Smartphone-Based Biosensor Devices for Healthcare: Technologies, Trends, and Adoption by End-Users
Abstract
:1. Introduction
2. Current State of Art and Trends in Smartphone-Based Sensors Field
3. Overview of Reviewed Technologies by Type of Sensors and Commercial Stage
3.1. Electrochemical Smartphone-Based Biosensors
3.2. Optical Smartphone-Based Biosensors
3.3. Paper-Based Biosensors That Uses Smartphones
4. Sensors at Commercial Stage
5. Technology to the Market
5.1. FDA Regulatory Compliance and CLINICAL Trials Positive Results
5.2. Technical Limitations of the Technology
5.3. User Adoption Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rackus, D.G.; Shamsi, M.H.; Wheeler, A.R. Electrochemistry, biosensors and microfluidics: A convergence of fields. Chem. Soc. Rev. 2015, 44, 5320–5340. [Google Scholar] [CrossRef]
- Zarei, M. Portable biosensing devices for point-of-care diagnostics: Recent developments and applications. TrAC-Trends Anal. Chem. 2017, 91, 26–41. [Google Scholar] [CrossRef]
- Kang, M.; Park, E.; Cho, B.H.; Lee, K. Recent patient health monitoring platforms incorporating internet of things-enabled smart devices. Int. Neurourol. J. 2018, 22, 76–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Miedany, Y. Telehealth and telemedicine: How the digital era is changing standard health care. Smart Homecare Technol. TeleHealth 2017, 4, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Sun, A.C.; Hall, D.A. Point-of-Care Smartphone-based Electrochemical Biosensing. Electroanalysis 2019, 31, 2–16. [Google Scholar] [CrossRef] [Green Version]
- Ray, T.R.; Choi, J.; Bandodkar, A.J.; Krishnan, S.; Gutruf, P.; Tian, L.; Ghaffari, R.; Rogers, J.A. Bio-integrated wearable systems: A comprehensive review. Chem. Rev. 2019, 119, 5461–5533. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Ajami, S.; Teimouri, F. Features and application of wearable biosensors in medical care. J. Res. Med. Sci. 2015, 20, 1208–1215. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jia, W.; Wang, J. Tattoo-Based Wearable Electrochemical Devices: A Review. Electroanalysis 2015, 27, 562–572. [Google Scholar] [CrossRef]
- Nag, A.; Mukhopadhyay, S.C.; Kosel, J. Wearable Flexible Sensors: A Review. IEEE Sens. J. 2017, 17, 3949–3960. [Google Scholar] [CrossRef] [Green Version]
- Tamsin, M. Wearable Biosensor Technologies. Int. J. Innov. Sci. Res. 2015, 13, 697–703. [Google Scholar]
- Rodrigues, D.; Barbosa, A.I.; Rebelo, R.; Kwon, I.K.; Reis, R.L.; Correlo, V.M. Skin-integrated wearable systems and implantable biosensors: A comprehensive review. Biosensors 2020, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.; Fortunato, G.; Radacsi, N. Wearable flexible sweat sensors for healthcare monitoring: A review. J. R. Soc. Interface 2019, 16, 20190217. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned Paper as a Platform for Inexpensive, Low-Volume, Portable Bioassays. Angew. Chem. 2007, 119, 1340–1342. [Google Scholar] [CrossRef]
- Liu, J.; Geng, Z.; Fan, Z.; Liu, J.; Chen, H. Point-of-care testing based on smartphone: The current state-of-the-art (2017–2018). Biosens. Bioelectron. 2019, 132, 17–37. [Google Scholar] [CrossRef]
- Halpern, M.; Zhu, Y.; Reddi, V.J. Mobile CPU’s rise to power: Quantifying the impact of generational mobile CPU design trends on performance, energy, and user satisfaction. In Proceedings of the 2016 IEEE International Symposium on High Performance Computer Architecture (HPCA), Barcelona, Spain, 12–16 March 2016; pp. 64–76. [Google Scholar] [CrossRef]
- Ginny; Kumar, C.; Naik, K. Smartphone Processor Architecture, Operations, and Functions: Current State-of-the-Art and Future Outlook: Energy Performance Trade-Off. J. Supercomput. 2021, 77, 2411–2502. [Google Scholar] [CrossRef]
- Wang, H.; Heintzmann, R.; Diederich, B. The power in your pocket-uncover smartphones for use as cutting-edge microscopic instruments in science and research. Adv. Opt. Technol. 2021, 10, 89–108. [Google Scholar] [CrossRef]
- Steinberg, M.D.; Kassal, P.; Steinberg, I.M. System Architectures in Wearable Electrochemical Sensors. Electroanalysis 2016, 28, 1149–1169. [Google Scholar] [CrossRef]
- Sun, A.; Venkatesh, A.G.; Hall, D.A. A Multi-Technique Reconfigurable Electrochemical Biosensor: Enabling Personal Health Monitoring in Mobile Devices. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Guo, J. Uric acid monitoring with a smartphone as the electrochemical analyzer. Anal. Chem. 2016, 88, 11986–11989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, D.; Sun, A.C. Reconfigurable, Multi-Technique Electrochemical Portable Biosensor. U.S. Patent No. 11,166,653, 9 November 2021. [Google Scholar]
- Sun, A.C.; Yao, C.; Ag, V.; Hall, D.A. An efficient power harvesting mobile phone-based electrochemical biosensor for point-of-care health monitoring. Sens. Actuators B Chem. 2016, 235, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Aronoff-Spencer, E.; Venkatesh, A.G.; Sun, A.; Brickner, H.; Looney, D.; Hall, D.A. Detection of Hepatitis C core antibody by dual-affinity yeast chimera and smartphone-based electrochemical sensing. Biosens. Bioelectron. 2016, 86, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.; Hsu, C.-L.; Sun, A.; Zhao, Y.; Aronoff-Spencer, E. Electrochemical Biosensor Array Devices, Systems, and Methods for Point-of-Care Detection. U.S. Patent Application No. 16/976,464, 25 March 2021. [Google Scholar]
- Guo, J. Smartphone-Powered Electrochemical Dongle for Point-of-Care Monitoring of Blood β-Ketone. Anal. Chem. 2017, 89, 8609–8613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Lu, Y.; Zhang, Q.; Liu, L.; Li, S.; Yao, Y.; Jiang, J.; Liu, G.L.; Liu, Q. Protein detecting with smartphone-controlled electrochemical impedance spectroscopy for point-of-care applications. Sens. Actuators B Chem. 2016, 222, 994–1002. [Google Scholar] [CrossRef]
- Raman, K.; Salahandish, R.; Wang, G.; Bhat, S.; Vastarey, N.S.; Kapoor, A.S. Portable Electrochemical-Sensor System for Analyzing User Health Conditions and Method Thereof. U.S. Patent Application No. 17/261,476, 2 September 2021. [Google Scholar]
- Bandodkar, A.J.; Imani, S.; Nuñez-Flores, R.; Kumar, R.; Wang, C.; Mohan, A.M.V.; Wang, J.; Mercier, P.P. Re-usable electrochemical glucose sensors integrated into a smartphone platform. Biosens. Bioelectron. 2018, 101, 181–187. [Google Scholar] [CrossRef]
- Aymerich, J.; Márquez, A.; Terés, L.; Muñoz-Berbel, X.; Jiménez, C.; Domínguez, C.; Serra-Graells, F.; Dei, M. Cost-effective smartphone-based reconfigurable electrochemical instrument for alcohol determination in whole blood samples. Biosens. Bioelectron. 2018, 117, 736–742. [Google Scholar] [CrossRef]
- Shin Low, S.; Pan, Y.; Ji, D.; Li, Y.; Lu, Y.; He, Y.; Chen, Q.; Liu, Q. Smartphone-based portable electrochemical biosensing system for detection of circulating microRNA-21 in saliva as a proof-of-concept. Sens. Actuators B Chem. 2020, 308, 127718. [Google Scholar] [CrossRef]
- Abdolahad, M.; Miripour, Z.S.; NajafiKhoshnoo, S. Real-time and label free analyzer for in-vitro and in-vivo detecting of cancer. U.S. Patent No. US 20,180,299,401 A1, 18 October 2018. [Google Scholar]
- Miripour, Z.S.; Sarrami-Forooshani, R.; Sanati, H.; Makarem, J.; Taheri, M.S.; Shojaeian, F.; Eskafi, A.H.; Abbasvandi, F.; Namdar, N.; Ghafari, H.; et al. Real-time diagnosis of reactive oxygen species (ROS) in fresh sputum by electrochemical tracing; correlation between COVID-19 and viral-induced ROS in lung/respiratory epithelium during this pandemic. Biosens. Bioelectron. 2020, 165, 112435. [Google Scholar] [CrossRef]
- Abdolahad, M.; Miripour, Z.S.; Koloukhi, H.S.; Zanjani, F.Z.S.; Ghafari, H.; Habashi, N.N. Electrochemical Approach for COVID-19 Detection. U.S. Patent No. 10,845,336, 24 November 2020. [Google Scholar]
- Zhao, H.; Liu, F.; Xie, W.; Zhou, T.C.; OuYang, J.; Jin, L.; Li, H.; Zhao, C.Y.; Zhang, L.; Wei, J.; et al. Ultrasensitive supersandwich-type electrochemical sensor for SARS-CoV-2 from the infected COVID-19 patients using a smartphone. Sens. Actuators B Chem. 2021, 327, 128899. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R. Development of Point-of-Care Biosensors for COVID-19. Front. Chem. 2020, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Gowri, A.; Ashwin Kumar, N.; Suresh Anand, B.S. Recent advances in nanomaterials based biosensors for point of care (PoC) diagnosis of Covid-19—A minireview. TrAC-Trends Anal. Chem. 2021, 137, 116205. [Google Scholar] [CrossRef]
- Cui, F.; Zhou, H.S. Diagnostic methods and potential portable biosensors for coronavirus disease 2019. Biosens. Bioelectron. 2020, 165, 112349. [Google Scholar] [CrossRef] [PubMed]
- Abad-Valle, P.; Fernández-Abedul, M.T.; Costa-García, A. Genosensor on gold films with enzymatic electrochemical detection of a SARS virus sequence. Biosens. Bioelectron. 2005, 20, 2251–2260. [Google Scholar] [CrossRef] [PubMed]
- Mahshid, S.S.; Flynn, S.E.; Mahshid, S. The potential application of electrochemical biosensors in the COVID-19 pandemic: A perspective on the rapid diagnostics of SARS-CoV-2. Biosens. Bioelectron. 2021, 176, 112905. [Google Scholar] [CrossRef] [PubMed]
- Baraket, A.; Lee, M.; Zine, N.; Sigaud, M.; Bausells, J.; Errachid, A. A fully integrated electrochemical biosensor platform fabrication process for cytokines detection. Biosens. Bioelectron. 2017, 93, 170–175. [Google Scholar] [CrossRef]
- Hosu, O.; Lettieri, M.; Papara, N.; Ravalli, A.; Sandulescu, R.; Cristea, C.; Marrazza, G. Colorimetric multienzymatic smart sensors for hydrogen peroxide, glucose and catechol screening analysis. Talanta 2019, 204, 525–532. [Google Scholar] [CrossRef]
- Chan, W.; Ming, K. Wireless communication device-based detection system. WIPO Application Patent WO2014/089700 A1, 19 June 2014. [Google Scholar]
- Ming, K.; Kim, J.; Biondi, M.J.; Syed, A.; Chen, K.; Lam, A.; Ostrowski, M.; Rebbapragada, A.; Feld, J.J.; Chan, W.C.W. Integrated quantum dot barcode smartphone optical device for wireless multiplexed diagnosis of infected patients. ACS Nano 2015, 9, 3060–3074. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Sia, S.; Xie, S.; Meng, F.; Yeager, K. Diagnostic devices, systems, and methods. WIPO Application Patent WO 2016/025698 A1, 18 February 2016. [Google Scholar]
- Guo, T.; Patnaik, R.; Kuhlmann, K.; Rai, A.J.; Sia, S.K. Smartphone dongle for simultaneous measurement of hemoglobin concentration and detection of HIV antibodies. Lab Chip 2015, 15, 3514–3520. [Google Scholar] [CrossRef]
- Nicolini, A.M.; Fronczek, C.F.; Yoon, J.Y. Droplet-based immunoassay on a “sticky” nanofibrous surface for multiplexed and dual detection of bacteria using smartphones. Biosens. Bioelectron. 2015, 67, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.; Srivastava, N.; Yue, M.; Priye, A.; Nagle, R. Analytical Instrument Systems. U.S. Patent Application No. 776,567, 14 March 2014. [Google Scholar]
- Priye, A.; Bird, S.W.; Light, Y.K.; Ball, C.S.; Negrete, O.A.; Meagher, R.J. A smartphone-based diagnostic platform for rapid detection of Zika, chikungunya, and dengue viruses. Sci. Rep. 2017, 7, 44778. [Google Scholar] [CrossRef] [PubMed]
- Tenda, K.; Arts, R.; Citterio, D.; Tomimuro, K.; Merkx, M. Detection device for bioluminescent detection of biomarkers from a biological fluid sample using luminescent sensing protein. WIPO Application Patent WO 2019038375 A1, 28 February 2019. [Google Scholar]
- Arts, R.; Den Hartog, I.; Zijlema, S.E.; Thijssen, V.; Van Der Beelen, S.H.E.; Merkx, M. Detection of Antibodies in Blood Plasma Using Bioluminescent Sensor Proteins and a Smartphone. Anal. Chem. 2016, 88, 4525–4532. [Google Scholar] [CrossRef] [PubMed]
- Michelini, E.; Roda, A.; Dolci, L.; Cevenini, L.; Mezzanotte, L. Portable device based on immobilized cells for the detection of analytes. US Application Patent No. 045,835 A1, 23 February 2012. [Google Scholar]
- Michelini, E.; Calabretta, M.M.; Cevenini, L.; Lopreside, A.; Southworth, T.; Fontaine, D.M.; Simoni, P.; Branchini, B.R.; Roda, A. Smartphone-based multicolor bioluminescent 3D spheroid biosensors for monitoring inflammatory activity. Biosens. Bioelectron. 2019, 123, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, C.; Shi, K.; Edwards, P. Compact spectrometer including a diffractive optical element with dual dispersion and focusing functionality. US Patent No. US 8,861,086 B2, 14 October 2014. [Google Scholar]
- Edwards, P.; Zhang, C.; Zhang, B.; Hong, X.; Nagarajan, V.K.; Yu, B.; Liu, Z. Smartphone based optical spectrometer for diffusive reflectance spectroscopic measurement of hemoglobin. Sci. Rep. 2017, 7, 12224. [Google Scholar] [CrossRef] [Green Version]
- Edwards, P.; Liu, Z. Mobile reflectance optical spectroscopy device and process of using and assembling mobile reflectance optical spectroscopy device. US Application Patent No. US 2016/0296118 A1, 13 October 2016. [Google Scholar]
- Varghese, J.; Campagna, J.; Bilousova, T.; Spilman, P.; Heinzelman, P.; Hatami, A.; Alam, M. 3D-exoquant method for the analysis of surface molecules and quantification of tissue-specific exosomes in biological fluids. US Application Patent No. US 20190025330 A1, 24 January 2019. [Google Scholar]
- Liu, Y.; Liu, Q.; Chen, S.; Cheng, F.; Wang, H.; Peng, W. Surface plasmon resonance biosensor based on smart phone platforms. Sci. Rep. 2015, 5, 12864. [Google Scholar] [CrossRef] [Green Version]
- Rogers, J.; Reeder, J.; Bandodkar, A.; Kim, S.; Sekine, Y.; Choi, J.; Ray, T.; Hourlier-Fargette, A.; Gutruf, P.; Lee, K.; et al. Epidermalsensing systems for optical redout, visualization and analysis of biofluids. US Application Patent No. US20210145352 A1, 20 May 2021. [Google Scholar]
- Sekine, Y.; Kim, S.B.; Zhang, Y.; Bandodkar, A.J.; Xu, S.; Choi, J.; Irie, M.; Ray, T.R.; Kohli, P.; Kozai, N.; et al. A fluorometric skin-interfaced microfluidic device and smartphone imaging module for: In situ quantitative analysis of sweat chemistry. Lab Chip 2018, 18, 2178–2186. [Google Scholar] [CrossRef]
- Sandhu, A. Method of forming and visualizing latent image. US Application Patent No. US 20120141746 A1, 7 June 2012. [Google Scholar]
- Sharma, J.; Ono, T.; Yukino, R.; Miyashita, H.; Hanyu, N.; Handa, H.; Sandhu, A. Smartphone based platform for real-time sharing of medical diagnostics information by optical detection of functionalized fluorescent magnetic nanoparticles. Biomed. Phys. Eng. Express 2019, 5, 35014. [Google Scholar] [CrossRef]
- Sandhu, A. Marker for biosensor, biosensor, and marker detection method for biosensor. Japanise Application Patent No. JP2008128677A, 5 June 2008. [Google Scholar]
- Sandhu, A.; Sharma, J.; Takamura, T.; Yukino, R.; Hanyu, N.; Yasuno, H.; Hama, N.; Tanaka, T.; Handa, H. Biosensing method and device using magnetic particles. WIPO Application Patent No. WO2017141503 A1, 24 August 2017. [Google Scholar]
- Mahato, K.; Srivastava, A.; Chandra, P. Paper based diagnostics for personalized health care: Emerging technologies and commercial aspects. Biosens. Bioelectron. 2017, 96, 246–259. [Google Scholar] [CrossRef]
- Tian, T.; Wei, X.; Jia, S.; Zhang, R.; Li, J.; Zhu, Z.; Zhang, H.; Ma, Y.; Lin, Z.; Yang, J. Integration of target responsive hydrogel with cascaded enzymatic reactions and microfluidic paper-based analytic devices (mPADs) for point-of-care testing (POCT). Biosens. Bioelectron. 2015, 77, 537–542. [Google Scholar] [CrossRef]
- Carvalhal, R.F.; Kfouri, M.S.; De Piazetta, M.H.O.; Gobbi, A.L.; Kubota, L.T. Electrochemical detection in a paper-based separation device. Anal. Chem. 2010, 82, 1162–1165. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Carrilho, E.; Thomas, S.W.; Sindi, H.; Whitesides, G.M. Simple telemedicine for developing regions: Camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal. Chem. 2008, 80, 3699–3707. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Huang, X.; Guo, J.; Ma, X. Automatic smartphone-based microfluidic biosensor system at the point of care. Biosens. Bioelectron. 2018, 110, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-C.; Han, Y.-D.; Kim, J. Optical Probe for Bio-Sensor, Optical Bio-Sensor Including Optical Probe, and Method for Manufacturing Optical Probe for Bio-Sensor. U.S. Patent Application No. 16/065,147, 27 December 2018. [Google Scholar]
- Kim, S.; Chun, H.J.; Lee, K.W.; Yoon, H.C. Smartphone-integrated urinary CTX-II immunosensor based on wavelength filtering from chromogenic reaction. Biosens. Bioelectron. 2020, 150, 111932. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Chatterjee, S.; Patel, S.; Chakraborty, S. Smartphone Based Blood Hemoglobin Estimation System. WIPO Patent WO2021019553A1, 2020. [Google Scholar]
- Biswas, S.K.; Laha, S.; Chatterjee, S.; Pedireddi, V.M.; Patel, S.; Saha, S.; Chakraborty, S. System for Estimation of Plasma Glucose by Integrated Paper-Based Device and Method thereof. WIPO Patent WO/2021/019552, 17 July 2020. [Google Scholar]
- Biswas, S.K.; Chatterjee, S.; Bandyopadhyay, S.; Kar, S.; Som, N.K.; Saha, S.; Chakraborty, S. Smartphone-Enabled Paper-Based Hemoglobin Sensor for Extreme Point-of-Care Diagnostics. ACS Sens. 2021, 6, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Branchini, B.R.; Southworth, T.L.; DeAngelis, J.P.; Roda, A.; Michelini, E. Isolated luciferase gene of L. italica. U.S. Patent No 8,642,272, 4 February 2014. [Google Scholar]
- Calabretta, M.M.; Álvarez-Diduk, R.; Michelini, E.; Roda, A.; Merkoçi, A. Nano-lantern on paper for smartphone-based ATP detection. Biosens. Bioelectron. 2020, 150, 111902. [Google Scholar] [CrossRef]
- Ünal, B.; Camci-Unal, G.; Lantigua, D. Devices and Methods for Therapeutic drug monitoring. US Application Patent No. US20210382048 A1, 9 December 2021. [Google Scholar]
- Ünal, B.; Camci-Unal, G.; Mahmud, K. Paper-Based Microfluidic Devices: Low-Cost Platforms for Rapid Biochemical Detection. Mil. Med. 2021, 186, 716–721. [Google Scholar] [CrossRef]
- Pan, D.; Moitra, P.; Alafeef, M.M.S.; Dighe, K. Rapid Diagnostic System Using Targeted Antisense Oligonucleotide Capped Plasmonic Nanoparticles. U.S. Patent Application No. 17/325,839, 16 December 2021. [Google Scholar]
- Alafeef, M.; Dighe, K.; Moitra, P.; Pan, D. Rapid, Ultrasensitive, and Quantitative Detection of SARS-CoV-2 Using Antisense Oligonucleotides Directed Electrochemical Biosensor Chip. ACS Nano 2020, 14, 17028–17045. [Google Scholar] [CrossRef]
- Ruzgas, T.; Arnebrant, T.; Sotres, J.; Laiwattanapaisal, W.; Gonzalez, J.F.; Shafaat, A.; Ducpham, A.; Larpant, N. Improved Biosensor and Method for Manufacturing Such. WIPO Patent WO2019139537A1, 2019. [Google Scholar]
- Larpant, N.; Kalambate, P.K.; Ruzgas, T.; Laiwattanapaisal, W. Paper-based competitive immunochromatography coupled with an enzyme-modified electrode to enable the wireless monitoring and electrochemical sensing of cotinine in urine. Sensors 2021, 21, 1659. [Google Scholar] [CrossRef]
- De La Rica Quesada, R.; Patiño Francy, A.A.; Adrover-Jaume, C. Procedure for Storing and Releasing Protein-Decorated Nanoparticles on Paper Substrates. WIPO Patent Application WO/2021/048087, 2021. [Google Scholar]
- Adrover-Jaume, C.; Alba-Patiño, A.; Clemente, A.; Santopolo, G.; Vaquer, A.; Russell, S.M.; Barón, E.; González del Campo, M.; del Ferrer, J.M.; Berman-Riu, M.; et al. Paper biosensors for detecting elevated IL-6 levels in blood and respiratory samples from COVID-19 patients. Sens. Actuators B Chem. 2021, 330, 129333. [Google Scholar] [CrossRef]
- Philips Wireless Remote Sensing Device. Available online: https://www.usa.philips.com/healthcare/product/HC989803196871/wearable-biosensor-wireless-remote-sensing-device (accessed on 30 November 2021).
- EverSense, “Continuous Glucose Monitoring”. Available online: https://www.ascensiadiabetes.com/eversense/eversense-cgm-system (accessed on 30 November 2021).
- DeHennis, A. Continuous Analyte Monitoring System. US Patent 11,116,402 B2, 2021. [Google Scholar]
- Nemaura_Medical Meet sugarBEAT®. Available online: https://nemauramedical.com/#glucose-monitoring (accessed on 30 November 2021).
- EtectRX, I. etectRx. Available online: https://ichgcp.net/clinical-trials-registry/NCT03653897 (accessed on 30 November 2021).
- Euliano, N.R.; Príncipe, J.C.; Meka, V.V.; Stahl, M.W., Jr. Medication Compliance System and Associated Methods. U.S. Patent No. 7,796,043, 14 September 2010. [Google Scholar]
- PROTEUS DIGITAL HEALTH Smart Pill. Available online: https://pharma.nridigital.com/pharma_apr20/proteus_digital_health_a_sharp_lesson_for_smart_pills# (accessed on 30 November 2021).
- Atmo Biosciences Atmo Gas Sensing Capsule. Available online: https://atmobiosciences.com/#introducing (accessed on 30 November 2021).
- Kalantar-Zadeh, K.; Berean, K.; Ha, N.; Ou, J.Z. Gas Sensor Capsule 2018. WIPO Patent WO 2018/032032 A1, 2018. [Google Scholar]
- MIT News Ingestible “Bacteria on A Chip” Could Help Diagnose Disease. Available online: https://news.mit.edu/2018/ingestible-bacteria-on-a-chip-help-diagnose-disease-0524 (accessed on 30 December 2021).
- Mimee, M.; Nadeau, P.; Hayward, A.; Carim, S.; Flanagan, S.; Jerger, L.; Collins, J.; Mcdonnell, S.; Swartwout, R.; Citorik, R.J.; et al. An ingestible bacterial-electronic system to monitor gastrointestinal health. Science 2018, 918, 915–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuan-Ta Lu, T.; Mimee, M.K.; Nadeau, P.; Chandrakasan, A.P. Predictive Digital Autoranging Analog-to-Digital Converter. Patent Application Publication US 2019/0253069 A1, 2019. [Google Scholar]
- Profusa, I. Wireless Lumee® Oxygen Platform. Available online: https://profusa.com/lumee/ (accessed on 30 November 2021).
- Griss, R.; Schena, A.; Reymond, L.; Patiny, L.; Werner, D.; Tinberg, C.E.; Backer, D.; Johnsson, K. Bioluminescent sensor proteins for point-of-care therapeutic drug monitoring. Nat. Chem. Biol. 2014, 10, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, K.P.; Schena, A.; Griss, R. Means and Methods for Bioluminescence Resonance Energy Transfer (bret) Analysis in a Biological Sample. WIPO Patent Wo 2015/007317A1, 2015. [Google Scholar]
- MIT Tattoo Biosensor. Available online: https://www.media.mit.edu/projects/d-Abyss/overview/ (accessed on 30 October 2021).
- Gupta, S.K. Medical Device Regulations: A Current Perspective. J. Young Pharm. 2016, 8, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Manita; Deep, A.; Vikram; Rana, A.C.; Sharma, P.C. Regulation and Clinical Investigation of Medical Device in the European Union. Appl. Clin. Res. Clin. Trials Regul. Aff. 2019, 6, 163–181. [Google Scholar] [CrossRef]
- Contains Nonbinding Recommendations. The Least Burdensome Provisions: Concept and Principles Guidance for Industry and Food and Drug Administration Staff. 2019, 1–24. Available online: https://www.federalregister.gov/documents/2019/02/05/2019-01022/the-least-burdensome-provisions-concept-and-principles-guidance-for-industry-and-food-and-drug (accessed on 30 October 2021).
- FDA. Device Advice: Comprehensive Regulatory Assistance. Available online: https://www.fda.gov/medical-devices/device-advice-comprehensive-regulatory-assistance (accessed on 30 October 2021).
- Faris, O.; Shuren, J. An FDA Viewpoint on Unique Considerations for Medical-Device Clinical Trials. N. Engl. J. Med. 2017, 376, 1350–1357. [Google Scholar] [CrossRef] [Green Version]
- Zuckerman, D.M.; Brown, P.; Nissen, S.E. Medical device recalls and the FDA approval process. Arch. Intern. Med. 2011, 171, 1006–1011. [Google Scholar] [CrossRef] [Green Version]
- Cipriani, A.; Ioannidis, J.P.A.; Rothwell, P.M.; Glasziou, P.; Li, T.; Hernandez, A.F.; Tomlinson, A.; Simes, J.; Naci, H. Generating comparative evidence on new drugs and devices after approval. Lancet 2020, 395, 998–1010. [Google Scholar] [CrossRef]
- Rathi, V.K.; Krumholz, H.M.; Masoudi, F.A.; Ross, J.S. Characteristics of clinical studies conducted over the total product life cycle of high-risk therapeutic medical devices receiving FDA Premarket Approval in 2010 and 2011. JAMA 2015, 314, 604–612. [Google Scholar] [CrossRef]
- Van Norman, G.A. Drugs, Devices, and the FDA: Part 2: An Overview of Approval Processes: FDA Approval of Medical Devices. JACC Basic Transl. Sci. 2016, 1, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Taherdoost, H. A review of technology acceptance and adoption models and theories. Procedia Manuf. 2018, 22, 960–967. [Google Scholar] [CrossRef]
- Norman, D.A.; Drapen, S.W. User Centered System Design: New Perspectives on Human-Computer Interaction. Lawrence Erlbaum Associates Inc.: Mahwah, NJ, USA, 1986; ISBN 978-0-89859-781-3. [Google Scholar]
- Pateraki, M.; Fysarakis, K.; Sakkalis, V.; Spanoudakis, G.; Varlamis, I.; Maniadakis, M.; Lourakis, M.; Ioannidis, S.; Cummins, N.; Schuller, B.; et al. Biosensors and Internet of Things in smart healthcare applications: Challenges and opportunities. Wearable Implant. Med. Devices 2020, 7, 25–53. [Google Scholar] [CrossRef] [Green Version]
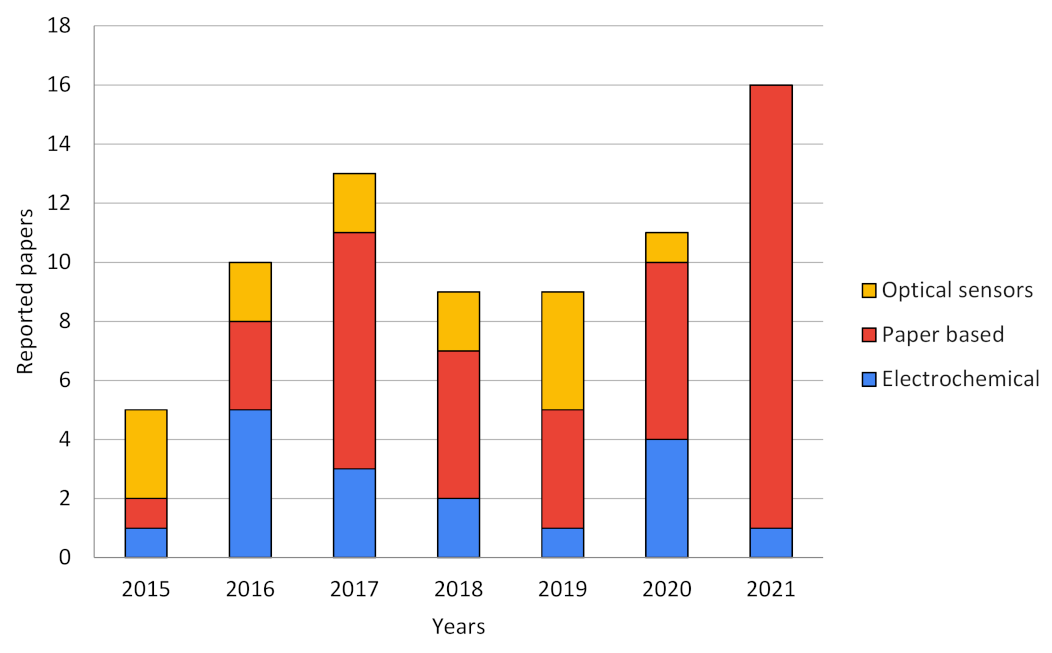
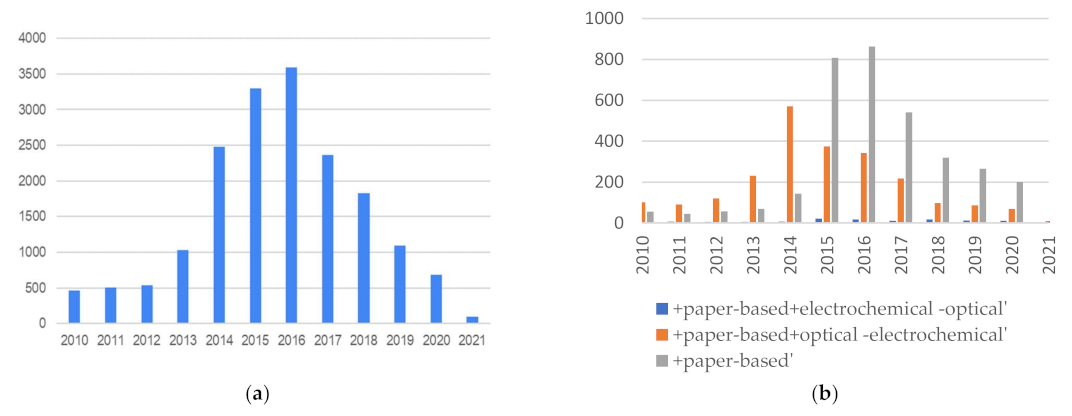
| Application | Biosensor Type | Evaluated in Real Samples? | Pat. Nº, Year, State | Improvements of Smart Sensor vs. Benchtop Techniques | Refs. |
|---|---|---|---|---|---|
| Secretory leukocyte protease inhibitor (SLPI) but can be applied to different applications | Immunological | No. Tested in solutions of different concentrations of the biomarker secretory leukocyte protease inhibitor (SLPI) | US11166653B2, 2016/2021 [24] | Electronic module containing a low-power potentiostat that interfaces efficiently with a wide variety of phones through the audio jack to obtain power and communicate. The system uses a microcontroller. Total power consumption: 6.9 mW. Compared with a commercial potentiostat: current from ±300 pA to ±20 µA with a 100 kΩ gain. It can be used to obtain voltammograms. The platform can be used with different brands of smartphones and allows the use of electrochemical biosensors for different applications. | [25,26] |
| US20210087614A1, 2019 Pending [27] | |||||
| Blood β-ketone (blood β-hydroxybutyrate) | Enzymatic: β-hydroxybutyrate dehydrogenase method | Yes. Tested in finger blood | Electrochemical dongle, which is powered by the smartphone through an OTG. It takes chronoamperometric measurements of blood ketone. Linear regression coefficient of 0.987 for a range of 0 to 4 mmol/L of blood β-hydroxybutyrate. The authors were able to demonstrate that the preciseness and stability of the measured data are highly reliable and applicable for clinical use. | [28] | |
| For protein detection: bull serum albumin (BSA) and thrombin | Immunological for BSA detection and Enzymatic for Thrombin detection | No. Tested with solutions of different concentrations of BSA and thrombin | Portable transducer and a handheld detector connected via Bluetooth to the smartphone. The detector can perform electrochemical impedance spectroscopy (EIS) (10 Hz to 10 kHz). The system can detect very low concentrations of BSA (1.78 µg/mL) and thrombin (2.97 ng/mL). They related the charge transfer resistance (Rct) with the concentration of BSA or thrombin. The smartphone delivers control commands, receive data signals, and display the Nyquist graph. A designed Android App serves as an interactive interface between the users and the biosensor system. It allows the use of other electrochemical biosensors. | [29] | |
| Glucose concentration | Enzyme-carbon composite pellets | No. Tested with solutions of different glucose concentrations | US20210270766A1, 2018 Pending [30] | Electrochemical sensor strips consist of carbon electrodes and a second part is composed of the carbon paste GOx biosensor, which can be replaced in each measurement. The biosensor is a compact carbon/GOx/rhodium pill. Measurement compartment: 3D-fabricated smartphone case with a permanently-attached passive sensor strip and a compartment where the biosensor magnetic pellet is placed for each measurement. They developed a portable potentiostat (Texas Instruments CC2541 BLE System on-Chip) communicated wirelessly with the smartphone. Android-based smartphone application developed. | [31] |
| Alcohol in whole blood samples | Enzymatic: two enzymes are used, HRP and alcohol oxidase | Yes. Tested with whole blood | The system combines a three-electrode microfluidic chip with a secondary compact PCB module as a µPotentiostat. Chronoamperometric and CV measurements. Communicated with the smartphone via USB. The novelty of the system is the reusable biosensor concept. Two enzymes, HRP and alcohol oxidase, are immobilized via in situ electrodeposition of a calcium alginate hydrogel for selective ethanol detection. A constant potential of 0 V was applied between WE and Pt RE. The smartphone acts as a simple graphical interface and for cloud connectivity. | [32] | |
| Cancer biomarker microRNAs (miRNAs) | Genetic: Tris(2-carboxyethyl)phosphine hydrochloride (TCEP)-treated ssDNA probe drop casted onto an rGO/Au composite-modified WE | No. Tested with miR-21 spiked artificial saliva | The system presents a circuit board as the potentiostat, powered through smartphone On-The-Go (OTG) port and a graphene oxide/gold composite-modified electrode as the biosensor. The circuit board communicates via Bluetooth with the smartphone. A specially designed Android application shows the results. The detection is facilitated via a synthetic ssDNA probe immobilized onto the GO/Au electrode. Good linearity (R2 = 0.99) for the detection of 1×10−4 M to 1×10−12 M of [miR-21]. The sample must be incubated at 40 °C for 1 h for hybridization before electrochemical measurement. | [33] | |
| Reactive oxygen species (ROS) for COVID-19 detection | MWCNTs on the tip of steel needles of 3 electrodes | Yes. Tested in Fresh sputum or bronchoalveolar lavage fluids | US11181499B2, 2017/2021 [34] | The system includes a previously patented electrochemical ROS/H2O2 system consisting of an electrochemical readout board (+/−0.8 mV, 100 mV s−1, and a sensing disposable sensor. The group presented an application Patent in 2020 for the electrochemical approach to detect COVID-19, which was granted in 2021. | [35] |
| US11047824B2, 2020/2021 [36] | |||||
| RNA from SARS-CoV-2 virus | Genetic: The sequences were provided by the Chinese Center for Disease Control and Prevention (CDC) | Yes. Tested with extracts from SARS-CoV-2-confirmed patients and recovered patients | It is an ultrasensitive electrochemical biosensor for the detection of the RNA of SARS-CoV-2 by using a smartphone. They used a super sandwich-type recognition strategy without the need for nucleic acid amplification and reverse transcription. For this biosensor, only two copies (10 μL) of SARS-CoV-2 were required per assay to detect a positive sample. Calibrated with concentrations between 10−17–10−12 M, LOD: 3 aM. LOD of the clinical specimen: 200 copies/mL, which was the lowest LOD among the published RNA measurement of SARS-CoV-2 at this moment | [37] |
| Application | Biosensor Type | Evaluated in Real Samples? | Pat. Nº, Year, State | Improvements of Smart Sensor vs. Benchtop Techniques | Ref. |
|---|---|---|---|---|---|
| H2O2 , Glucose and Catechol biosensor | Enzymatic: GOX and tyrosinase over poly(aniline-co-anthranilic acid) | Yes. Food and pharmaceutical samples | Polymeric substrate material and image processing software provided a great correlation with benchtop techniques and higher LOD. | [44] | |
| HIV and Hepatitis B biosensor | DNA/RNA-linked biosensor | Yes. Plasma samples | WO2014089700A1, 2013 Pending [45] | They were able to detect between 103 to 109 copies/mL over a 20 µL sample and differentiate patients with HIV from those with HBV on the mono-infection assay and multiplexed detection of both of them in a co-infection assay. The results were quite well-correlated compared to benchtop equipment measurements. | [46] |
| Hemoglobin and HIV biosensor | Immunosensor | Yes. Blood samples | WO2016025698A1, 2014 Pending [47] | It consists of a combined pure optical assay and an immunoassay at the same time, and in the same device, without a difficult procedure for handling samples and reagents. The results are in good agreement with their commercial equivalents supported by smartphone technologies. | [48] |
| E. coli and S. typhimurium biosensor | Immunosensor | No | For the first time, a device capable of detecting two genetically related bacteria within a single sample drop is reported, with a LOD of 10−2 CFU/mL, in a fairly short time (12 min), and with a good consistency in comparison with the results obtained in laboratory experiments. | [49] | |
| Zika, Dengue, Chikungunya detector | DNA/RNA-linked biosensor | No. Tested in artificial blood, urine, and saliva samples | US20160025630A1, 2014 Pending [50] | Detection technique that involves quenching of unincorporated amplification signal reporters (QUASR). Distinctively to other reported LAMP detection modalities, QUASR offers very bright signals, reduces the detection of false-positive amplification, and offers the ability to multiplex two or more targets per reaction. These features can highly reduce reagent costs and dilution needs when sample volume is limiting. A personalized smartphone application (app) controls the isothermal heating module and a LED excitation module via Bluetooth. The app processes images through a novel detection algorithm for multiplexed QUASR assay signals with greater accuracy than conventional image analysis software. | [51] |
| HIV1-p17, hemagglutinin (HA), and dengue virus type I detector | Bioluminiscent reporter | Yes. Blood plasma samples | WO2019038375A1, 2018 Pending [52] | The design shows to be an attractive analytical platform for point-of-care antibody detection that dispenses with liquid handling steps that are related to the major issues in immunoassays. | [53] |
| Inflammation and cell viability biosensors | Bioluminiscent reporters | No. Simulated proinflammatory and toxic samples. | US20120045835A1, 2009 Pending [54] | A limit of detection for tumor necrosis factor (TNFα) of 0.15 ± 0.05 ng/mL was achieved. This proposal promises to be a useful platform to preliminary screen environmental samples or other types of compounds for on-site detection. | [55] |
| Hemoglobin sensor | Label-free detection | No. Simulated samples. | US8861086B2, 2014 [56] | It stands out for its compact size, portability, low cost, the efficiency of optical spectroscopy for quantitative measurement, and ease of data collection, management, and computation. | [57] |
| US20160296118A1, 2015 Pending [58] | |||||
| Bovine immunoglobulinG (IgG) | Immunosensor | No. Spiked buffer solution of IgG protein | US20190025330A1, 2917 Pending [59] | In addition to the ability to detect immunoglobulins G, the device can be applied to the sensing of other analytes by properly functionalizing the gold film. The results and sensitivity obtained were comparable to commercial SPR instruments, so being a portable SPR system, it makes it an extremely useful device. | [60] |
| Chloride, sodium, and zinc in sweat | Fluorescence | Yes. Sweat | US20210145352A1, 2018 Pending [61] | Through an ultrathin, skin-compatible adhesive layer, the device allows sweat to be collected and distributed to different areas with fluorescent reagents. The device makes it possible to quantitatively determine, in a simple and low-cost device, several biomarkers of sweat at the same time. | [62] |
| Prostatespecific antigen (PSA) | Fluorescence | No. Spiked solution with PSA | US20120141746A1, 2009 Pending [63] | The device allows, through simple steps, to quantify different concentrations of PSA by means of fluorescence measurement with a smartphone. This sends the data to the cloud for processing and gives a result in about 1 min. It is not a practical device since it needs an objective lens (magnification 40×) to be able to capture the images with the smartphone. | [64] |
| JP2008128677A, 2006 Pending [65] | |||||
| WO2017141503A1, 2016 Pending [66] |
| Applications | Biosensor Type | Evaluated in Real Samples? | Pat. Nº, Year, State | Improvements of Smart Sensor vs. Benchtop Techniques | Ref. |
|---|---|---|---|---|---|
| µCTX-II in urine | Immunological | No. Tested with artificial urine solution (AUS) with the same composition as real urine | US20180371529A1, 2015 Pending [72] | Effective smart optical biosensor, highly correlated with benchtop techniques and higher LOD for the use in patients with complications of renal insufficiency and also for the diagnosis and/or prognosis of osteoarthritis. | [73] |
| Hemoglobin | Colorimetric | Yes. Finger-pricked blood | WO2021019553A1, 2019 Pending [74] WO2021019552A1, 2020 Pending [75] | Fast, sensitive, and specific device for the detection of anemia with good correlation with the results of an automated hematology analyzer and on par with other POC test platforms. The results differ from the pathological estimates within the range of 0.5 g/dL for all severely anemic samples and <1.5 g/dL for the rest of the samples. | [76] |
| Urinary microbial ATP | Bioluminescent | No. A urine sample inoculated with E.Coli was used to simulate a urinary tract infection. | US8642272B2, 2014 [77] | First device bioluminescent on paper for the detection of low-cost ATP, based on the reaction of Luciferase/D-Luciferina that exploits the smartphone camera as a detector. The ATP sensing paper includes an Innovator Lyophilized “Nano-Lantern” With Reaction Components. The mentioned patent does not correspond to the device but is related to its manufacturing materials. | [78] |
| Human IgM and IgG | Immunological | Yes. Human serum | US20210382048A1, 2021 Pending [79] | This paper device has a detection limit of 100 fg/mL demonstrated for the biomarkers of the IgG and IgM protein, which is higher than the one achieved with a traditional Benchtop ELISA test. It is also a much faster method (<5 min), portable, resistant, stable, and low cost, which uses serum without sample preparation and can be easily discarded. | [80] |
| SARS-CoV-2 | Genetic: AuNPs capped with highly specific antisense oligonucleotides (ssDNA) | Yes. Samples collected from Vero cells infected with SARS-CoV-2 virus and clinical samples | US20210388454A1, 2020 Pending [81] | This device can successfully and precisely distinguish the positive samples from Covid-19 from negatives, with sensitivity and specificity of almost 100%. It also presents sensing feasibility even for virus genomic mutation events due to the use of AuNPs, covered with highly specific antisense oligonucleotides (SSDNA) that are simultaneously directed to two separate regions of the same SAR-CoV-2 N gene | [82] |
| Cotinine in Urine | Immunological-Electrochemical | Yes. Urine samples of smoker and non-smokers patients | WO2019139537A1, 2019 Pending [83] | A simple lateral flow competitive immunochromatography was successfully integrated with the AgNP/HRP/AuNP-modified electrode. Immunoreaction can be monitored by either electrochemical measurement or wireless detection. Wireless sensing was realized for cotinine in the range of 100–1000 ng/mL (R2 = 0.96) in PBS medium. For 1:8 diluted urine samples, the device differentiated positive and negative samples and exhibited cotinine discrimination at levels higher than 12 ng/mL. | [84] |
| IL-6 levels in blood and respiratory samples | Immunological | Yes. Human blood and bronchial aspirate samples | WO2021048087A1, 2019 Pending [85] | Paper immunosensor interfaced with a smartphone that generates intense colorimetric signals when the sample contains ultralow concentrations of IL-6. The device combines a paper-based signal amplification mechanism with polymer-filled reservoirs for dispensing antibody-decorated nanoparticles and a bespoken app for color quantification. Semi-quantitative measurements of IL-6 can be facilitated in 10 min with a LOD of 1.3 pg mL−1 and a dynamic range of up to 102 pg mL−1 in diluted blood samples. | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madrid, R.E.; Ashur Ramallo, F.; Barraza, D.E.; Chaile, R.E. Smartphone-Based Biosensor Devices for Healthcare: Technologies, Trends, and Adoption by End-Users. Bioengineering 2022, 9, 101. https://doi.org/10.3390/bioengineering9030101
Madrid RE, Ashur Ramallo F, Barraza DE, Chaile RE. Smartphone-Based Biosensor Devices for Healthcare: Technologies, Trends, and Adoption by End-Users. Bioengineering. 2022; 9(3):101. https://doi.org/10.3390/bioengineering9030101
Chicago/Turabian StyleMadrid, Rossana E., Fernando Ashur Ramallo, Daniela E. Barraza, and Roberto E. Chaile. 2022. "Smartphone-Based Biosensor Devices for Healthcare: Technologies, Trends, and Adoption by End-Users" Bioengineering 9, no. 3: 101. https://doi.org/10.3390/bioengineering9030101
APA StyleMadrid, R. E., Ashur Ramallo, F., Barraza, D. E., & Chaile, R. E. (2022). Smartphone-Based Biosensor Devices for Healthcare: Technologies, Trends, and Adoption by End-Users. Bioengineering, 9(3), 101. https://doi.org/10.3390/bioengineering9030101









