Exosomes in the Pathogenesis, Progression, and Treatment of Osteoarthritis
Abstract
:1. Introduction
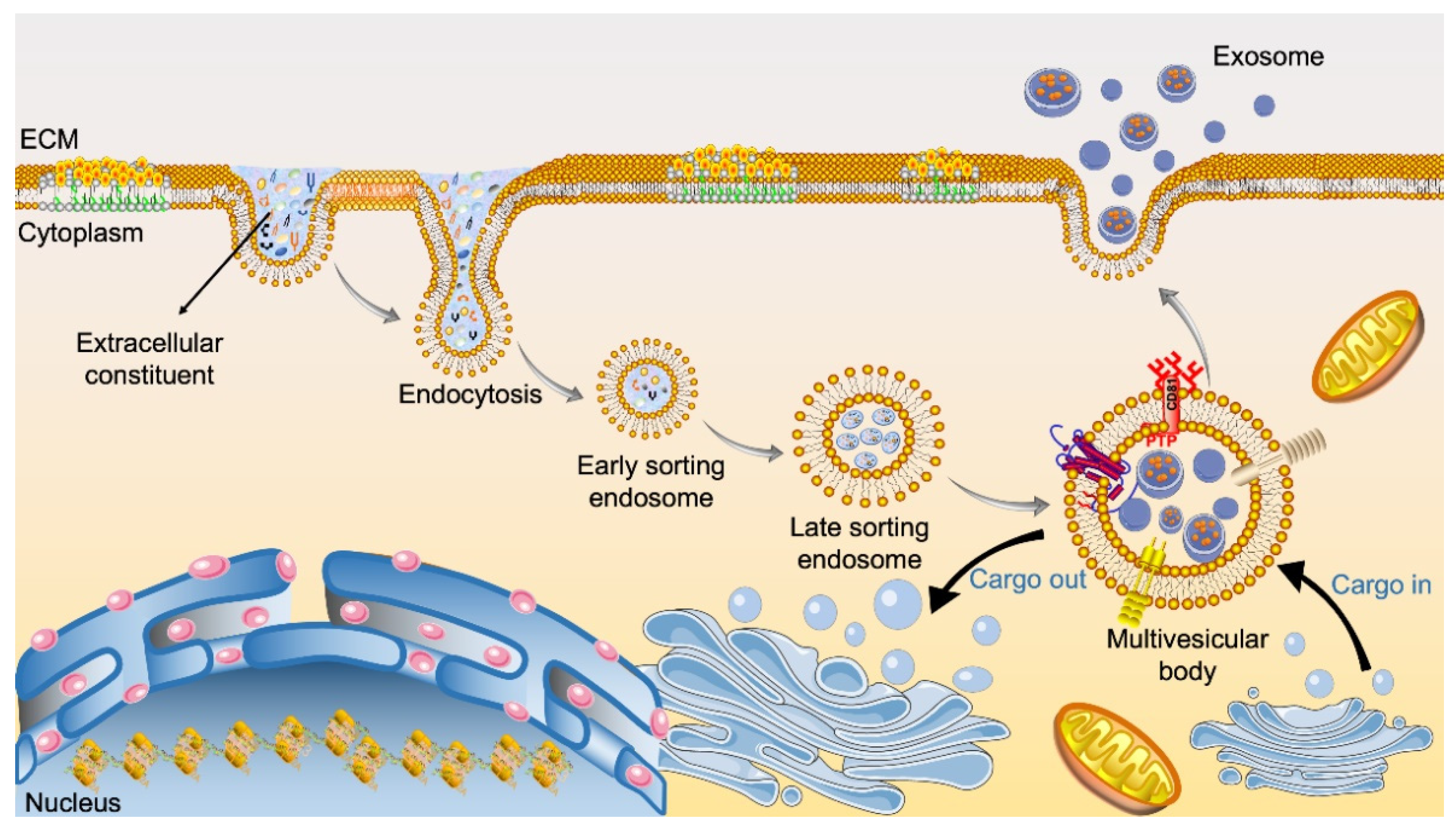
2. Formation and Origin of Exosomes
2.1. Biogenesis of Exosomes
2.2. Origins of Exosomes and Their Roles in OA
2.2.1. Exosomes Derived from Different Types of MSCs
2.2.2. Exosomes Derived from Chondrocytes and Chondrogenic Progenitor Cells
2.2.3. Exosomes Derived from SFBs and Macrophages
2.2.4. Exosomes Derived from Osteoblasts and Osteocytes
2.2.5. Exosomes Derived from Adipose Tissue
2.2.6. Exosomes Derived from PRP
2.2.7. Exosomes Derived from Other Cells
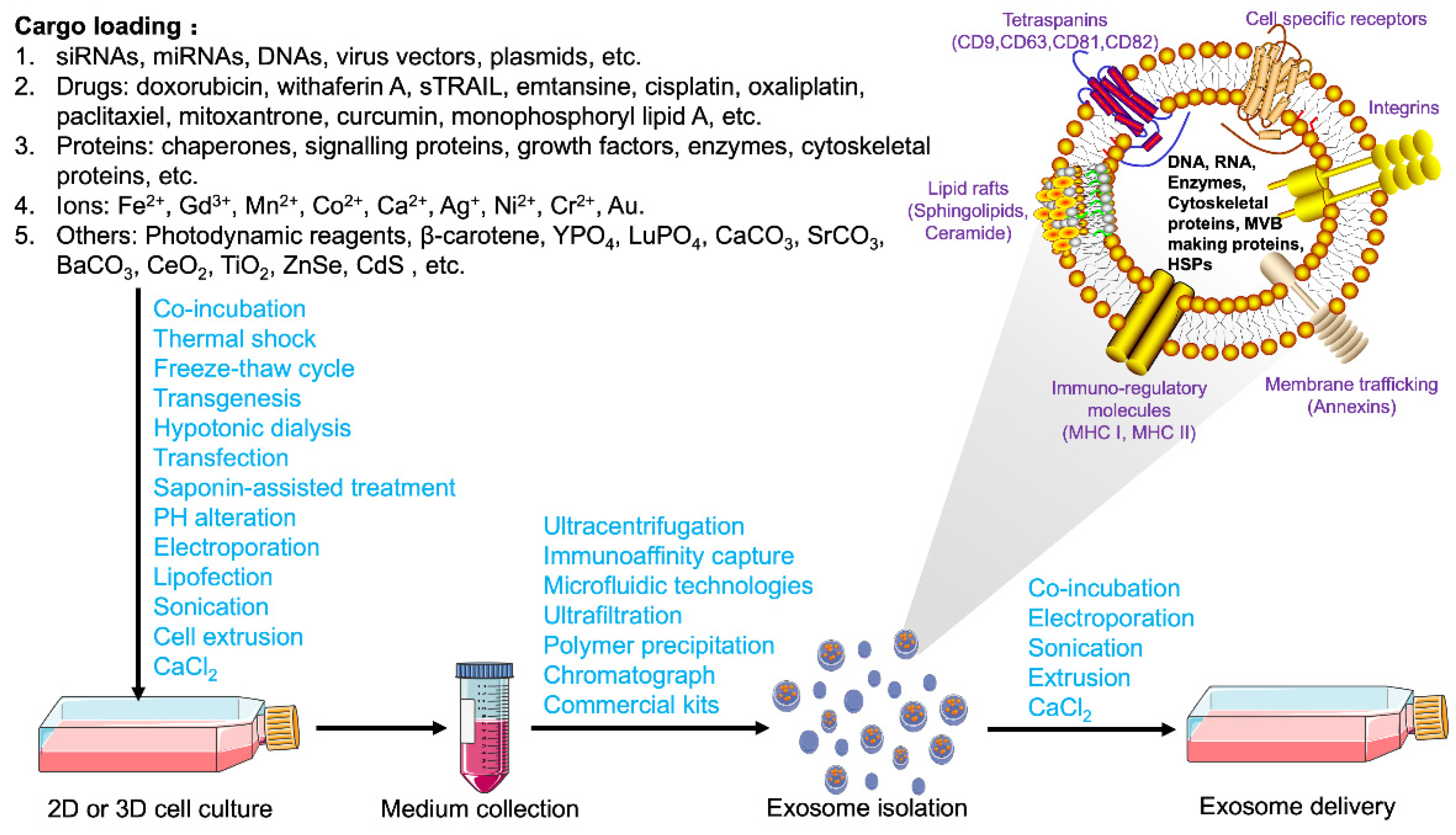
3. Extraction, Bioengineering Modification, and Delivery of Exosomes
3.1. Extraction, Identification, and Storage of Exosomes
3.2. Contents and Loading Strategies for Exosomes
3.3. Bioengineered Modification and Delivery Strategies of Exosomes
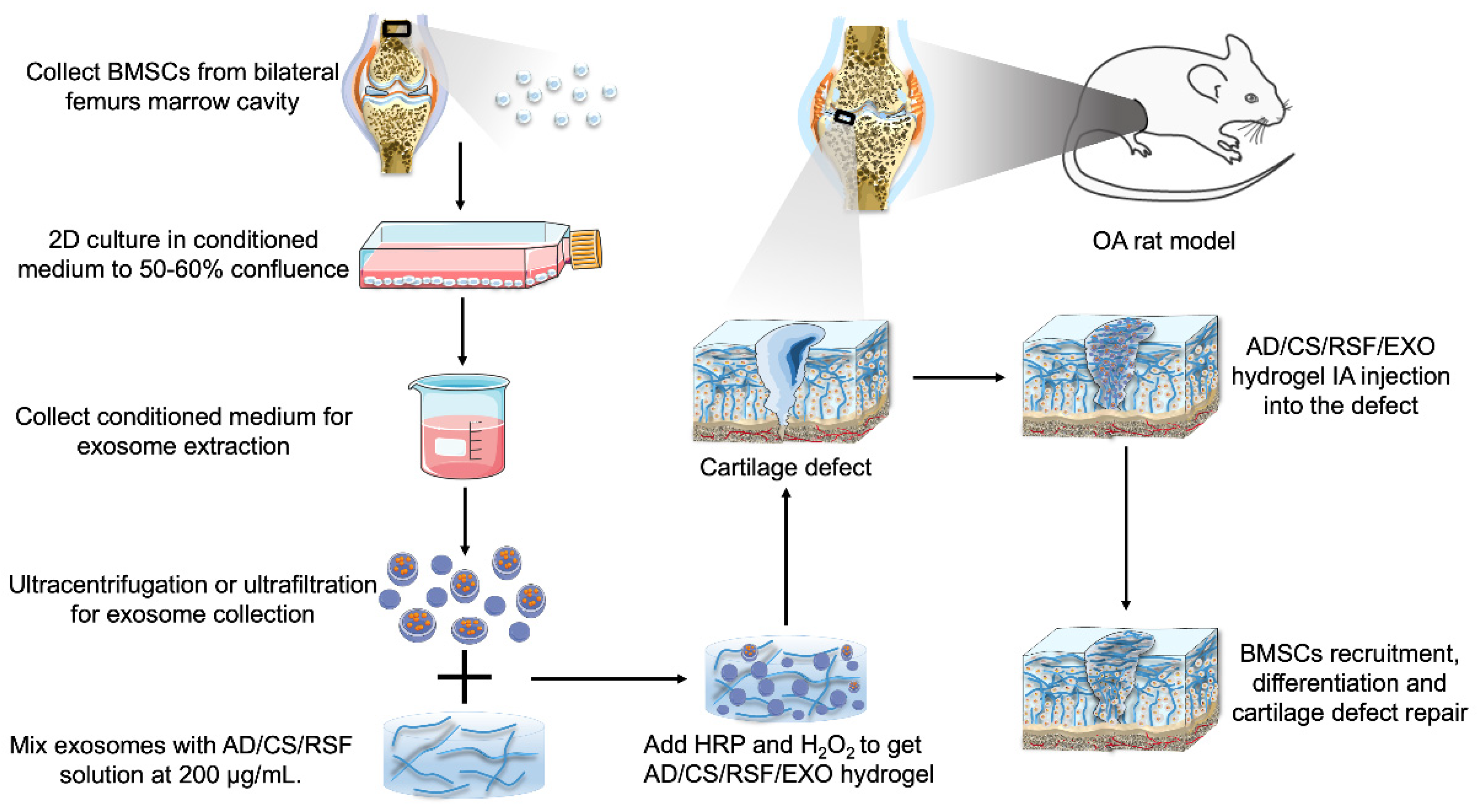
4. In Vivo Efficacy of Exosomes for OA Treatment
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Y.; Makarczyk, M.J.; Lin, H. Role of mitochondria in mediating chondrocyte response to mechanical stimuli. Life Sci. 2020, 263, 118602. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Z.; Alexander, P.G.; Ocasio-Nieves, B.D.; Yocum, L.; Lin, H.; Tuan, R.S. Pathogenesis of osteoarthritis: Risk factors, regulatory pathways in chondrocytes, and experimental models. Biol. 2020, 9, 194. [Google Scholar] [CrossRef]
- Zhao, X.; Shah, D.; Gandhi, K.; Wei, W.; Dwibedi, N.; Webster, L.; Sambamoorthi, U. Clinical, humanistic, and economic burden of osteoarthritis among noninstitutionalized adults in the United States. Osteoarthr. Cartil. 2019, 27, 1618–1626. [Google Scholar] [CrossRef]
- Bosch, M.H.V.D. Osteoarthritis year in review 2020: Biology. Osteoarthr. Cartil. 2021, 29, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Favero, M.; El-Hadi, H.; Belluzzi, E.; Granzotto, M.; Porzionato, A.; Sarasin, G.; Rambaldo, A.; Iacobellis, C.; Cigolotti, A.; Fontanella, C.G.; et al. Infrapatellar fat pad features in osteoarthritis: A histopathological and molecular study. Rheumatology (Oxford) 2017, 56, 1784–1793. [Google Scholar] [CrossRef] [Green Version]
- Prieto-Alhambra, D.; Judge, A.; Javaid, M.; Cooper, C.; Diez-Perez, A.; Arden, N.K. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: Influences of age, gender and osteoarthritis affecting other joints. Ann. Rheum. Dis. 2013, 73, 1659–1664. [Google Scholar] [CrossRef] [Green Version]
- Vina, E.; Kwoh, C.K. Epidemiology of osteoarthritis: Literature update. Curr. Opin. Rheumatol. 2018, 30, 160–167. [Google Scholar] [CrossRef]
- Van Spil, W.E.; Kubassova, O.; Boesen, M.; Bay-Jensen, A.-C.; Mobasheri, A. Osteoarthritis phenotypes and novel therapeutic targets. Biochem. Pharmacol. 2019, 165, 41–48. [Google Scholar] [CrossRef]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, diagnosis, and treatment options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Latourte, A.; Kloppenburg, M.; Richette, P. Emerging pharmaceutical therapies for osteoarthritis. Nat. Rev. Rheumatol. 2020, 16, 673–688. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Salgado, C.; Jordan, O.; Allémann, E. Osteoarthritis in vitro models: Applications and implications in development of intra-articular drug delivery systems. Pharmaceutics 2021, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3.22.1–3.22.29. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, L.; Zeng, X.; Schwarz, H.; Nanda, H.S.; Peng, X.; Zhou, Y. Exosomes, a new star for targeted delivery. Front. Cell Dev. Biol. 2021, 9, 751079. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Kim, J.-H. A comprehensive review on factors influences biogenesis, functions, therapeutic and clinical implications of exosomes. Int. J. Nanomed. 2021, 16, 1281–1312. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Ma, C.; Wang, G.; Zhang, Y.; Sun, S. Exosomes derived from platelet-rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via Wnt/β-catenin signaling pathway. J. Orthop. Surg. Res. 2019, 14, 470. [Google Scholar] [CrossRef] [Green Version]
- Qiu, B.; Xu, X.; Yi, P.; Hao, Y. Curcumin reinforces MSC-derived exosomes in attenuating osteoarthritis via modulating the miR-124/NF-kB and miR-143/ROCK1/TLR9 signalling pathways. J. Cell. Mol. Med. 2020, 24, 10855–10865. [Google Scholar] [CrossRef]
- Ni, Z.; Zhou, S.; Li, S.; Kuang, L.; Chen, H.; Luo, X.; Ouyang, J.; He, M.; Du, X.; Chen, L. Exosomes: Roles and therapeutic potential in osteoarthritis. Bone Res. 2020, 8, 25. [Google Scholar] [CrossRef]
- Trams, E.G.; Lauter, C.J.; Salem, N., Jr.; Heine, U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta 1981, 645, 63–70. [Google Scholar] [CrossRef]
- Pan, B.-T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Fan, J.; Lee, C.-S.; Kim, S.; Chen, C.; Aghaloo, T.; Lee, M. Generation of small RNA-modulated exosome mimetics for bone regeneration. ACS Nano 2020, 14, 11973–11984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Liang, G.; Kan, S.; Zhu, Y.; Feng, S.; Feng, W.; Gao, S. Engineered exosome-mediated delivery of functionally active miR-26a and its enhanced suppression effect in HepG2 cells. Int. J. Nanomed. 2018, 13, 585–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morishita, M.; Takahashi, Y.; Matsumoto, A.; Nishikawa, M.; Takakura, Y. Exosome-based tumor antigens–adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomater. 2016, 111, 55–65. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [Green Version]
- Mustonen, A.-M.; Nieminen, P. Extracellular vesicles and their potential significance in the pathogenesis and treatment of osteoarthritis. Pharmaceuticals 2021, 14, 315. [Google Scholar] [CrossRef]
- Li, D.; Gupta, P.; Sgaglione, N.; Grande, D. Exosomes derived from non-classic sources for treatment of post-traumatic osteoarthritis and cartilage injury of the knee: In vivo review. J. Clin. Med. 2021, 10, 2001. [Google Scholar] [CrossRef]
- Yang, R.-Z.; Zheng, H.-L.; Xu, W.-N.; Zheng, X.-F.; Li, B.; Jiang, L.-S.; Jiang, S.-D. Vascular endothelial cell-secreted exosomes facilitate osteoarthritis pathogenesis by promoting chondrocyte apoptosis. Aging (Albany NY) 2021, 13, 4647–4662. [Google Scholar] [CrossRef]
- Ni, Z.; Kuang, L.; Chen, H.; Xie, Y.; Zhang, B.; Ouyang, J.; Wu, J.; Zhou, S.; Chen, L.; Su, N.; et al. The exosome-like vesicles from osteoarthritic chondrocyte enhanced mature IL-1β production of macrophages and aggravated synovitis in osteoarthritis. Cell Death Dis. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Zheng, L.; Wang‡, Y.; Qiu, P.; Xia, C.; Fang, Y.; Mei, S.; Fang, C.; Shi, Y.; Wu, K.; Chen, Z.; et al. Primary chondrocyte exosomes mediate osteoarthritis progression by regulating mitochondrion and immune reactivity. Nanomedicine (Lond) 2019, 14, 3193–3212. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Crawford, R.; Xiao, Y.; Mao, X.; Prasadam, I. Osteoarthritic subchondral bone release exosomes that promote cartilage degeneration. Cells 2021, 10, 251. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, L.; Zou, R.; Wen, C.; Wang, Z.; Lin, F. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle 2018, 17, 2411–2422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; He, T.; Xing, J.; Zhou, Q.; Fan, L.; Liu, C.; Chen, Y.; Wu, D.; Tian, Z.; Liu, B.; et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res. Ther. 2020, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Cosenza, S.; Ruiz, M.; Toupet, K.; Jorgensen, C.; Noël, D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zheng, L.; Wang, Y.; Tao, M.; Xie, Z.; Xia, C.; Gu, C.; Chen, J.; Qiu, P.; Mei, S.; et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 2019, 9, 2439–2459. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, K.; Ge, G.; Zhang, D.; Bai, J.; Guo, X.; Zhou, J.; Xu, T.; Xu, M.; Long, X.; et al. Exosomes derived from miR-155-5p–overexpressing synovial mesenchymal stem cells prevent osteoarthritis via enhancing proliferation and migration, attenuating apoptosis, and modulating extracellular matrix secretion in chondrocytes. Cell Biol. Toxicol. 2021, 37, 85–96. [Google Scholar] [CrossRef]
- Tao, S.-C.; Yuan, T.; Zhang, Y.-L.; Yin, W.-J.; Guo, S.-C.; Zhang, C.-Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 2017, 7, 180–195. [Google Scholar] [CrossRef]
- Zhang, S.; Teo, K.Y.W.; Chuah, S.J.; Lai, R.C.; Lim, S.K.; Toh, W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 2019, 200, 35–47. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, D.; Liu, Z.; Zhou, F.; Dai, J.; Wu, B.; Zhou, J.; Heng, B.C.; Zou, X.H.; Ouyang, H.; et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther. 2017, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Wang, Y.; Zhao, B.; Niu, X.; Hu, B.; Li, Q.; Zhang, J.; Ding, J.; Chen, Y.; Wang, Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 2017, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Liu, G.; Wu, X. Exosomes derived from umbilical cord mesenchymal stem cells in mechanical environment show improved osteochondral activity via upregulation of LncRNA H19. J. Orthop. Transl. 2021, 26, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Chen, J.Y.; Peng, W.M.; Yuan, B.; Bi, Q.; Xu, Y.J. Exosomes from adipose-derived stem cells promote chondrogenesis and suppress inflammation by upregulating miR-145 and miR-221. Mol. Med. Rep. 2020, 21, 1881–1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Kuang, L.; Chen, C.; Yang, J.; Zeng, W.-N.; Li, T.; Chen, H.; Huang, S.; Fu, Z.; Li, J.; et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials 2019, 206, 87–100. [Google Scholar] [CrossRef]
- Woo, C.H.; Kim, H.K.; Yang, S.; Park, J.H.; Jo, D.; Cho, Y.W.; Jung, G.Y.; Jung, Y.J.; Lee, K.S.; Yun, Y.E.; et al. Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J. Extracell. Vesicles 2020, 9, 1735249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavatti, M.; Beretti, F.; Casciaro, F.; Bertucci, E.; Maraldi, T. Comparison of the therapeutic effect of amniotic fluid stem cells and their exosomes on monoiodoacetate-induced animal model of osteoarthritis. BioFactors 2020, 46, 106–117. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, X.; Li, X.; Xiong, J.; Li, B.; Duan, L.; Wang, D.; Xia, J. Chondrocyte-targeted microRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl. Mater. Interfaces 2020, 12, 36938–36947. [Google Scholar] [CrossRef]
- Wang, R.; Jiang, W.; Zhang, L.; Xie, S.; Zhang, S.; Yuan, S.; Jin, Y.; Zhou, G. Intra-articular delivery of extracellular vesicles secreted by chondrogenic progenitor cells from MRL/MpJ superhealer mice enhances articular cartilage repair in a mouse injury model. Stem Cell Res. Ther. 2020, 11, 93. [Google Scholar] [CrossRef]
- Zeng, G.; Deng, G.; Xiao, S.; Li, F. Fibroblast-like synoviocytes-derived exosomal PCGEM1 accelerates IL-1β-induced apoptosis and cartilage matrix degradation by miR-142-5p/RUNX2 in chondrocytes. Immunol. Investig. 2021, 10, 1–18. [Google Scholar] [CrossRef]
- Zhou, Y.; Ming, J.; Li, Y.; Li, B.; Deng, M.; Ma, Y.; Chen, Z.; Zhang, Y.; Li, J.; Liu, S. Exosomes derived from miR-126-3p-overexpressing synovial fibroblasts suppress chondrocyte inflammation and cartilage degradation in a rat model of osteoarthritis. Cell Death Discov. 2021, 7, 37. [Google Scholar] [CrossRef]
- Otahal, A.; Kramer, K.; Kuten-Pella, O.; Weiss, R.; Stotter, C.; Lacza, Z.; Weber, V.; Nehrer, S.; De Luna, A. Characterization and chondroprotective effects of extracellular vesicles from plasma- and serum-based autologous blood-derived products for osteoarthritis therapy. Front. Bioeng. Biotechnol. 2020, 8, 584050. [Google Scholar] [CrossRef]
- Wang, Y.; He, G.; Guo, Y.; Tang, H.; Shi, Y.; Bian, X.; Zhu, M.; Kang, X.; Zhou, M.; Lyu, J.; et al. Exosomes from tendon stem cells promote injury tendon healing through balancing synthesis and degradation of the tendon extracellular matrix. J. Cell. Mol. Med. 2019, 23, 5475–5485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, T.; Xu, M.; Bai, J.; Lin, J.; Yu, B.; Liu, Y.; Guo, X.; Shen, J.; Sun, H.; Hao, Y.; et al. Tenocyte-derived exosomes induce the tenogenic differentiation of mesenchymal stem cells through TGF-β. Cytotechnology 2019, 71, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Maruyama, K.; Sakisaka, Y.; Suzuki, S.; Tada, H.; Suto, M.; Saito, M.; Yamada, S.; Nemoto, E. Cyclic stretch force induces periodontal ligament cells to secrete exosomes that suppress IL-1β production through the inhibition of the NF-κB signaling pathway in macrophages. Front. Immunol. 2019, 10, 1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Dai, W.; Wang, H.; Xue, C.; Feng, J.; He, Y.; Wang, P.; Li, S.; Bai, D.; Shu, R. Periodontal ligament fibroblasts regulate osteoblasts by exosome secretion induced by inflammatory stimuli. Arch. Oral Biol. 2019, 105, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, Q.; Zhao, Z.; Guan, X.; Bai, Y. Periodontal ligament fibroblast-derived exosomes induced by compressive force promote macrophage M1 polarization via Yes-associated protein. Arch. Oral Biol. 2021, 132, 105263. [Google Scholar] [CrossRef] [PubMed]
- Lopa, S.; Colombini, A.; Moretti, M.; De Girolamo, L. Injective mesenchymal stem cell-based treatments for knee osteoarthritis: From mechanisms of action to current clinical evidences. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2003–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara-Barba, E.; Araya, M.J.; Hill, C.N.; Bustamante-Barrientos, F.A.; Ortloff, A.; García, C.; Galvez-Jiron, F.; Pradenas, C.; Luque-Campos, N.; Maita, G.; et al. Role of microRNA shuttled in small extracellular vesicles derived from mesenchymal stem/stromal cells for osteoarticular disease treatment. Front. Immunol. 2021, 12, 768771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef]
- Basu, J.; Ludlow, J.W. Exosomes for repair, regeneration and rejuvenation. Expert Opin. Biol. Ther. 2016, 16, 489–506. [Google Scholar] [CrossRef]
- Zhang, L.-Q.; Zhao, G.-Z.; Xu, X.-Y.; Fang, J.; Chen, J.-M.; Li, J.-W.; Gao, X.-J.; Hao, L.-J.; Chen, Y.-Z. Integrin-β1 regulates chondrocyte proliferation and apoptosis through the upregulation of GIT1 expression. Int. J. Mol. Med. 2015, 35, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Tofiño-Vian, M.; Guillén, M.I.; del Caz, M.D.P.; Castejón, M.A.; Alcaraz, M.J. Extracellular vesicles from adipose-derived mesenchymal stem cells downregulate senescence features in osteoarthritic osteoblasts. Oxidative Med. Cell. Longev. 2017, 2017, 7197598. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-Y.; Vesvoranan, O.; Yin, X.; Montoya, A.; Londono, V.; Sawatari, Y.; Garcia-Godoy, F. Anti-inflammatory effects of conditioned medium of periodontal ligament-derived stem cells on chondrocytes, synoviocytes, and meniscus cells. Stem Cells Dev. 2021, 30, 537–547. [Google Scholar] [CrossRef]
- Shao, J.; Zhu, J.; Chen, Y.; Fu, Q.; Li, L.; Ding, Z.; Wu, J.; Han, Y.; Li, H.; Qian, Q.; et al. Exosomes from kartogenin-pretreated infrapatellar fat pad mesenchymal stem cells enhance chondrocyte anabolism and articular cartilage regeneration. Stem Cells Int. 2021, 2021, 6624874. [Google Scholar] [CrossRef]
- Huri, P.Y.; Hamsici, S.; Ergene, E.; Huri, G.; Doral, A.M.N. Infrapatellar fat pad-derived stem cell-based regenerative strategies in orthopedic surgery. Knee Surg. Relat. Res. 2018, 30, 179–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Wang, L.; Lin, J.; Liu, Q. The role of extracellular vesicles in the pathogenesis, diagnosis, and treatment of osteoarthritis. Molecules 2021, 26, 4987. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, I.M.; Landis, W.J.; Risbud, M.V. Matrix vesicles: Are they anchored exosomes? Bone 2015, 79, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Mitton, E.; Gohr, C.M.; McNally, M.T.; Rosenthal, A.K. Articular cartilage vesicles contain RNA. Biochem. Biophys. Res. Commun. 2009, 388, 533–538. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wang, Y.; Xiang, S.; Zheng, Z.; Bian, Y.; Feng, B.; Weng, X. Chondrocytes-derived exosomal miR-8485 regulated the Wnt/β-catenin pathways to promote chondrogenic differentiation of BMSCs. Biochem. Biophys. Res. Commun. 2020, 523, 506–513. [Google Scholar] [CrossRef]
- Koelling, S.; Kruegel, J.; Irmer, M.; Path, J.R.; Sadowski, B.; Miro, X.; Miosge, N. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell 2009, 4, 324–335. [Google Scholar] [CrossRef] [Green Version]
- Kato, T.; Miyaki, S.; Ishitobi, H.; Nakamura, Y.; Nakasa, T.; Lotz, M.K.; Ochi, M. Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res. Ther. 2014, 16, R163. [Google Scholar] [CrossRef] [Green Version]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuno, H.; Suematsu, N.; Sato, T.; Arito, M.; Matsui, T.; Iizuka, N.; Omoteyama, K.; Okamoto, K.; Tohma, S.; Kurokawa, M.S.; et al. Effects of methotrexate and salazosulfapyridine on protein profiles of exosomes derived from a human synovial sarcoma cell line of SW982. Proteom. Clin. Appl. 2015, 10, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Wang, D.; Yuan, Z. The fibroblast-like synoviocyte derived exosomal long non-coding RNA H19 alleviates osteoarthritis progression through the miR-106b-5p/TIMP2 axis. Inflammation 2020, 43, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, W.; Yong, H.; He, M.; Yang, Y.; Deng, Z.; Li, Y. Macrophages in osteoarthritis: Pathophysiology and therapeutics. Am J Transl Res 2020, 12, 261–268. [Google Scholar]
- Barr, A.J.; Campbell, T.M.; Hopkinson, D.; Kingsbury, S.R.; Bowes, M.A.; Conaghan, P.G. A systematic review of the relationship between subchondral bone features, pain and structural pathology in peripheral joint osteoarthritis. Arthritis Res. Ther. 2015, 17, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Suzuki, T.; Kawano, M.; Tamura, M. Circulating osteocyte-derived exosomes contain miRNAs which are enriched in exosomes from MLO-Y4 cells. Biomed. Rep. 2017, 6, 223–231. [Google Scholar] [CrossRef]
- Lv, P.-Y.; Gao, P.-F.; Tian, G.-J.; Yang, Y.-Y.; Mo, F.-F.; Wang, Z.-H.; Sun, L.; Kuang, M.-J.; Wang, Y.-L. Osteocyte-derived exosomes induced by mechanical strain promote human periodontal ligament stem cell proliferation and osteogenic differentiation via the miR-181b-5p/PTEN/AKT signaling pathway. Stem Cell Res. Ther. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Qin, Y.; Peng, Y.; Zhao, W.; Pan, J.; Ksiezak-Reding, H.; Cardozo, C.; Wu, Y.; Pajevic, P.D.; Bonewald, L.F.; Bauman, W.A.; et al. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: A novel mechanism in muscle-bone communication. J. Biol. Chem. 2017, 292, 11021–11033. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-Y.; Xia, K.; Wang, Z.-X.; Xie, H.; Xu, R. Osteocyte exosomes accelerate benign prostatic hyperplasia development. Mol. Cell. Endocrinol. 2021, 531, 111301. [Google Scholar] [CrossRef]
- Chang, J.; Liao, Z.; Lu, M.; Meng, T.; Han, W.; Ding, C. Systemic and local adipose tissue in knee osteoarthritis. Osteoarthr. Cartil. 2018, 26, 864–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-E.; Moon, P.-G.; Lee, I.-K.; Baek, M.-C. Proteomic analysis of extracellular vesicles released by adipocytes of Otsuka Long-Evans Tokushima fatty (OLETF) rats. J. Protein Chem. 2015, 34, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Sano, S.; Izumi, Y.; Yamaguchi, T.; Yamazaki, T.; Tanaka, M.; Shiota, M.; Osada-Oka, M.; Nakamura, Y.; Wei, M.; Wanibuchi, H.; et al. Lipid synthesis is promoted by hypoxic adipocyte-derived exosomes in 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2014, 445, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Kita, S.; Maeda, N.; Shimomura, I. Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. J. Clin. Investig. 2019, 129, 4041–4049. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Wang, X.; Liu, X.; Du, H.; Sun, C.; Shao, X.; Tian, J.; Gu, X.; Wang, H.; Tian, J.; et al. Adipose-derived exosomes exert proatherogenic effects by regulating macrophage foam cell formation and polarization. J. Am. Hear. Assoc. 2018, 7, e007442. [Google Scholar] [CrossRef]
- Deng, Z.-B.; Poliakov, A.; Hardy, R.W.; Clements, R.; Liu, C.; Liu, Y.; Wang, J.; Xiang, X.; Zhang, S.; Zhuang, X.; et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes 2009, 58, 2498–2505. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Shang, Q.; Pan, Z.; Bai, Y.; Li, Z.; Zhang, H.; Zhang, Q.; Guo, C.; Zhang, L.; Wang, Q. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes 2018, 67, 235–247. [Google Scholar] [CrossRef] [Green Version]
- Fotouhi, A.; Maleki, A.; Dolati, S.; Aghebati-Maleki, A.; Aghebati-Maleki, L. Platelet rich plasma, stromal vascular fraction and autologous conditioned serum in treatment of knee osteoarthritis. Biomed. Pharmacother. 2018, 104, 652–660. [Google Scholar] [CrossRef]
- Shen, L.; Yuan, T.; Chen, S.; Xie, X.; Zhang, C. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: Systematic review and meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2017, 12, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Koseoglu, S.; Flaumenhaft, R. Advances in platelet granule biology. Curr. Opin. Hematol. 2013, 20, 464–471. [Google Scholar] [CrossRef]
- Ambrosio, A.L.; Di Pietro, S.M. Mechanism of platelet α-granule biogenesis: Study of cargo transport and the VPS33B-VPS16B complex in a model system. Blood Adv. 2019, 3, 2617–2626. [Google Scholar] [CrossRef]
- Michael, B.N.R.; Kommoju, V.; Ganapathy, C.K.; Negi, V.S. Characterization of cell-derived microparticles in synovial fluid and plasma of patients with rheumatoid arthritis. Rheumatol. Int. 2019, 39, 1377–1387. [Google Scholar] [CrossRef]
- Oba, R.; Isomura, M.; Igarashi, A.; Nagata, K. Circulating CD3+HLA-DR+extracellular vesicles as a marker for Th1/Tc1-Type immune responses. J. Immunol. Res. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Distler, J.H.W.; Jüngel, A.; Huber, L.C.; Seemayer, C.A.; Reich, C.F.; Gay, R.E.; Michel, B.A.; Fontana, A.; Gay, S.; Pisetsky, D.S.; et al. The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc. Natl. Acad. Sci. USA 2005, 102, 2892–2897. [Google Scholar] [CrossRef] [Green Version]
- Störch, H.; Zimmermann, B.; Resch, B.; Tykocinski, L.-O.; Moradi, B.; Horn, P.; Kaya, Z.; Blank, N.; Rehart, S.; Thomsen, M.; et al. Activated human B cells induce inflammatory fibroblasts with cartilage-destructive properties and become functionally suppressed in return. Ann. Rheum. Dis. 2016, 75, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Hirota, K.; Sakaguchi, S. Synovial tissue inflammation mediated by autoimmune T cells. Front. Immunol. 2019, 10, 1989. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-X.; Wu, Y.-J.; Zhang, J.; Wei, W. T-cells interact with B cells, dendritic cells, and fibroblast-like synoviocytes as hub-like key cells in rheumatoid arthritis. Int. Immunopharmacol. 2019, 70, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Asghar, S.; Litherland, G.J.; Lockhart, J.C.; Goodyear, C.S.; Crilly, A. Exosomes in intercellular communication and implications for osteoarthritis. Rheumatology (Oxford) 2019, 59, 57–68. [Google Scholar] [CrossRef]
- Wu, X.; Showiheen, S.A.A.; Sun, A.R.; Crawford, R.; Xiao, Y.; Mao, X.; Prasadam, I. Exosomes extraction and identification. Methods Mol. Biol. 2019, 2054, 81–91. [Google Scholar] [CrossRef]
- Macías, M.; Rebmann, V.; Mateos, B.; Varo, N.; Perez-Gracia, J.L.; Alegre, E.; González, Á. Comparison of six commercial serum exosome isolation methods suitable for clinical laboratories. Effect in cytokine analysis. Clin. Chem. Lab. Med. (CCLM) 2019, 57, 1539–1545. [Google Scholar] [CrossRef]
- Greening, D.W.; Xu, R.; Ji, H.; Tauro, B.J.; Simpson, R.J. A Protocol for exosome isolation and characterization: Evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol. Biol. 2015, 1295, 179–209. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, W.; Zhang, H.; Zhang, F.; Chen, L.; Ma, L.; Larcher, L.M.; Chen, S.; Liu, N.; Zhao, Q.; et al. Progress, opportunity, and perspective on exosome isolation—efforts for efficient exosome-based theranostics. Theranostics 2020, 10, 3684–3707. [Google Scholar] [CrossRef] [PubMed]
- Helwa, I.; Cai, J.; Drewry, M.D.; Zimmerman, A.; Dinkins, M.B.; Khaled, M.L.; Seremwe, M.; Dismuke, W.M.; Bieberich, E.; Stamer, W.D.; et al. A comparative study of serum exosome isolation using differential ultracentrifugation and three commercial reagents. PLoS ONE 2017, 12, e0170628. [Google Scholar] [CrossRef] [PubMed]
- Taghikhani, A.; Farzaneh, F.; Sharifzad, F.; Mardpour, S.; Ebrahimi, M.; Hassan, Z.M. Engineered tumor-derived extracellular vesicles: Potentials in cancer immunotherapy. Front. Immunol. 2020, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Deng, W.; Ii, D.J.K. Exosomes: Improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Analyst 2015, 140, 6631–6642. [Google Scholar] [CrossRef] [Green Version]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Kang, M.-H.; Jeyaraj, M.; Qasim, M.; Kim, J.-H. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells 2019, 8, 307. [Google Scholar] [CrossRef] [Green Version]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-’t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef]
- Bahr, M.M.; Amer, M.S.; Abo-El-Sooud, K.; Abdallah, A.N.; El-Tookhy, O.S. Preservation techniques of stem cells extracellular vesicles: A gate for manufacturing of clinical grade therapeutic extracellular vesicles and long-term clinical trials. Int. J. Veter- Sci. Med. 2020, 8, 1–8. [Google Scholar] [CrossRef]
- Yamashita, T.; Takahashi, Y.; Takakura, Y. Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol. Pharm. Bull. 2018, 41, 835–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charoenviriyakul, C.; Takahashi, Y.; Nishikawa, M.; Takakura, Y. Preservation of exosomes at room temperature using lyophilization. Int. J. Pharm. 2018, 553, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, G.D.; Barabadi, M.; Tan, J.; Morton, D.; Frith, J.; Lim, R. To protect and to preserve: Novel preservation strategies for extracellular vesicles. Front. Pharmacol. 2018, 9, 1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrell, C.R.; Markovic, B.S.; Fellabaum, C.; Arsenijevic, A.; Volarevic, V. Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomed. Pharmacother. 2019, 109, 2318–2326. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, J.; Han, J.; Zhang, W.; Ma, J. Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. Am. J. Transl. Res. 2019, 11, 6275–6289. [Google Scholar]
- Mianehsaz, E.; Mirzaei, H.R.; Mahjoubin-Tehran, M.; Rezaee, A.; Sahebnasagh, R.; Pourhanifeh, M.H.; Mirzaei, H.; Hamblin, M.R. Mesenchymal stem cell-derived exosomes: A new therapeutic approach to osteoarthritis? Stem Cell Res. Ther. 2019, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.; Wen, G.; Sun, W.; Jiang, K.; Chen, T.; Chen, S.; Wen, J. Mechanical stress promotes angiogenesis through fibroblast exosomes. Biochem. Biophys. Res. Commun. 2020, 533, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, Q.; Zhang, T.; Wang, X.; Cheng, K.; Gao, M.; Xia, P.; Li, X. Low-intensity pulsed ultrasound promotes chondrogenesis of mesenchymal stem cells via regulation of autophagy. Stem Cell Res. Ther. 2019, 10, 41. [Google Scholar] [CrossRef] [Green Version]
- Rong, Y.; Zhang, J.; Jiang, D.; Ji, C.; Liu, W.; Wang, J.; Ge, X.; Tang, P.; Yu, S.; Cui, W.; et al. Hypoxic pretreatment of small extracellular vesicles mediates cartilage repair in osteoarthritis by delivering miR-216a-5p. Acta Biomater. 2021, 122, 325–342. [Google Scholar] [CrossRef]
- Li, S.; Stöckl, S.; Lukas, C.; Herrmann, M.; Brochhausen, C.; König, M.A.; Johnstone, B.; Grässel, S. Curcumin-primed human BMSC-derived extracellular vesicles reverse IL-1β-induced catabolic responses of OA chondrocytes by upregulating miR-126-3p. Stem Cell Res. Ther. 2021, 12, 252. [Google Scholar] [CrossRef]
- Xu, X.; Liang, Y.; Li, X.; Ouyang, K.; Wang, M.; Cao, T.; Li, W.; Liu, J.; Xiong, J.; Li, B.; et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials 2021, 269, 120539. [Google Scholar] [CrossRef]
- Wang, R.; Xu, B. TGF-β1-modified MSC-derived exosomal miR-135b attenuates cartilage injury via promoting M2 synovial macrophage polarization by targeting MAPK6. Cell Tissue Res. 2021, 384, 113–127. [Google Scholar] [CrossRef]
- Wang, Y.; He, S.; Liang, X.; Zhang, X.; Li, S.; Li, T. ATF4 -modified serum exosomes derived from osteoarthritic mice inhibit osteoarthritis by inducing autophagy. IUBMB Life 2021, 73, 146–158. [Google Scholar] [CrossRef]
- Panagopoulos, P.; Lambrou, G. The involvement of microRNAs in osteoarthritis and recent developments: A narrative review. Mediterr. J. Rheumatol. 2018, 29, 67–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolhe, R.; Hunter, M.; Liu, S.; Jadeja, R.N.; Pundkar, C.; Mondal, A.K.; Mendhe, B.; Drewry, M.; Rojiani, M.V.; Liu, Y.; et al. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, L.; Xu, L.; Xu, X.; Qin, Z.; Zhou, X.; Xiao, Y.; Liang, Y.; Xia, J. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale 2020, 13, 1387–1397. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Xiao, Y.; Crawford, R.; Mao, X.; Prasadam, I. Extracellular vesicles: Potential role in osteoarthritis regenerative medicine. J. Orthop. Transl. 2020, 21, 73–80. [Google Scholar] [CrossRef]
- Qiu, M.; Liu, D.; Fu, Q. MiR-129-5p shuttled by human synovial mesenchymal stem cell-derived exosomes relieves IL-1β induced osteoarthritis via targeting HMGB1. Life Sci. 2021, 269, 118987. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Xu, Y.; Long, D.; Sun, H.; Li, H.; Xin, R.; Zhang, Z.; Li, Z.; Yang, Z.; Kang, Y. Exosome-transported circRNA_0001236 enhances chondrogenesis and suppress cartilage degradation via the miR-3677-3p/Sox9 axis. Stem Cell Res. Ther. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Kanchanapally, R.; Deshmukh, S.K.; Chavva, S.R.; Tyagi, N.; Srivastava, S.K.; Patel, G.K.; Singh, A.P.; Singh, S. Drug-loaded exosomal preparations from different cell types exhibit distinctive loading capability, yield, and antitumor efficacies: A comparative analysis. Int. J. Nanomed. 2019, 14, 531–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, A.; Aqil, F.; Jeyabalan, J.; Spencer, W.A.; Beck, J.; Gachuki, B.W.; Alhakeem, S.; Oben, K.; Munagala, R.; Bondada, S.; et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine 2017, 13, 1627–1636. [Google Scholar] [CrossRef]
- Familtseva, A.; Jeremic, N.; Tyagi, S.C. Exosomes: Cell-created drug delivery systems. Mol. Cell. Biochem. 2019, 459, 1–6. [Google Scholar] [CrossRef]
- Jafari, D.; Shajari, S.; Jafari, R.; Mardi, N.; Gomari, H.; Ganji, F.; Moghadam, M.F.; Samadikuchaksaraei, A. Designer exosomes: A new platform for biotechnology therapeutics. BioDrugs 2020, 34, 567–586. [Google Scholar] [CrossRef]
- Xi, X.-M.; Xia, S.-J.; Lu, R. Drug loading techniques for exosome-based drug delivery systems. Pharmazie 2021, 76, 61–67. [Google Scholar] [PubMed]
- Liu, Y.; Bai, L.; Guo, K.; Jia, Y.; Zhang, K.; Liu, Q.; Wang, P.; Wang, X. Focused ultrasound-augmented targeting delivery of nanosonosensitizers from homogenous exosomes for enhanced sonodynamic cancer therapy. Theranostics 2019, 9, 5261–5281. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Zaro, J.; Shen, Y. Advances in exosome-based drug delivery and tumor targeting: From tissue distribution to intracellular fate. Int. J. Nanomed. 2020, 15, 9355–9371. [Google Scholar] [CrossRef]
- Kenari, A.N.; Kastaniegaard, K.; Greening, D.; Shambrook, M.; Stensballe, A.; Cheng, L.; Hill, A.F. Proteomic and post-translational modification profiling of exosome-mimetic nanovesicles compared to exosomes. Proteom. 2019, 19, e1800161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haraszti, R.A.; Miller, R.; Stoppato, M.; Sere, Y.Y.; Coles, A.; Didiot, M.-C.; Wollacott, R.; Sapp, E.; Dubuke, M.L.; Li, X.; et al. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol. Ther. 2018, 26, 2838–2847. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.-H.; Ji, A.-L.; Wang, Z.-X.; Qiang, G.-H.; Qu, Z.; Jiang, C.-P. Exosome-mimetic nanovesicles from hepatocytes promote hepatocyte proliferation in vitro and liver regeneration in vivo. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalimuthu, S.; Gangadaran, P.; Rajendran, R.L.; Zhu, L.; Oh, J.M.; Lee, H.W.; Gopal, A.; Baek, S.H.; Jeong, S.Y.; Lee, S.-W.; et al. A new approach for loading anticancer drugs into mesenchymal stem cell-derived exosome mimetics for cancer therapy. Front. Pharmacol. 2018, 9, 1116. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, Y.; Xu, S.; Gao, X.; Kong, F.; Xu, K.; Tang, B. Homotypic cell membrane-cloaked biomimetic nanocarrier for the targeted chemotherapy of hepatocellular carcinoma. Theranostics 2019, 9, 5828–5838. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Noguchi, K.; Fujii, I.; Futaki, S. Vectorization of biomacromolecules into cells using extracellular vesicles with enhanced internalization induced by macropinocytosis. Sci. Rep. 2016, 6, 34937. [Google Scholar] [CrossRef] [Green Version]
- Sawada, S.-I.; Sato, Y.T.; Kawasaki, R.; Yasuoka, J.-I.; Mizuta, R.; Sasaki, Y.; Akiyoshi, K. Nanogel hybrid assembly for exosome intracellular delivery: Effects on endocytosis and fusion by exosome surface polymer engineering. Biomater. Sci. 2020, 8, 619–630. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, J.; Gu, W.; Huang, Y.; Tong, Z.; Huang, L.; Tan, J. Exosome-liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv. Sci. 2018, 5, 1700611. [Google Scholar] [CrossRef]
- Yang, Y.; Hong, Y.; Nam, G.-H.; Chung, J.H.; Koh, E.; Kim, I.-S. Virus-mimetic fusogenic exosomes for direct delivery of integral membrane proteins to target cell membranes. Adv. Mater. 2017, 29, 29. [Google Scholar] [CrossRef]
- Lu, M.; Zhao, X.; Xing, H.; Liu, H.; Lang, L.; Yang, T.; Xun, Z.; Wang, D.; Ding, P. Cell-free synthesis of connexin 43-integrated exosome-mimetic nanoparticles for siRNA delivery. Acta Biomater. 2019, 96, 517–536. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Y.; Sun, X.; Xing, Y.; Wang, X.; Yang, Q. Immunomodulation of MSCs and MSC-derived extracellular vesicles in osteoarthritis. Front. Bioeng. Biotechnol. 2020, 8, 8. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Li, Y.; Niu, X.; Zhao, B.; Wang, Y.; Bao, C.; Xie, Z.; Lin, Q.; Zhu, L. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale 2017, 9, 4430–4438. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, X.; Chen, X.; Wei, Y.; Du, W.; Wang, Y.; Liu, L.; Zhao, W.; Han, Z.; Kong, D.; et al. Enhanced therapeutic effects of mesenchymal stem cell-derived exosomes with an injectable hydrogel for hindlimb ischemia treatment. ACS Appl. Mater. Interfaces 2018, 10, 30081–30091. [Google Scholar] [CrossRef]
- Henriques-Antunes, H.; Cardoso, R.M.S.; Zonari, A.; Correia, J.; Leal, E.C.; Jiménez-Balsa, A.; Lino, M.M.; Barradas, A.; Kostic, I.; Gomes, C.; et al. The kinetics of small extracellular vesicle delivery impacts skin tissue regeneration. ACS Nano 2019, 13, 8694–8707. [Google Scholar] [CrossRef]
- Lenzini, S.; Bargi, R.; Chung, G.; Shin, J.-W. Matrix mechanics and water permeation regulate extracellular vesicle transport. Nat. Nanotechnol. 2020, 15, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-X.; Liu, P.; Ding, W.; Meng, Q.-B.; Su, D.-H.; Zhang, Q.-C.; Lian, R.-X.; Yu, B.-Q.; Zhao, M.-D.; Dong, J.; et al. Injectable Mussel-Inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomater. 2021, 278, 121169. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Choi, J.; Kim, K. Mesenchymal stem cell-derived exosomes for effective cartilage tissue repair and treatment of osteoarthritis. Biotechnol. J. 2020, 15, e2000082. [Google Scholar] [CrossRef] [PubMed]
- Xiaomin, W.; Bian, B.; Lin, Z.; Wu, C.; Sun, Y.; Pan, Y.; Dai, Y.; Lui, T.H.; Zhuang, T.; Pan, X. Identification of exosomal mRNA, lncRNA and circRNA signatures in an osteoarthritis synovial fluid-exosomal study. Exp. Cell Res. 2021, 410, 112881. [Google Scholar] [CrossRef]
- Jeon, O.H.; Wilson, D.R.; Clement, C.C.; Rathod, S.; Cherry, C.; Powell, B.; Lee, Z.; Khalil, A.M.; Green, J.J.; Campisi, J.; et al. Senescence cell–associated extracellular vesicles serve as osteoarthritis disease and therapeutic markers. JCI Insight 2019, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Geiger, B.C.; Wang, S.; Padera, R.F., Jr.; Grodzinsky, A.J.; Hammond, P.T. Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis. Sci. Transl. Med. 2018, 10, eaat8800. [Google Scholar] [CrossRef] [Green Version]
- Bajpayee, A.G.; Scheu, M.; Grodzinsky, A.J.; Porter, R.M. A rabbit model demonstrates the influence of cartilage thickness on intra-articular drug delivery and retention within cartilage. J. Orthop. Res. 2015, 33, 660–667. [Google Scholar] [CrossRef]
- Ng, C.Y.; Chai, J.Y.; Foo, J.B.; Yahaya, N.H.M.; Yang, Y.; Ng, M.H.; Law, J.X. Potential of exosomes as cell-free therapy in articular cartilage regeneration: A review. Int. J. Nanomed. 2021, 16, 6749–6781. [Google Scholar] [CrossRef]
- Bajpayee, A.G.; Wong, C.R.; Bawendi, M.G.; Frank, E.H.; Grodzinsky, A.J. Avidin as a model for charge driven transport into cartilage and drug delivery for treating early stage post-traumatic osteoarthritis. Biomater. 2014, 35, 538–549. [Google Scholar] [CrossRef] [Green Version]
- Bajpayee, A.G.; Grodzinsky, A.J. Cartilage-targeting drug delivery: Can electrostatic interactions help? Nat. Rev. Rheumatol. 2017, 13, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Vedadghavami, A.; Zhang, C.; Bajpayee, A.G. Overcoming negatively charged tissue barriers: Drug delivery using cationic peptides and proteins. Nano Today 2020, 34, 100898. [Google Scholar] [CrossRef] [PubMed]
- Vedadghavami, A.; Wagner, E.; Mehta, S.; He, T.; Zhang, C.; Bajpayee, A.G. Cartilage penetrating cationic peptide carriers for applications in drug delivery to avascular negatively charged tissues. Acta Biomater. 2019, 93, 258–269. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Zhang, C.; Vedadghavami, A.; Mehta, S.; Clark, H.A.; Porter, R.M.; Bajpayee, A.G. Multi-arm Avidin nano-construct for intra-cartilage delivery of small molecule drugs. J. Control. Release 2020, 318, 109–123. [Google Scholar] [CrossRef]
- Gupta, A.; Maffulli, N.; Rodriguez, H.C.; Carson, E.W.; Bascharon, R.A.; Delfino, K.; Levy, H.J.; El-Amin, S.F. Safety and efficacy of umbilical cord-derived Wharton’s jelly compared to hyaluronic acid and saline for knee osteoarthritis: Study protocol for a randomized, controlled, single-blind, multi-center trial. J. Orthop. Surg. Res. 2021, 16, 1–8. [Google Scholar] [CrossRef]
- Gupta, A.; Maffulli, N.; Rodriguez, H.C.; Lee, C.E.; Levy, H.J.; El-Amin, S.F. Umbilical cord-derived Wharton’s jelly for treatment of knee osteoarthritis: Study protocol for a non-randomized, open-label, multi-center trial. J. Orthop. Surg. Res. 2021, 16, 1–7. [Google Scholar] [CrossRef]

| Cells | Source | Extraction | Dose | Delivery Method | Target Cells | Results | Ref |
|---|---|---|---|---|---|---|---|
| VECs | Conditioned medium | Ultrafiltration | 100 μg | Co-incubation for 24 h | Primary chondrocytes | Promoted OA progression by inhibiting chondrocyte autophagy, downregulating p21 expression, and increasing ROS production and apoptosis. | [29] |
| OA chondrocytes | Culture supernatant | Ultracentrifugation | 1 × 106/mL | Co-incubation | Synovial macrophages | Promoted OA progression by stimulating inflammasome activation and upregulating mature IL-1β production in synovial macrophages | [30] |
| Primary chondrocytes | Conditioned medium | Ultracentrifugation | 200 μg/mL | Co-incubation for 48 h Intra-articular injection | Chondrocytes | Prevented OA via the restoration of mitochondrial function and macrophage polarization toward the M2 phenotype | [31] |
| OA osteoblasts | Conditioned medium | Ultracentrifugation | 20 μg/mL | Co-incubation for 14 d | Chondrocytes | Promoted OA progression by suppressing oxygen consumption by chondrocytes via miR-210-5p. | [32] |
| BM-MSCs | Conditioned medium | Ultracentrifugation | 10 μg/mL | Co-incubation for 24 h | Chondrocytes | Promoted proliferation and inhibited apoptosis of chondrocyte via miR-206/GIT1 axis | [33,34] |
| BM-MSCs | Conditioned medium | Ultracentrifugation | 250 ng | Intra-articular injection | Chondrocytes | Prevented OA development by inhibiting the degradation of cartilage and the formation of osteophyte | [35] |
| BM-MSCs | Conditioned medium | Ultracentrifugation | 200 μg/mL | 3D printed ECM/GelMA/exosome scaffolds | Osteochondral defect rabbit model | Prevented OA development by facilitating cartilage regeneration and restoring chondrocyte mitochondrial function | [36] |
| SMSCs | Conditioned medium | Ultracentrifugation | 5 μg | Co-incubation for 12 h | Chondrocytes | Prevented the development of OA by facilitating migration, proliferation and ECM secretion and suppressing chondrocyte apoptosis | [37] |
| SMSCs | Conditioned medium | Ultracentrifugation | 1010 particles | Intra-articular injection | DMM mice model | Prevented OA development by enhancing cartilage tissue regeneration via miR-140-5p upregulation of Wnt and YAP | [38] |
| ESC-MSCs | Conditioned medium | Ultrafiltration | 5 μg/mL 100 μg | Co-incubation for 48 h Intra-articular injection | TMJ condylar chondrocytes | Prevented OA development via inflammation attenuation and matrix homeostasis restoration | [39] |
| ESC-MSCs | Conditioned medium | Ultracentrifugation | 881 ng | Intra-articular injection | DMM OA model | Prevented OA development by balancing cartilage ECM synthesis and degradation | [40] |
| iPSC-MSCs | Conditioned medium | Ultracentrifugation | 8 μL 1010/mL | Intra-articular injection | Collagenase-induced OA model | Prevented OA development by promoting migration and proliferation of chondrocytes | [41] |
| UC-MSCs | Conditioned medium | Ultracentrifugation | 10 μg/mL 100 μg | Co-incubation for 72 h Intra-articular injection | Rat cartilage defect model | Mechanical stimulation increased the expression level of LncRNA H19 in exosomes, which promoted chondrocyte proliferation, matrix synthesis, and inhibited apoptosis | [42] |
| ADSCs | Conditioned medium | Ultracentrifugation | 400 µg/mL | Co-incubation for 48 h | Chondrocytes | Prevented OA development by promoting chondrogenesis and suppressing inflammation via upregulating miR-221 and miR-145 | [43] |
| ADSCs | Conditioned medium | Ultracentrifugation | 108 particles | Intra-articular injection | DMM and MIA induced OA model | Prevented OA development by inhibiting proteoglycan degradation and cartilage destruction and ameliorating gait abnormality | [44,45] |
| AFSC | Conditioned medium | Precipitation | 30 μg 100 μg | Co-incubation for 72 h Intra-articular injection | MIA-induced OA mice model | Prevent the development of OA by promoting chondrocyte proliferation, cartilage matrix synthesis, and polarizing macrophages to M2 phenotype | [46] |
| Engineered CAP-Lamp2b exosomes | Conditioned medium | Ultracentrifugation | 10 μg 100 μg | Co-incubation for 3 h Intra-articular injection | Chondrocytes DMM OA rat model | Prevented OA development by delivering miR-140 to deep cartilage regions and inhibiting cartilage-degrading proteases | [47] |
| CPCs | Conditioned medium | Ultracentrifugation | 108/mL 8 × 107 particle | Co-incubation for 3 h Intra-articular injection | Chondrocytes | Enhanced articular cartilage repair by stimulating chondrocyte proliferation and migration via upregulating miRNA 221-3p | [48] |
| Synoviocytes | Conditioned medium | Ultracentrifugation | 20 μg/mL | Co-incubation for 24 h | Chondrocytes | Promoted OA progression by inducing apoptosis and cartilage matrix degradation via upregulating miR-142-5p/RUNX2 | [49] |
| Synovial fibroblasts | Patient synovial fluid | Ultracentrifugation | 2 × 109/mL 20 μg | Co-incubation for 48 h Intra-articular injection | ACLT + MMx OA rat model | Prevented OA development by suppressing chondrocyte apoptosis, constraining inflammation, and cartilage degeneration | [50] |
| PRP | PRP | exoEasy Maxi Kit | 50 μg/mL 100 μg/mL | Co-incubation for 24 h Intra-articular injection | Chondrocytes | Prevented OA development by facilitating proliferation and reducing apoptosis of chondrocyte via Wnt/β-catenin | [17] |
| CPRP | Whole blood | Ultracentrifugation | 1.42 × 109 particles | Co-incubation for 48 h | OA chondrocytes | Prevented OA development by inducing chondrogenic gene expression changes and preventing proinflammatory cytokine release | [51] |
| IPFP | IPFP | Ultracentrifugation | 10 μL 1010/mL | Intra-articular injection | DMM mice model | Prevented OA development by alleviating articular cartilage damage via miR-100-5p downregulation of mTOR | [44] |
| Tenocyte | Conditioned medium | Ultracentrifugation | 486.3 μg/mL | Co-incubation for 48 h | Tendon stem cells | Promoted tendon healing by regulating tendon ECM metabolism and inducing the tenogenic differentiation of MSCs via upregulating transforming growth factor-beta | [52,53] |
| Periodontal ligament cells | PureExo® exosome isolation kit | Precipitation | 5 μg/mL | Co-incubation for 48 h | Macrophage | Regulated macrophage function and maintained inflammation homeostasis by suppressing IL-1β via inhibiting NF-κB signaling pathway | [54] |
| LPS-pretreated PDLFs | Conditioned medium | Ultracentrifugation | 100 μg/mL | Co-incubation for 48 h | Osteoblast | Prevented bone remodeling by inducing inflammation and inhibiting osteogenic activity of osteoblasts, promoting macrophage polarization toward M1 via YAP | [55,56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Li, Z.; He, Y. Exosomes in the Pathogenesis, Progression, and Treatment of Osteoarthritis. Bioengineering 2022, 9, 99. https://doi.org/10.3390/bioengineering9030099
Fan Y, Li Z, He Y. Exosomes in the Pathogenesis, Progression, and Treatment of Osteoarthritis. Bioengineering. 2022; 9(3):99. https://doi.org/10.3390/bioengineering9030099
Chicago/Turabian StyleFan, Yishu, Zhong Li, and Yuchen He. 2022. "Exosomes in the Pathogenesis, Progression, and Treatment of Osteoarthritis" Bioengineering 9, no. 3: 99. https://doi.org/10.3390/bioengineering9030099







