged in 1% SDS and shaken at 70 rpm at 25 °C for 3 weeks, with the 1% SDS solution refreshed weekly | Promote the arrangement and differentiation of human and mouse skeletal muscle cells | Skeletal muscle | Mouse and human muscle cells | [49] |
| Potato | Submerged in 1% SDS and shaken at 70 rpm at 25 °C for 3 weeks, with the 1% SDS solution refreshed weekly | Promote cell arrangement and differentiation | Skeletal muscle | Mouse and human muscle cells | [49] |
| Apple | Submerged in 0.5% SDS for 48 h | Promote the growth and differentiation of osteoblasts | Connective tissue | Pluripotent stem cells | [18] |
| Broccoli florets | 10% SDS, 3% Tween-20, and 10% bleach for 48 h | Promote cell adhesion | Connective tissue | Primary bovine satellite cells | [51] |
| Asparagus/ Green onion/Leek/Celery | Submerged in 1% SDS and shaken at 70 rpm at 25 °C for 3 weeks, with the 1% SDS solution refreshed weekly | Promote cell arrangement and differentiation | Skeletal muscle | Mouse and human muscle cells | [49] |
| Bamboo stems | (a) 10% SDS in 1% sodium hypochlorite (b) 1% Triton X-100 in 1% sodium hypochlorite (c) 10%SDS and 1% Triton X-100 in 1% sodium hypochlorite (d) 1% sodium hypochlorite | Promote cell adhesion, proliferation and osteogenic differentiation, and enhance bone tissue regeneration | Connective tissue | Mesenchymal stem cells | [52] |
| Grass blades | Agitated in 1% SDS, 1% Tween-20, and 10% bleach for 1 to 2 days | Maintain cell viability; induce cell alignment; support cell attachment, proliferation, and differentiation | Skeletal muscle | Murine C2C12 myoblasts | [53] |
| Jackfruit | Treated with 10% SDS for 5 days with gentle shaking, followed by washing in 0.1% Triton X-100 for 2 days | Promote cell adhesion and culture primary porcine myogenic cells with a rough surface | Skeletal muscle | Porcine myoblasts | [29] |
| Macrofungi-derived decellularized scaffolds | |||||
| Macrofungi Agaricus bisporus | Sodium Deoxycholate and Nucleases | Induce osteogenic differentiation | Connective tissue | Human mesenchymal stem cells (hMSCs) | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Ying, K.; Shi, Y.; Liu, D.; Chen, Q. Bioprocessing by Decellularized Scaffold Biomaterials in Cultured Meat: A Review. Bioengineering 2022, 9, 787. https://doi.org/10.3390/bioengineering9120787
Lu H, Ying K, Shi Y, Liu D, Chen Q. Bioprocessing by Decellularized Scaffold Biomaterials in Cultured Meat: A Review. Bioengineering. 2022; 9(12):787. https://doi.org/10.3390/bioengineering9120787
Chicago/Turabian StyleLu, Hongyun, Keqin Ying, Ying Shi, Donghong Liu, and Qihe Chen. 2022. "Bioprocessing by Decellularized Scaffold Biomaterials in Cultured Meat: A Review" Bioengineering 9, no. 12: 787. https://doi.org/10.3390/bioengineering9120787
APA StyleLu, H., Ying, K., Shi, Y., Liu, D., & Chen, Q. (2022). Bioprocessing by Decellularized Scaffold Biomaterials in Cultured Meat: A Review. Bioengineering, 9(12), 787. https://doi.org/10.3390/bioengineering9120787