Development of Novel Tetrapyrrole Structure Photosensitizers for Cancer Photodynamic Therapy
Abstract
:1. Introduction
2. The Meaning and Mechanism of Cell Damage during Photodynamic Therapy
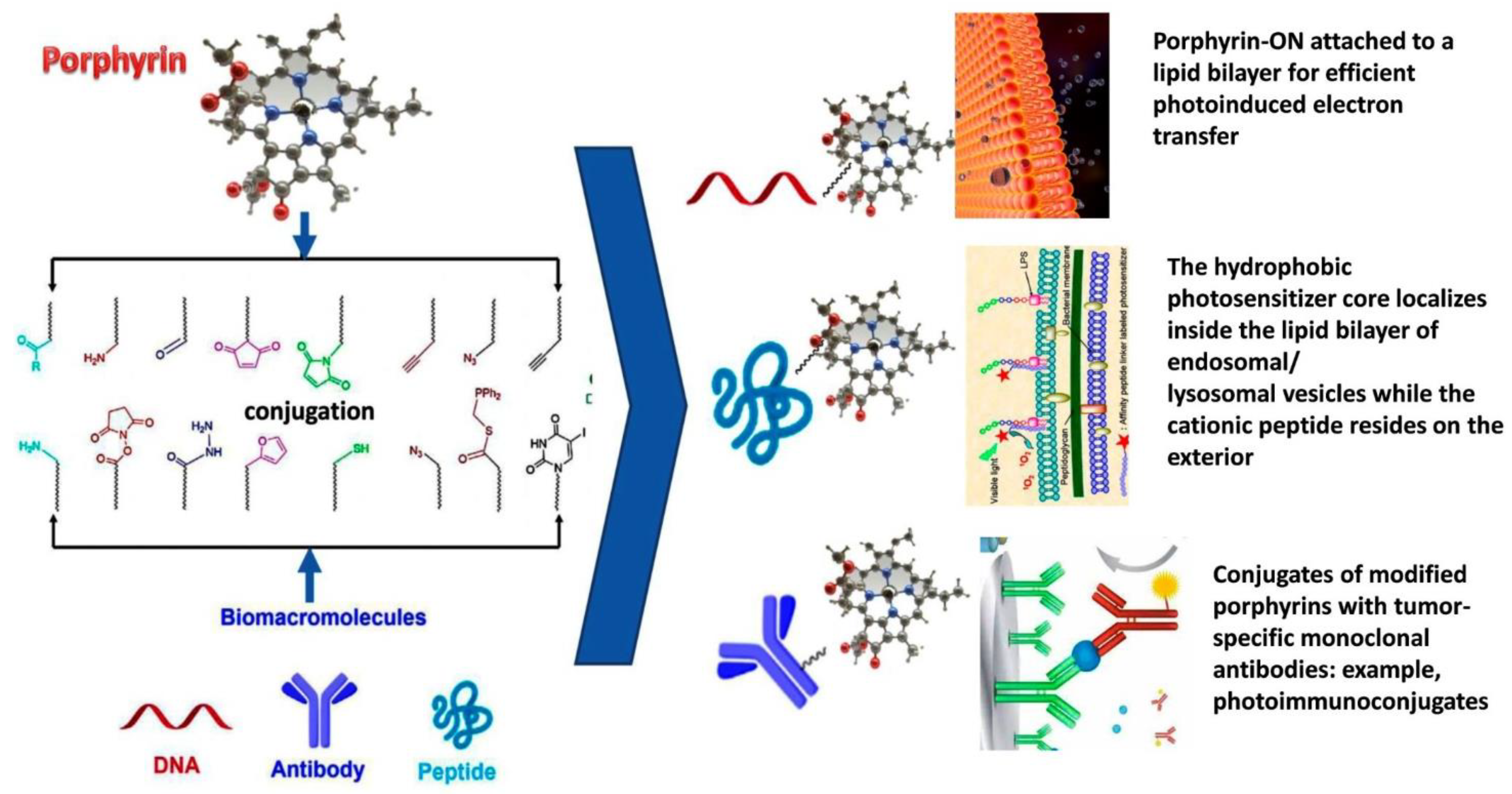
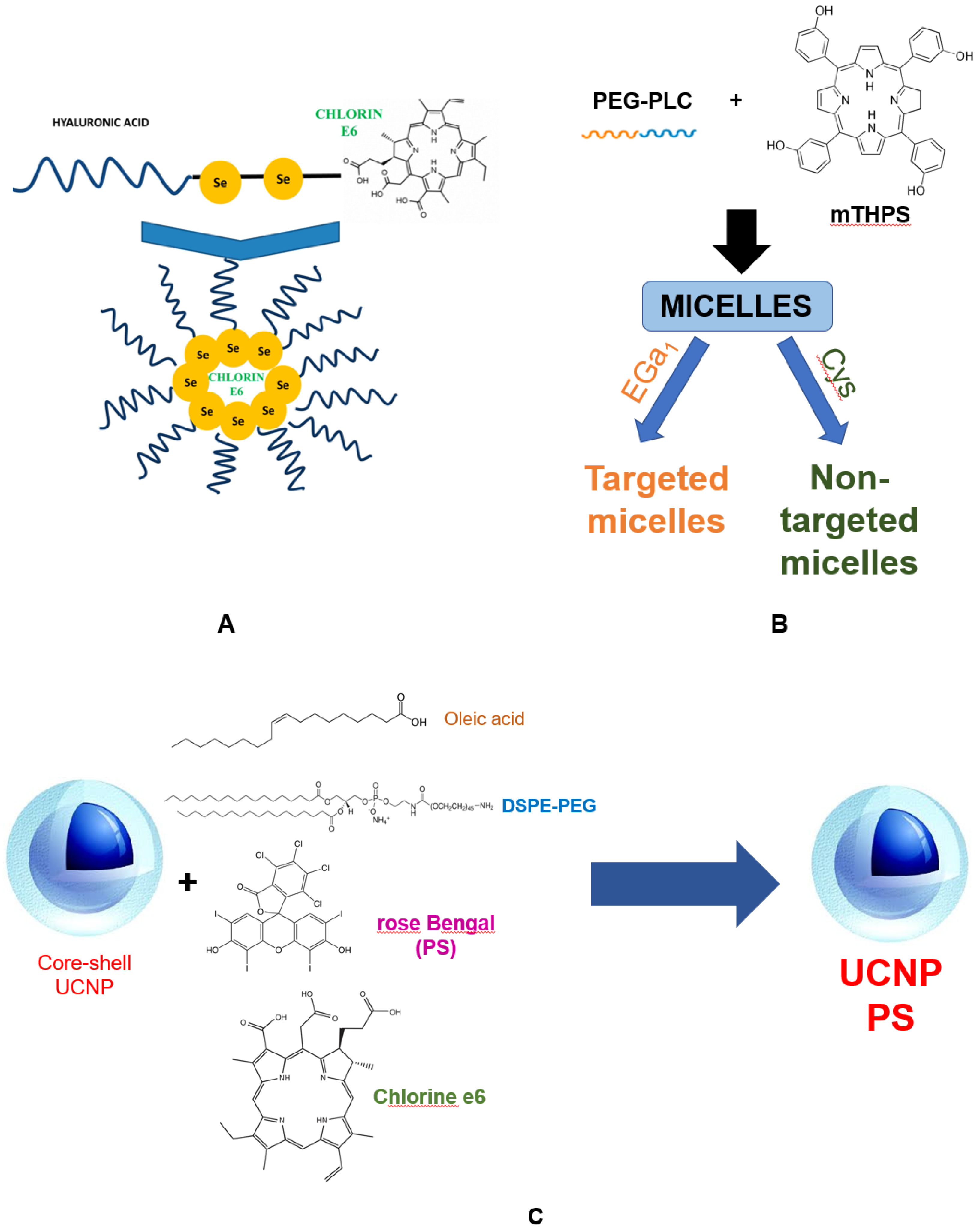
3. Porphyrin Photosensitizers for Cancer Photodynamic Therapy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Raghupathi, W.; Raghupathi, V. An empirical study of chronic diseases in the United States: A visual analytics approach to public health. Int. J. Environ. Res. Public Health 2018, 15, 431. [Google Scholar] [CrossRef] [Green Version]
- Oleinick, N.L.; Morris, R.L.; Belichenko, I. The role of apoptosis in response to photodynamic therapy: What, where, why, and how. Photochem. Photobiol. Sci. 2002, 1, 1–21. [Google Scholar] [CrossRef]
- Krammer, B. Vascular effects of photodynamic therapy. Anticancer Res. 2001, 21, 4271–4277. [Google Scholar] [PubMed]
- Zhang, X.H.; Zhang, L.J.; Sun, J.J.; Yan, Y.J.; Zhang, L.X.; Chen, N.; Chen, Z.L. Photodynamic efficiency of a chlorophyll-a derivative in vitro and in vivo. Biomed. Pharmacother. 2016, 81, 265–272. [Google Scholar]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Jiang, C.; Longo, J.P.F.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sin. 2018, 8, 137–146. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591. [Google Scholar] [CrossRef] [Green Version]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Biophysical and Biological Features of Third Generation Photosensitizers Used in Anticancer Photodynamic Therapy. Photodiagn. Photodyn. Ther. 2021, 102091. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.; Li, J.Z.; Shim, Y.K. Advance in photosensitizers and light delivery for photodynamic therapy. Clin. Endosc. 2013, 46, 7. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part two—Cellular signaling, cell metabolism and modes of cell death. Photodiagn. Photodyn. Ther. 2005, 2, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Weishaupt, K.R.; Gomer, C.J.; Dougherty, T.J. Identification of singlet oxygen as the cytotoxic agent in photo-inactivation of a murine tumor. Cancer Res. 1976, 36, 2326–2329. [Google Scholar] [PubMed]
- Henderson, B.W.; Miller, A.C. Effects of scavengers of reactive oxygen and radical species on cell survival following photodynamic treatment in vitro: Comparison to ionizing radiation. Radiat. Res. 1986, 108, 196–205. [Google Scholar] [CrossRef]
- Grune, T.; Klotz, L.O.; Gieche, J.; Rudeck, M.; Sies, H. Protein oxidation and proteolysis by the nonradical oxidants singlet oxygen or peroxynitrite. Free Radic. Biol. Med. 2001, 30, 1243–1253. [Google Scholar] [CrossRef]
- Sigdestad, C.P.; Fingar, V.H.; Wieman, T.J.; Lindberg, R.D. Chemical modification of normal tissue damage induced by photodynamic therapy. Br. J. Cancer Suppl. 1996, 27, S89. [Google Scholar] [PubMed]
- Cadet, J.; Davies, K.J.; Medeiros, M.H.; Di Mascio, P.; Wagner, J.R. Formation and repair of oxidatively generated damage in cellular DNA. Free Radic. Biol. Med. 2017, 107, 13–34. [Google Scholar] [CrossRef]
- Huang, H.; Banerjee, S.; Qiu, K.; Zhang, P.; Blacque, O.; Malcomson, T.; Sadler, P.J. Targeted photoredox catalysis in cancer cells. Nat. Chem. 2019, 11, 1041–1048. [Google Scholar] [CrossRef]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef]
- McCaughan, B.; Rouanet, C.; Fowley, C.; Nomikou, N.; McHale, A.P.; McCarron, P.A.; Callan, J.F. Enhanced ROS production and cell death through combined photo-and sono-activation of conventional photosensitising drugs. Bioorganic Med. Chem. Lett. 2011, 21, 5750–5752. [Google Scholar] [CrossRef]
- Callmann, C.E.; LeGuyader, C.L.; Burton, S.T.; Thompson, M.P.; Hennis, R.; Barback, C.; Gianneschi, N.C. Antitumor activity of 1, 18-octadecanedioic acid-paclitaxel complexed with human serum albumin. J. Am. Chem. Soc. 2019, 141, 11765–11769. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, M.E.; Zhang, P.; Azizuddin, K.; Delos Santos, G.B.; Chiu, S.M.; Xue, L.Y.; Oleinick, N.L. Structural factors and mechanisms underlying the improved photodynamic cell killing with silicon phthalocyanine photosensitizers directed to lysosomes versus mitochondria. Photochem. Photobiol. 2009, 85, 1189–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez De Pinillos Bayona, A.; Mroz, P.; Thunshelle, C.; Hamblin, M.R. Design features for optimization of tetrapyrrole macrocycles as antimicrobial and anticancer photosensitizers. Chem. Biol. Drug Des. 2019, 89, 192–206. [Google Scholar] [CrossRef] [Green Version]
- Tasso, T.T.; Schlothauer, J.C.; Junqueira, H.C.; Matias, T.A.; Araki, K.; Liandra-Salvador, E.; Baptista, M.S. Photobleaching efficiency parallels the enhancement of membrane damage for porphyrazine photosensitizers. J. Am. Chem. Soc. 2019, 141, 15547–15556. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic therapy review: Principles, photosensitizers, applications, and future directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Zhang, Q.C.; Da Rocha, S.T.; Flynn, R.A.; Bharadwaj, M.; Calabrese, J.M.; Chang, H.Y. Systematic discovery of Xist RNA binding proteins. Cell 2015, 161, 404–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgerald, F. (Ed.) Photodynamic Therapy (PDT): Principles, Mechanisms and Applications; Nova Biomedical: Waltham, MA, USA, 2017. [Google Scholar]
- Lee, C.N.; Hsu, R.; Chen, H.; Wong, T.W. Daylight photodynamic therapy: An update. Molecules 2020, 25, 5195. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Huang, Y. Imaging in Photodynamic Therapy; Taylor Francis Group: Boca Raton, FL, USA, 2017. [Google Scholar]
- Privalov, V.A.; Lappa, A.V.; Kochneva, E.V. Five years’ experience of photodynamic therapy with new chlorin photosensitizer. In Therapeutic Laser Applications and Laser-Tissue Interactions. Int. Soc. Opt. Photonics 2005, 5863, 586310. [Google Scholar] [CrossRef]
- Kessel, D. Apoptosis, paraptosis and autophagy: Death and survival pathways associated with photodynamic therapy. Photochem. Photobiol. 2019, 95, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Golab, J. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Targeting immunogenic cancer cell death by photodynamic therapy: Past, present and future. J. Immunother. Cancer 2021, 9. [Google Scholar] [CrossRef]
- Pucelik, B.; Sułek, A.; Dąbrowski, J.M. Bacteriochlorins and their metal complexes as NIR-absorbing photosensitizers: Properties, mechanisms, and applications. Coord. Chem. Rev. 2020, 416, 213340. [Google Scholar] [CrossRef]
- Pinel, S.; Thomas, N.; Boura, C.; Barberi-Heyob, M. Approaches to physical stimulation of metallic nanoparticles for glioblastoma treatment. Adv. Drug Deliv. Rev. 2019, 138, 344–357. [Google Scholar] [CrossRef]
- Pushpan, S.K.; Venkatraman, S.; Anand, V.G.; Sankar, J.; Parmeswaran, D.; Ganesan, S.; Chandrashekar, T.K. Porphyrins in photodynamic therapy-a search for ideal photosensitizers. Curr. Med. Chem.-Anti-Cancer Agents 2002, 2, 187–207. [Google Scholar] [CrossRef]
- Turubanova, V.D.; Balalaeva, I.V.; Mishchenko, T.A.; Catanzaro, E.; Alzeibak, R.; Peskova, N.N.; Krysko, D.V. Immunogenic cell death induced by a new photodynamic therapy based on photosens and photodithazine. J. Immunother. Cancer 2019, 7, 1–13. [Google Scholar] [CrossRef]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Zhang, H. Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef] [PubMed]
- Chizenga, E.P.; Abrahamse, H. Nanotechnology in modern photodynamic therapy of cancer: A review of cellular resistance patterns affecting the therapeutic response. Pharmaceutics 2020, 12, 632. [Google Scholar] [CrossRef]
- Tsukagoshi, K.; Wakayama, S.; Oto, K.; Takaoka, S.; Murase, K.; Gamo, K. Magnetotransport through disordered and anisotropic antidot lattices in GaAs/Alx Ga1−x as heterostructures. Phys. Rev. B 1995, 52, 8344. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.; Wu, Y.H.; Levine, Z.A.; Gundersen, M.A.; Vernier, P.T. Water influx and cell swelling after nanosecond electropermeabilization. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 1715–1722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Liu, Y.; Wang, L.; Ruan, X.; Wang, F.; Xu, D.; Liu, D. Evaluation on short-term therapeutic effect of 2 porphyrin Photosensitizer-Mediated photodynamic therapy for esophageal cancer. Technol. Cancer Res. Treat. 2019, 18. [Google Scholar] [CrossRef] [PubMed]
- Betsy, K.J.; Lazar, A.; Pavithran, A.; Vinod, C.P. CO2 Hydrogenation to Formate by Palladium Nanoparticles Supported on N-Incorporated Periodic Mesoporous Organosilica. ACS Sustain. Chem. Eng. 2020, 8, 14765–14774. [Google Scholar] [CrossRef]
- Miura, Y.; Takenaka, T.; Toh, K.; Wu, S.; Nishihara, H.; Kano, M.R.; Kataoka, K. Cyclic RGD-linked polymeric micelles for targeted delivery of platinum anticancer drugs to glioblastoma through the blood–brain tumor barrier. ACS Nano 2013, 7, 8583–8592. [Google Scholar] [CrossRef]
- Qiu, X.; Yu, H.; Karunakaran, M.; Pradeep, N.; Nunes, S.P.; Peinemann, K.V. Selective separation of similarly sized proteins with tunable nanoporous block copolymer membranes. ACS Nano 2013, 7, 768–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathak, P.; Zarandi, M.A.; Zhou, X.; Jayawickramarajah, J. Synthesis and Applications of Porphyrin-Biomacromolecule Conjugates. Front. Chem. 2021, 983. [Google Scholar] [CrossRef]
- Alonso, C.; Ross, W.B. Bioconjugates of porphyrins and related molecules for photodynamic Therapy. Handb. Porphyr. Sci. 2010, 122–190. [Google Scholar]
- Williams, T.M.; Sibrian-Vazquez, M.; Vicente, M.G.H. Design and synthesis of photosensitizer-peptide conjugates for PDT. In Handbook of Photodynamic Therapy: Updates on Recent Applications of Porphyrin-Based Compounds; World Scientific Publishers: Singapore, 2016; pp. 45–93. [Google Scholar]
- Dognini, P.; Coxon, C.R.; Alves, W.A.; Giuntini, F. Peptide-Tetrapyrrole Supramolecular Self-Assemblies: State of the Art. Molecules 2021, 26, 693. [Google Scholar] [CrossRef]
- Devaraj, N.K. The future of bioorthogonal chemistry. ACS Cent. Sci. 2018, 4, 952–959. [Google Scholar] [CrossRef] [Green Version]
- Aubert, N.; Troiani, V.; Gross, M.; Solladié, N. Novel porphyrinic peptides with assigned sequence of metallated chromophores, a further step towards redox switches. Tetrahedron Lett. 2002, 43, 8405–8408. [Google Scholar] [CrossRef]
- Aziat, F.; Rein, R.; Peón, J.; Rivera, E.; Solladié, N. Polypeptides with pendant porphyrins of defined sequence of chromophores: Towards artificial photosynthetic systems. J. Porphyr. Phthalocyanines 2008, 12, 1232–1241. [Google Scholar] [CrossRef]
- Solladié, N.; Hamel, A.; Gross, M. Synthesis of multiporphyrinic α-polypeptides: Towards the study of the migration of an excited state for the mimicking of the natural light harvesting device. Tetrahedron Lett. 2002, 41, 6075–6078. [Google Scholar] [CrossRef]
- Nair, V.S.; Pareek, Y.; Karunakaran, V.; Ravikanth, M.; Ajayaghosh, A. Cyclotriphosphazene appended porphyrins and fulleropyrrolidine complexes as supramolecular multiple photosynthetic reaction centers: Steady and excited states photophysical investigation. Phys. Chem. Chem. Phys. 2014, 16, 10149–10156. [Google Scholar] [CrossRef]
- Zhou, X.; Pathak, P.; Jayawickramarajah, J. Design, synthesis, and applications of DNA—Macrocyclic host conjugates. Chem. Commun. 2018, 54, 11668–11680. [Google Scholar] [CrossRef] [PubMed]
- Mew, D.; Lum, V.; Wat, C.K.; Towers, G.H.N.; Sun, C.C.; Walter, R.J.; Levy, J.G. Ability of specific monoclonal antibodies and conventional antisera conjugated to hematoporphyrin to label and kill selected cell lines subsequent to light activation. Cancer Res. 1985, 45, 4380–4386. [Google Scholar] [PubMed]
- Jiang, F.N.; Liu, D.J.; Neyndorff, H.; Chester, M.; Jiang, S.Y.; Levy, J.G. Photodynamic killing of human squamous cell carcinoma cells using a monoclonal antibody-photosensitizer conjugate. JNCI J. Natl. Cancer Inst. 1991, 83, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Berki, T.; Németh, P. Photo-immunotargeting with haematoporphyrin conjugates activated by a low-power He-Ne laser. Cancer Immunology. Immunotherapy 1992, 35, 69–74. [Google Scholar] [CrossRef]
- Pogrebniak, H.W.; Matthews, W.; Black, C.; Russo, A.; Mitchell, J.B.; Smith, P.; Pass, H.I. Targetted phototherapy with sensitizer-monoclonal antibody conjugate and light. Surg. Oncol. 1993, 2, 31–42. [Google Scholar] [CrossRef]
- Hemming, A.W.; Davis, N.L.; Dubois, B.; Quenville, N.F.; Finley, R.J. Photodynamic therapy of squamous cell carcinoma. An evaluation of a new photosensitizing agent, benzoporphyrin derivative and new photoimmunoconjugate. Surg. Oncol. 1993, 2, 187–196. [Google Scholar] [CrossRef]
- Jiang, F.N.; Richter, A.M.; Jain, A.K.; Levy, J.G.; Smits, C. Biodistribution of a benzoporphyrin derivative-monoclonal antibody conjugate in A549-tumor-bearing nude mice. Biotechnol. Ther. 1993, 4, 43–61. [Google Scholar]
- Goff, B.A.; Hermanto, U.; Rumbaugh, J.; Blake, J.; Bamberg, M.; Hasan, T. Photoimmunotherapy and biodistribution with an OC125-chlorin immunoconjugate in an in vivo murine ovarian cancer model. Br. J. Cancer 1994, 70, 474–480. [Google Scholar] [CrossRef] [Green Version]
- Linares, R.; Pacheco, J.R.; Good, T.A. Efficacy of different targeting agents in the photolysis of interleukin-2 receptor bearing cells. J. Photochem. Photobiol. B Biol. 2004, 77, 17–26. [Google Scholar] [CrossRef]
- Deonarain, M.; Stamati, I.; Yahioglu, G. Antibody-targeted photodynamic therapy. Mol. Cell. Ther. 2012, 4, 103–124. [Google Scholar]
- Boyle, R.W.; Xie, L.Y.; Dolphin, D. Meso-phenyl substituted porphocyanines: A new class of functionalized expanded porphyrins. Tetrahedron Lett. 1994, 35, 5377–5380. [Google Scholar] [CrossRef]
- Smith, K.; Malatesti, N.; Cauchon, N.; Hunting, D.; Lecomte, R.; van Lier, J.E.; Boyle, R.W. Mono-and tri-cationic porphyrin–monoclonal antibody conjugates: Photodynamic activity and mechanism of action. Immunology 2011, 132, 256–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, P.M.; Carvalho, J.J.; Silva, S.; Cavaleiro, J.A.; Schneider, R.J.; Fernandes, R.; Tomé, J.P. Porphyrin conjugated with serum albumins and monoclonal antibodies boosts efficiency in targeted destruction of human bladder cancer cells. Org. Biomol. Chem. 2014, 12, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Maruani, A.; Savoie, H.; Bryden, F.; Caddick, S.; Boyle, R.; Chudasama, V. Site-selective multi-porphyrin attachment enables the formation of a next-generation antibody-based photodynamic therapeutic. Chem. Commun. 2015, 51, 15304–15307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinoda, Y.; Kujirai, K.; Aoki, K.; Morita, M.; Masuda, M.; Zhang, L.; Fujiwara, Y. Novel Photosensitizer β-Mannose-Conjugated Chlorin e6 as a Potent Anticancer Agent for Human Glioblastoma U251 Cells. Pharmaceuticals 2020, 13, 316. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yang, M.; Zhang, J.; Zhu, S.; Shi, M.; Wang, K. Metalloporphyrin-indomethacin conjugates as new photosensitizers for photodynamic therapy. JBIC J. Biol. Inorg. Chem. 2019, 24, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, H.; Yang, M.; Wu, F. Palladium porphyrin complexes for photodynamic cancer therapy: Effect of porphyrin units and metal. Photochem. Photobiol. Sci. 2020, 19, 905–912. [Google Scholar] [CrossRef]
- Dudek-Perić, A.M.; Ferreira, G.B.; Muchowicz, A.; Wouters, J.; Prada, N.; Martin, S.; Agostinis, P. Antitumor immunity triggered by melphalan is potentiated by melanoma cell surface–associated calreticulin. Cancer Res. 2015, 75, 1603–1614. [Google Scholar] [CrossRef] [Green Version]
- McCaughan, J.S. Photodynamic therapy. Drugs Aging 1999, 15, 49–68. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Rompicharla, S.V.K.; Bhatt, H.; Ghosh, B.; Biswas, S. Development of chlorin e6-conjugated poly (ethylene glycol)-poly (d, l-lactide) nanoparticles for photodynamic therapy. Nanomedicine 2019, 14, 819–834. [Google Scholar] [CrossRef]
- Liu, J.; Liu, K.; Feng, L.; Liu, Z.; Xu, L. Comparison of nanomedicine-based chemotherapy, photodynamic therapy and photothermal therapy using reduced graphene oxide for the model system. Biomater. Sci. 2017, 5, 331–340. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, R.; Kim, E.; Lee, S.; Park, Y.I. Near-infrared light-triggered photodynamic therapy and apoptosis using upconversion nanoparticles with dual photosensitizers. Front. Bioeng. Biotechnol. 2020, 8, 275. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, D.; Wu, H.; Fu, Y.; Cao, Y.; Zhang, Y.; Yang, S. Functionalized Cu3BiS3 nanoparticles for dual-modal imaging and targeted photothermal/photodynamic therapy. Nanoscale 2018, 10, 4452–4462. [Google Scholar] [CrossRef]
- Xu, D.; Baidya, A.; Deng, K.; Li, Y.S.; Wu, B.; Xu, H.B. Multifunctional nanoparticle PEG-Ce6-Gd for MRI-guided photodynamic therapy. Oncol. Rep. 2021, 45, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Simões, J.C.; Sarpaki, S.; Papadimitroulas, P.; Therrien, B.; Loudos, G. Conjugated photosensitizers for imaging and PDT in cancer research. J. Med. Chem. 2020, 63, 14119–14150. [Google Scholar] [CrossRef] [PubMed]
- Peppicelli, S.; Andreucci, E.; Ruzzolini, J.; Bianchini, F.; Calorini, L. FDG uptake in cancer: A continuing debate. Theranostics 2020, 10, 2944. [Google Scholar] [CrossRef]
- Kataoka, H.; Nishie, H.; Tanaka, M.; Sasaki, M.; Nomoto, A.; Osaki, T.; Yano, S. Potential of Photodynamic Therapy Based on Sugar-Conjugated Photosensitizers. J. Clin. Med. 2021, 10, 841. [Google Scholar] [CrossRef]
- McCarthy, J.R.; Bhaumik, J.; Karver, M.R.; Erdem, S.S.; Weissleder, R. Targeted nanoagents for the detection of cancers. Mol. Oncol. 2010, 4, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Tang, X.; He, J.; Yi, X.; Xu, G.; Tian, L.; Yang, K. Polydopamine nanoparticle as a multifunctional nanocarrier for combined radiophotodynamic therapy of cancer. Part. Part. Syst. Charact. 2017, 34, 1600296. [Google Scholar] [CrossRef]
- Kostryukova, L.V.; Prozorovskiy, V.N.; Medvedeva, N.V.; Ipatova, O.M. Comparison of a new nanoform of the photosensitizer chlorin e6, based on plant phospholipids, with its free form. FEBS Open Bio 2018, 8, 201. [Google Scholar] [CrossRef] [Green Version]
- Gomaa, I.; Sebak, A.; Afifi, N.; Abdel-Kader, M. Liposomal delivery of ferrous chlorophyllin: A novel third generation photosensitizer for in vitro PDT of melanoma. Photodiagn. Photodyn. Ther. 2017, 18, 162–170. [Google Scholar] [CrossRef]
- Galliani, M.; Signore, G. Poly (Lactide-Co-Glycolide) Nanoparticles Co-Loaded with Chlorophyllin and Quantum Dots as Photodynamic Therapy Agents. ChemPlusChem 2019, 84, 1653–1658. [Google Scholar] [CrossRef]
- Yu, W.; Zhen, W.; Zhang, Q.; Li, Y.; Luo, H.; He, J.; Liu, Y. Porphyrin-Based Metal-Organic Framework Compounds as Promising Nanomedicines in Photodynamic Therapy. ChemMedChem 2020, 15, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Yang, Z.; Deng, T.; Chen, J.; Wen, Y.; Fu, X.; Yu, C. A natural polysaccharide mediated MOF-based Ce6 delivery system with improved biological properties for photodynamic therapy. J. Mater. Chem. B 2020, 8, 1481–1488. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plekhova, N.; Shevchenko, O.; Korshunova, O.; Stepanyugina, A.; Tananaev, I.; Apanasevich, V. Development of Novel Tetrapyrrole Structure Photosensitizers for Cancer Photodynamic Therapy. Bioengineering 2022, 9, 82. https://doi.org/10.3390/bioengineering9020082
Plekhova N, Shevchenko O, Korshunova O, Stepanyugina A, Tananaev I, Apanasevich V. Development of Novel Tetrapyrrole Structure Photosensitizers for Cancer Photodynamic Therapy. Bioengineering. 2022; 9(2):82. https://doi.org/10.3390/bioengineering9020082
Chicago/Turabian StylePlekhova, Natalia, Olga Shevchenko, Oksana Korshunova, Aleksandra Stepanyugina, Ivan Tananaev, and Vladimir Apanasevich. 2022. "Development of Novel Tetrapyrrole Structure Photosensitizers for Cancer Photodynamic Therapy" Bioengineering 9, no. 2: 82. https://doi.org/10.3390/bioengineering9020082
APA StylePlekhova, N., Shevchenko, O., Korshunova, O., Stepanyugina, A., Tananaev, I., & Apanasevich, V. (2022). Development of Novel Tetrapyrrole Structure Photosensitizers for Cancer Photodynamic Therapy. Bioengineering, 9(2), 82. https://doi.org/10.3390/bioengineering9020082





