Alginate Core–Shell Capsules for 3D Cultivation of Adipose-Derived Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
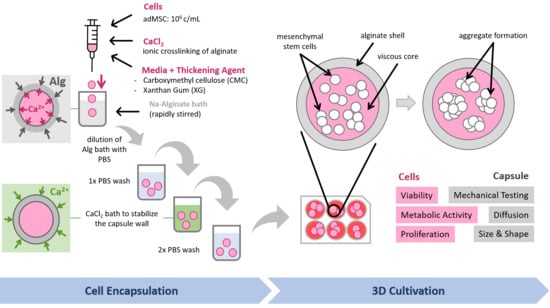
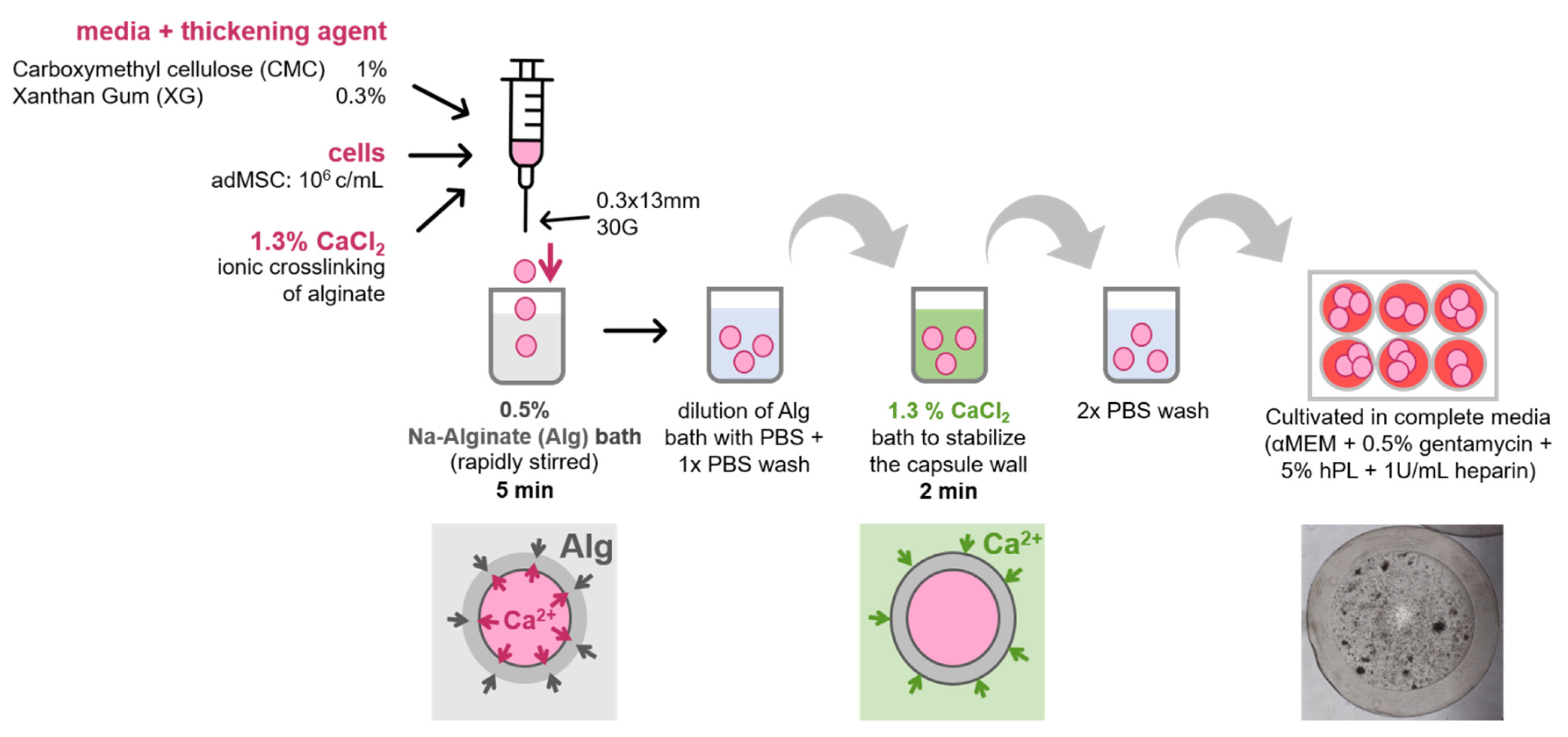
2.2. Core–Shell Capsule Production
2.3. Cell Encapsulation in Core–Shell Capsules
2.4. Determination of Capsule Dimensions
2.5. Mechanical Testing
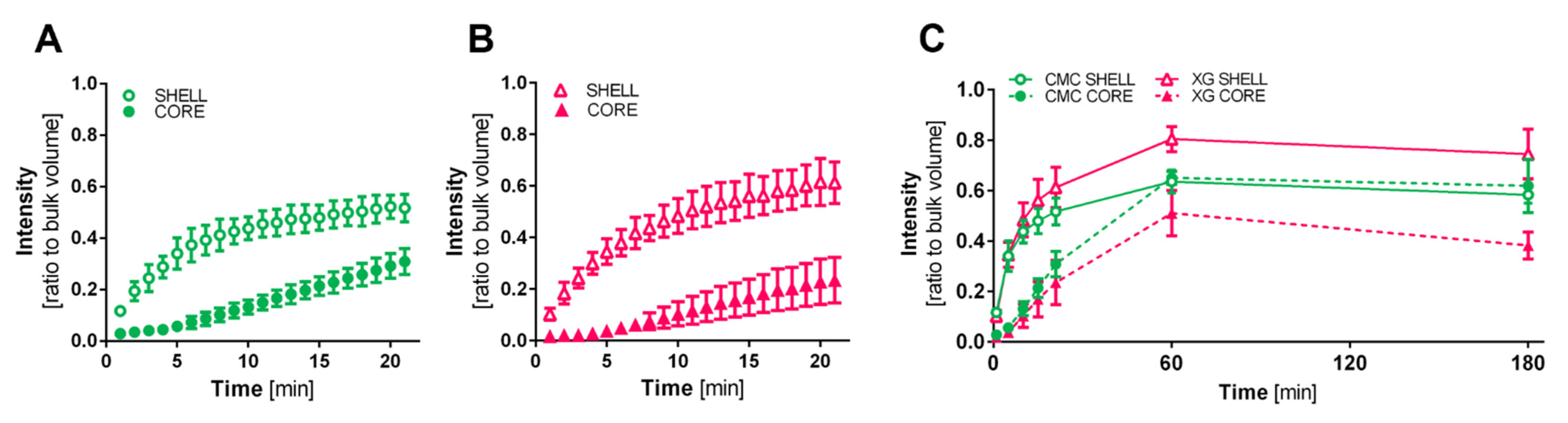
2.6. Diffusion Characteristics
2.7. Metabolic Activity
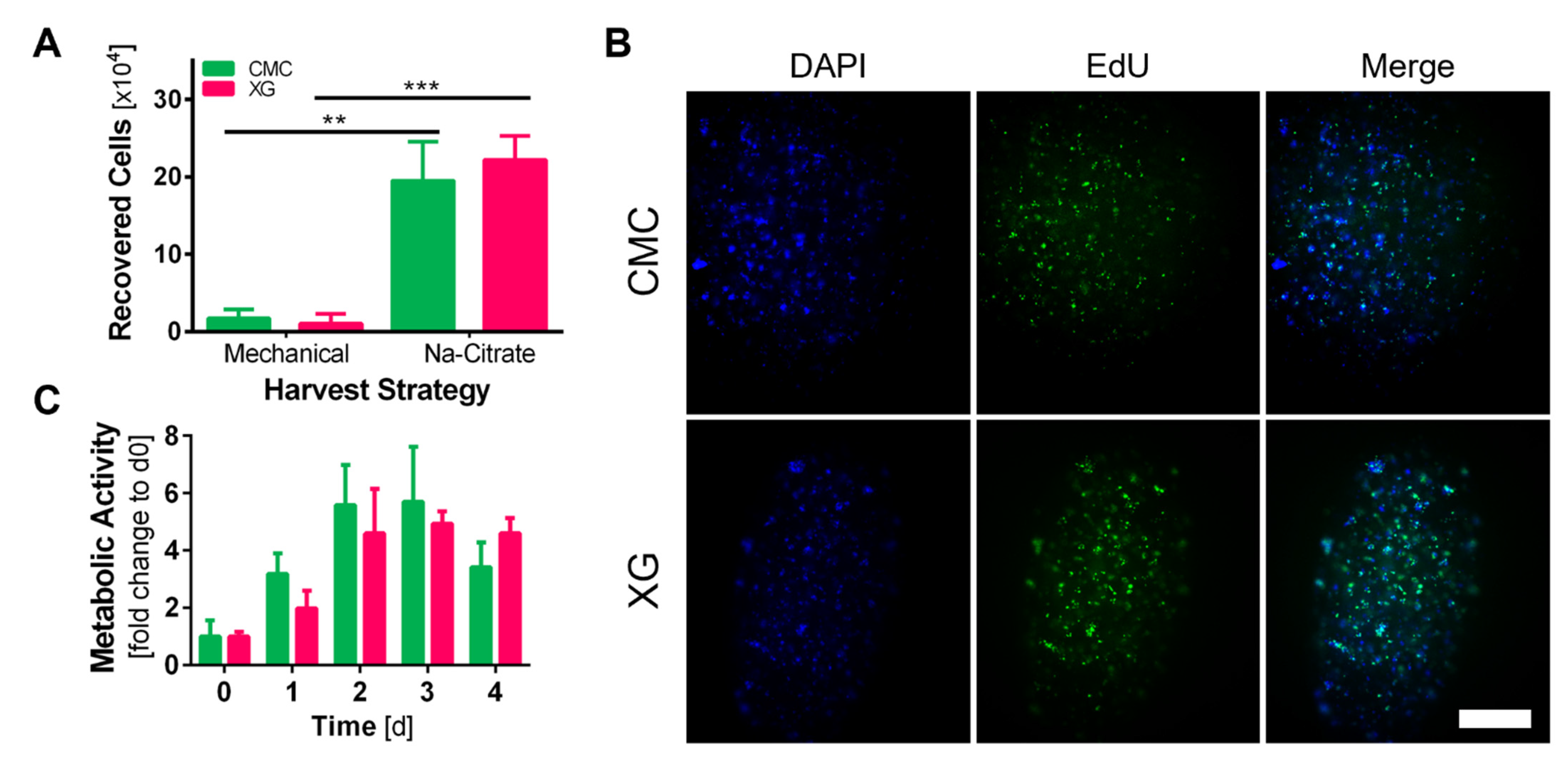
2.8. Cell Harvest
2.9. EdU Assay
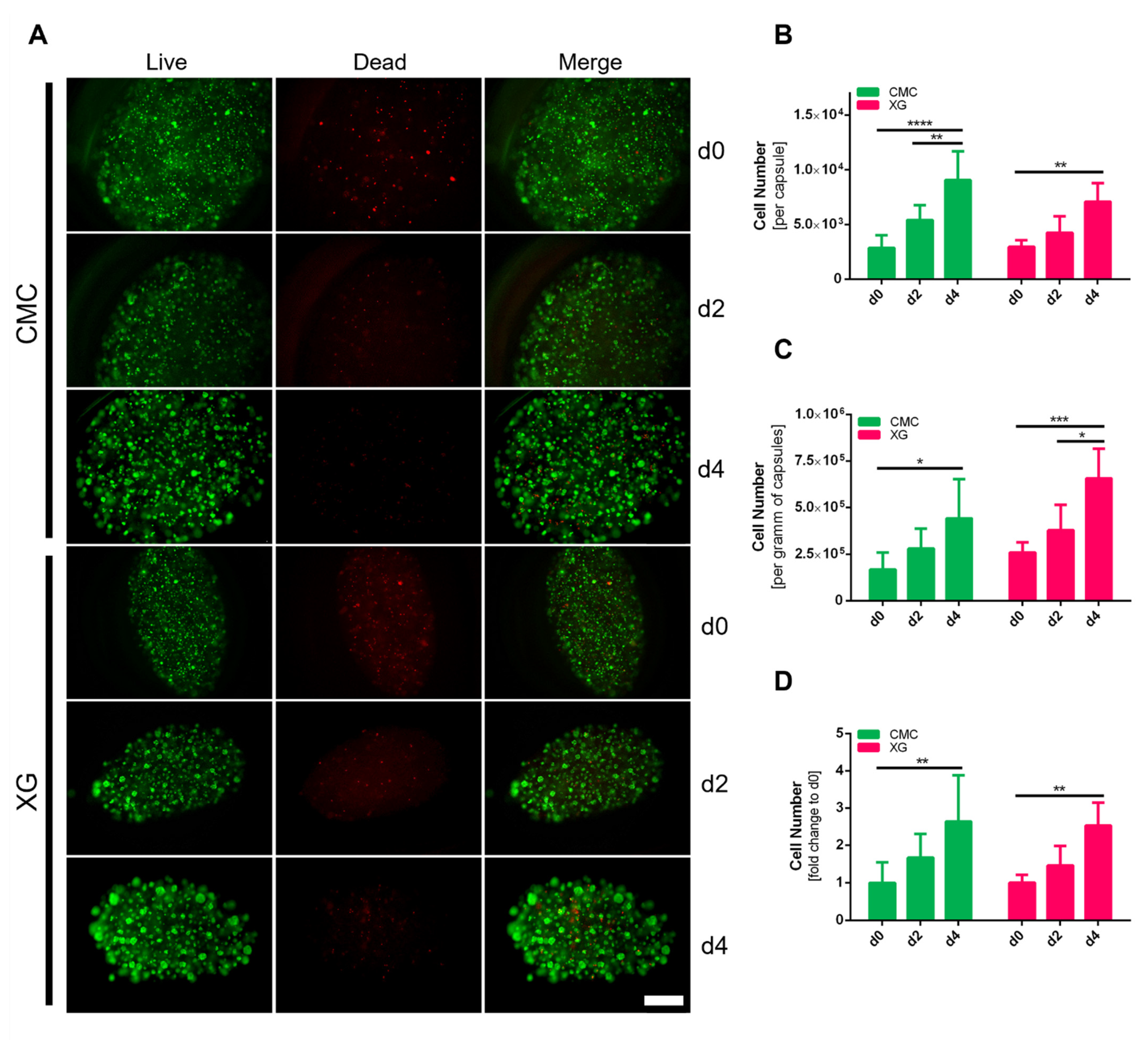
2.10. Live/Dead Staining
2.11. Statistical Analysis
3. Results
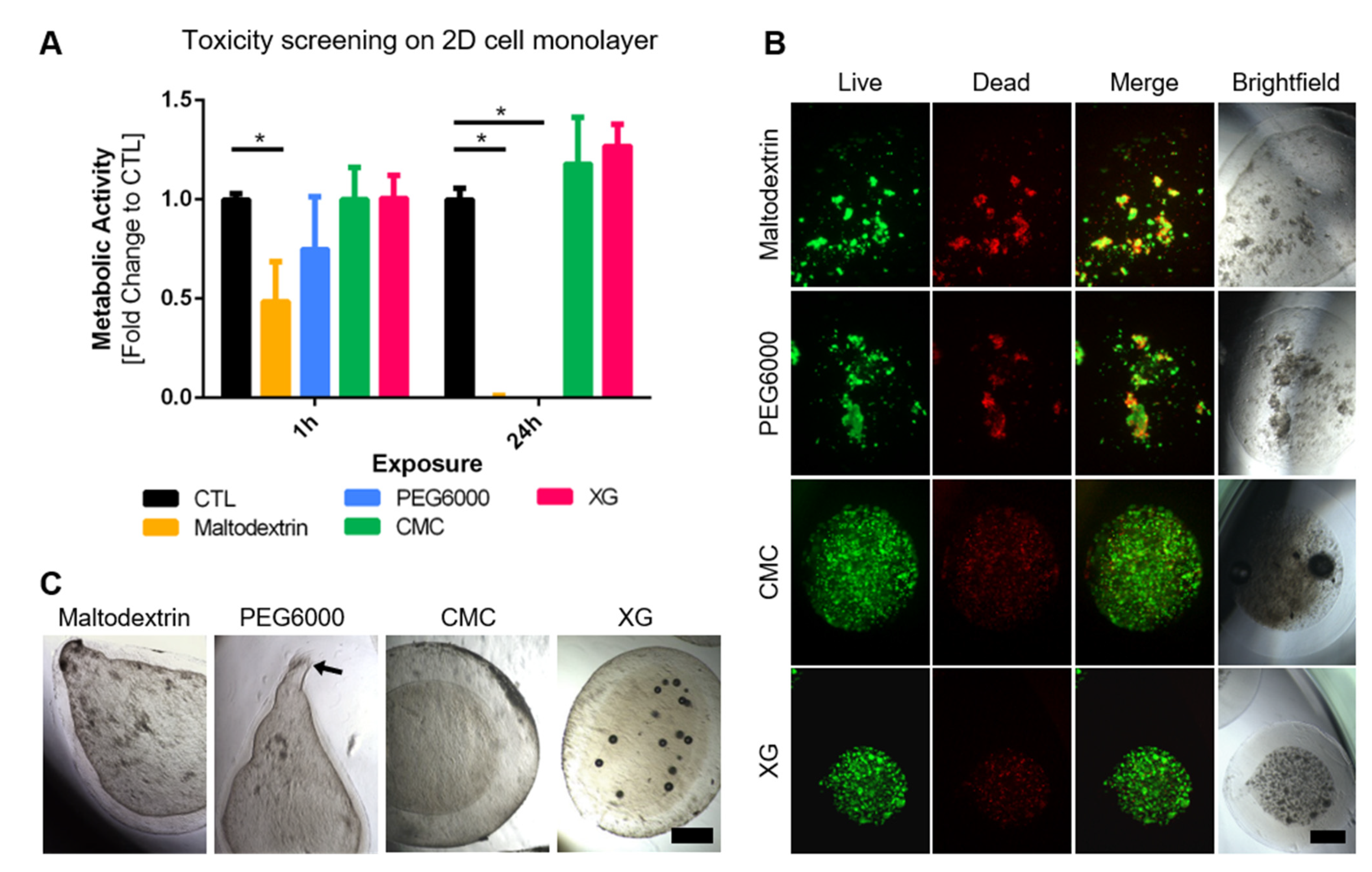
3.1. Tuning Viscosity of Capsule Interior
3.2. Encapsulation Procedure
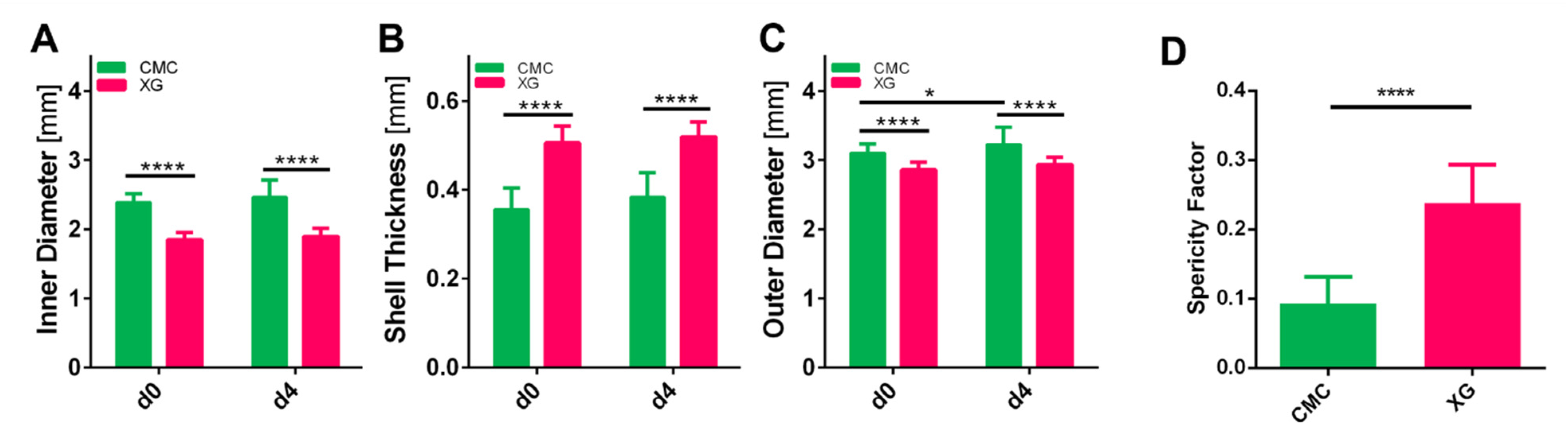
3.3. Assessment of Capsule Size and Shape
3.4. Capsule Characterization
3.5. Recovery of adMSCs from Core–Shell Alginate Capsules
3.6. Evaluation of Encapsulated adMSC Proliferation and Viability
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Disclosure
Conflicts of Interest
References
- Wu, M.; Han, Z.B.; Liu, J.F.; Wang, Y.W.; Zhang, J.Z.; Li, C.T.; Xin, P.L.; Han, Z.C.; Zhu, X.P. Serum-free media and the immunoregulatory properties of mesenchymal stem cells in vivo and in vitro. Cell. Physiol. Biochem. 2014, 33, 569–580. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Jones, E.; Yang, X. Mesenchymal stem cells and bone regeneration: Current status. Injury 2011, 42, 562–568. [Google Scholar] [CrossRef]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytom. Part A 2018, 93, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Bartosh, T.J.; Ylostalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef] [Green Version]
- Frith, J.E.; Thomson, B.; Genever, P.G. Dynamic Three-Dimensional Culture Methods Enhance Mesenchymal Stem Cell Properties and Increase Therapeutic Potential. Tissue Eng. Part C Methods 2010, 16, 735–749. [Google Scholar] [CrossRef]
- Kinney, M.A.; Sargent, C.Y.; McDevitt, T.C. The Multiparametric Effects of Hydrodynamic Environments on Stem Cell Culture. Tissue Eng. Part B Rev. 2011, 17, 249–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, P.; McAree, M.; Chang, W.; Yu, X. Mechanical forces in musculoskeletal tissue engineering. In Regenerative Engineering of Musculoskeletal Tissues and Interfaces; Elsevier: Amsterdam, The Netherlands, 2015; pp. 77–93. ISBN 9781782423140. [Google Scholar] [CrossRef]
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A tense situation: Forcing tumour progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar] [CrossRef]
- Maia, F.R.; Lourenço, A.H.; Granja, P.L.; Gonçalves, R.M.; Barrias, C.C. Effect of Cell Density on Mesenchymal Stem Cells Aggregation in RGD-Alginate 3D Matrices under Osteoinductive Conditions. Macromol. Biosci. 2014, 14, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.C.; Silva, D.N.; Moreira, A.F.; Correia, I.J. Optical clearing methods: An overview of the techniques used for the imaging of 3D spheroids. Biotechnol. Bioeng. 2019, 116, 2742–2763. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.J.R.; Luquet, E.; Pletenka, J.; Leonard, A.; Warter, E.; Gurchenkov, B.; Carrere, J.; Rieu, C.; Hardouin, J.; Moncaubeig, F.; et al. C-Stem: Engineering Niche-Like Micro-Compartments for Optimal and Scale-Independent Expansion of Human Pluripotent Stem Cells in Bioreactors. bioRxiv 2021. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Fu, D.-J.; An, D.; Chiu, A.; Schwartz, R.; Nikitin, A.Y.; Ma, M. Scalable Production and Cryostorage of Organoids Using Core-Shell Decoupled Hydrogel Capsules. Adv. Biosyst. 2017, 1, 1700165. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.K.; Son, Y.M.; Lee, N.-E. Hydrogel Encapsulation of Cells in Core-Shell Microcapsules for Cell Delivery. Adv. Healthc. Mater. 2015, 4, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Rabanel, J.-M.; Banquy, X.; Zouaoui, H.; Mokhtar, M.; Hildgen, P. Progress Technology in Microencapsulation Methods for Cell Therapy. Am. Inst. Chem. Eng. Biotechnol. Prog. 2009, 25, 946–963. [Google Scholar] [CrossRef]
- O’Shea, G.M.; Goosen, M.F.A.; Sun, A.M. Prolonged survival of transplanted islets of Langerhans encapsulated in a biocompatible membrane. Biochim. Biophys. Acta-Mol. Cell Res. 1984, 804, 133–136. [Google Scholar] [CrossRef]
- Sanandiya, N.D.; Vasudevan, J.; Das, R.; Lim, C.T.; Fernandez, J.G. Stimuli-responsive injectable cellulose thixogel for cell encapsulation. Int. J. Biol. Macromol. 2019, 130, 1009–1017. [Google Scholar] [CrossRef]
- Siltanen, C.; Diakataou, M.; Lowen, J.; Haque, A.; Rahimian, A.; Stybayeva, G.; Revzin, A. One step fabrication of hydrogel microcapsules with hollow core for assembly and cultivation of hepatocyte spheroids. Acta Biomater. 2017, 50, 428–436. [Google Scholar] [CrossRef] [Green Version]
- Alessandri, K.; Sarangi, B.R.; Gurchenkov, V.V.; Sinha, B.; Kiessling, T.R.; Fetler, L.; Rico, F.; Scheuring, S.; Lamaze, C.; Simon, A.; et al. Cellular capsules as a tool for multicellular spheroid production and for investigating the mechanics of tumor progression in vitro. Proc. Natl. Acad. Sci. USA 2013, 110, 14843–14848. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Ni, C.; Grist, S.M.; Bayly, C.; Cheung, K.C. Alginate core-shell beads for simplified three-dimensional tumor spheroid culture and drug screening. Biomed. Microdevices 2015, 17, 33. [Google Scholar] [CrossRef]
- Ma, M.; Chiu, A.; Sahay, G.; Doloff, J.C.; Dholakia, N.; Thakrar, R.; Cohen, J.; Vegas, A.; Chen, D.; Bratlie, K.M.; et al. Core–Shell Hydrogel Microcapsules for Improved Islets Encapsulation. Adv. Healthc. Mater. 2013, 2, 667–672. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhao, S.; Rao, W.; Snyder, J.; Choi, J.K.; Wang, J.; Khan, I.A.; Saleh, N.B.; Mohler, P.J.; Yu, J.; et al. A novel core–shell microcapsule for encapsulation and 3D culture of embryonic stem cells. J. Mater. Chem. B 2013, 1, 1002–1009. [Google Scholar] [CrossRef]
- Fattahi, P.; Rahimian, A.; Slama, M.Q.; Gwon, K.; Gonzalez-Suarez, A.M.; Wolf, J.; Baskaran, H.; Duffy, C.D.; Stybayeva, G.; Peterson, Q.P.; et al. Core-shell hydrogel microcapsules enable formation of human pluripotent stem cell spheroids and their cultivation in a stirred bioreactor. Sci. Rep. 2021, 11, 7177. [Google Scholar] [CrossRef] [PubMed]
- Leong, J.-Y.; Lam, W.-H.; Ho, K.-W.; Voo, W.-P.; Lee, M.F.-X.; Lim, H.-P.; Lim, S.-L.; Tey, B.-T.; Poncelet, D.; Chan, E.-S. Advances in fabricating spherical alginate hydrogels with controlled particle designs by ionotropic gelation as encapsulation systems. Particuology 2016, 24, 44–60. [Google Scholar] [CrossRef]
- Bezbaruah, A.N.; Shanbhogue, S.S.; Simsek, S.; Khan, E. Encapsulation of iron nanoparticles in alginate biopolymer for trichloroethylene remediation. J. Nanopart. Res. 2011, 13, 6673–6681. [Google Scholar] [CrossRef]
- Koyama, K.; Seki, M. Cultivation of Yeast and Plant Cells Entrapped in the Low-Viscous Liquid-Core of an Alginate Membrane Capsule Prepared Using Polyethylene Glycol. J. Biosci. Bioeng. 2004, 97, 111–118. [Google Scholar] [CrossRef]
- Nussinovitch, A.; Gershon, Z.; Nussinovitch, M. Liquid-core hydrocolloid capsules. Food Hydrocoll. 1996, 10, 21–26. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, J.D.; Mettu, S.; Dagastine, R.R. Precise measurements of capsule mechanical properties using indentation. Soft Matter 2017, 13, 1943–1947. [Google Scholar] [CrossRef]
- Leick, S.; Kemper, A.; Rehage, H. Alginate/poly-l-lysine capsules: Mechanical properties and drug release characteristics. Soft Matter 2011, 7, 6684. [Google Scholar] [CrossRef]
- Lee, B.-B.; Ibrahim, R.; Chu, S.-Y.; Zulkifli, N.A.; Ravindra, P. Alginate liquid core capsule formation using the simple extrusion dripping method. J. Polym. Eng. 2015, 35, 311–318. [Google Scholar] [CrossRef]
- Pereda, M.; Poncelet, D.; Renard, D. Characterization of Core-Shell Alginate Capsules. Food Biophys. 2019, 14, 467–478. [Google Scholar] [CrossRef]
- Wang, C.X.; Cowen, C.; Zhang, Z.; Thomas, C.R. High-speed compression of single alginate microspheres. Chem. Eng. Sci. 2005, 60, 6649–6657. [Google Scholar] [CrossRef]
- Park, H.-S.; Kim, J.-W.; Lee, S.-H.; Yang, H.K.; Ham, D.-S.; Sun, C.-L.; Hong, T.H.; Khang, G.; Park, C.-G.; Yoon, K.-H. Antifibrotic effect of rapamycin containing polyethylene glycol-coated alginate microcapsule in islet xenotransplantation. J. Tissue Eng. Regen. Med. 2017, 11, 1274–1284. [Google Scholar] [CrossRef]
- Lakey, J.; Botvinick, E.; Najdahmadi, A. Diffusion coefficient of alginate microcapsules used in pancreatic islet transplantation, a method to cure type 1 diabetes. In Proceedings of the Conference on Nanoscale Imaging, Sensing, and Actuation for Biomedical Applications XV, San Francisco, CA, USA, 30–31 January 2018; Cartwright, A.N., Nicolau, D.V., Fixler, D., Eds.; Society of Photo-Optical Instrumentation Engineers (SPIE): Bellingham, WA, USA, 2018; p. 49. [Google Scholar] [CrossRef]
- Chen, H.; Ouyang, W.; Martoni, C.; Prakash, S. Genipin Cross-Linked Polymeric Alginate-Chitosan Microcapsules for Oral Delivery: In-Vitro Analysis. Int. J. Polym. Sci. 2009, 2009, 617184. [Google Scholar] [CrossRef]
- Sart, S.; Tsai, A.-C.; Li, Y.; Ma, T. Three-Dimensional Aggregates of Mesenchymal Stem Cells: Cellular Mechanisms, Biological Properties, and Applications. Tissue Eng. Part B Rev. 2014, 20, 365–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passanha, F.R.; Gomes, D.B.; Piotrowska, J.; Moroni, L.; Baker, M.B.; LaPointe, V.L.S. A comparative study of mesenchymal stem cells cultured as cell-only aggregates and in encapsulated hydrogels. J. Tissue Eng. Regen. Med. 2021, 16, 14–25. [Google Scholar] [CrossRef]
- Freyei, J.P.; Sutherland, R.M. Regulation of Growth Saturation and Development of Necrosisin EMT6/R0 Multicellular Spheroids by the Glucose and Oxygen Supplyl. Cancer Res. 1986, 46, 3504–3512. [Google Scholar]
- Mineda, K.; Feng, J.; Ishimine, H.; Takada, H.; Doi, K.; Kuno, S.; Kinoshita, K.; Kanayama, K.; Kato, H.; Mashiko, T.; et al. Therapeutic Potential of Human Adipose-Derived Stem/Stromal Cell Microspheroids Prepared by Three-Dimensional Culture in Non-Cross-Linked Hyaluronic Acid Gel. Stem Cells Transl. Med. 2015, 4, 1511–1522. [Google Scholar] [CrossRef]
- Park, J.; Choe, G.; Oh, S.; Lee, J.Y. In Situ Formation of Proangiogenic Mesenchymal Stem Cell Spheroids in Hyaluronic Acid/Alginate Core-Shell Microcapsules. ACS Biomater. Sci. Eng. 2020, 6, 6938–6948. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 6th ed.; Garland Science: New York, NY, USA, 2014; Volume 20, ISBN 1317563751. [Google Scholar]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef] [Green Version]
- Segura, T.; Anderson, B.C.; Chung, P.H.; Webber, R.E.; Shull, K.R.; Shea, L.D. Crosslinked hyaluronic acid hydrogels: A strategy to functionalize and pattern. Biomaterials 2005, 26, 359–371. [Google Scholar] [CrossRef]
- Hollabaugh, C.B.; Burt, L.H.; Walsh, A.P. Carboxymethylcellulose. Uses and Applications. Ind. Eng. Chem. 1945, 37, 943–947. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of xanthan gum as polysaccharide in tissue engineering: A review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Jossen, V.; van den Bos, C.; Eibl, R.; Eibl, D. Manufacturing human mesenchymal stem cells at clinical scale: Process and regulatory challenges. Appl. Microbiol. Biotechnol. 2018, 102, 3981–3994. [Google Scholar] [CrossRef] [Green Version]
- Nold, P. Role of impeller design for the cultivation of stem cells in stirred-tank bioreactors. Cytotherapy 2019, 21, S80. [Google Scholar] [CrossRef]
- Reumann, M.; Linnemann, C.; Aspera-Werz, R.; Arnold, S.; Held, M.; Seeliger, C.; Nussler, A.; Ehnert, S. Donor Site Location Is Critical for Proliferation, Stem Cell Capacity, and Osteogenic Differentiation of Adipose Mesenchymal Stem/Stromal Cells: Implications for Bone Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 1868. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.J.; Kim, K.J.; Kim, M.K.; Lee, S.J.; Ryu, Y.H.; Seo, B.F.; Oh, D.Y.; Ahn, S.T.; Lee, H.Y.; Rhie, J.W. The Stem Cell Potential and Multipotency of Human Adipose Tissue-Derived Stem Cells Vary by Cell Donor and Are Different from Those of Other Types of Stem Cells. Cells Tissues Organs 2014, 199, 373–383. [Google Scholar] [CrossRef]
- Bijonowski, B.M.; Daraiseh, S.I.; Yuan, X.; Ma, T. Size-Dependent Cortical Compaction Induces Metabolic Adaptation in Mesenchymal Stem Cell Aggregates. Tissue Eng. Part A 2019, 25, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Strunk, D.; Koh, M.B.C.; Schallmoser, K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 2016, 76, 371–387. [Google Scholar] [CrossRef]
- van der Valk, J. Fetal bovine serum (FBS): Past—Present—Future. ALTEX 2018, 35, 99–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nebel, S.; Lux, M.; Kuth, S.; Bider, F.; Dietrich, W.; Egger, D.; Boccaccini, A.R.; Kasper, C. Alginate Core–Shell Capsules for 3D Cultivation of Adipose-Derived Mesenchymal Stem Cells. Bioengineering 2022, 9, 66. https://doi.org/10.3390/bioengineering9020066
Nebel S, Lux M, Kuth S, Bider F, Dietrich W, Egger D, Boccaccini AR, Kasper C. Alginate Core–Shell Capsules for 3D Cultivation of Adipose-Derived Mesenchymal Stem Cells. Bioengineering. 2022; 9(2):66. https://doi.org/10.3390/bioengineering9020066
Chicago/Turabian StyleNebel, Sabrina, Manuel Lux, Sonja Kuth, Faina Bider, Wolf Dietrich, Dominik Egger, Aldo R. Boccaccini, and Cornelia Kasper. 2022. "Alginate Core–Shell Capsules for 3D Cultivation of Adipose-Derived Mesenchymal Stem Cells" Bioengineering 9, no. 2: 66. https://doi.org/10.3390/bioengineering9020066
APA StyleNebel, S., Lux, M., Kuth, S., Bider, F., Dietrich, W., Egger, D., Boccaccini, A. R., & Kasper, C. (2022). Alginate Core–Shell Capsules for 3D Cultivation of Adipose-Derived Mesenchymal Stem Cells. Bioengineering, 9(2), 66. https://doi.org/10.3390/bioengineering9020066