Investigating the Processing Potential of Ethiopian Agricultural Residue Enset/Ensete ventricosum for Biobutanol Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Sample Preparation
2.2. Compositional Analysis
2.3. Pretreatment and Enzymatic Hydrolysis
2.4. Microorganism and Culture Maintenance
2.5. ABE Fermentation
2.6. Analytical Methods
3. Results
3.1. Compositional Analysis
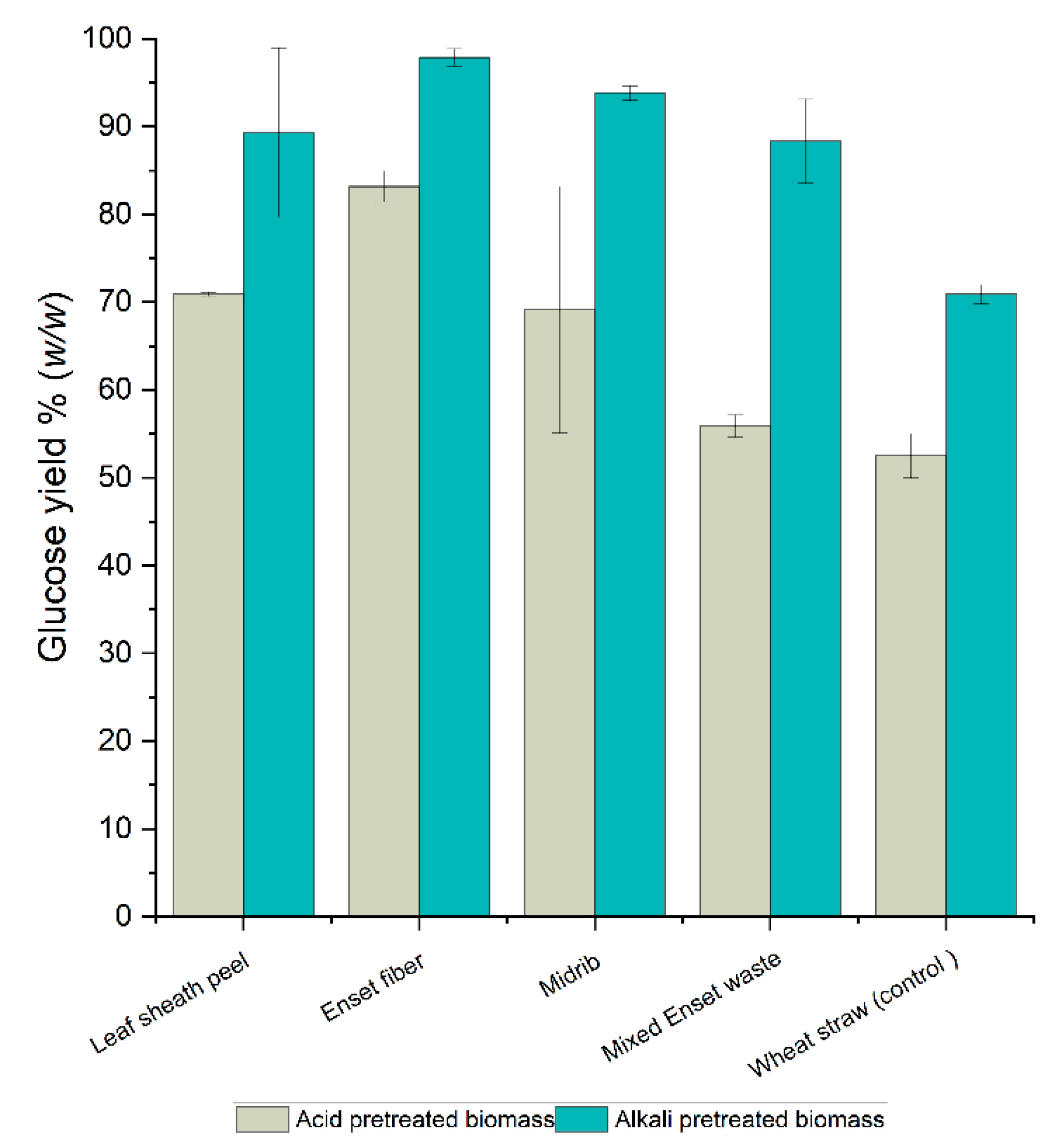
3.2. Effect of Dilute Alkali and Acid Pretreatment Method on Enzymatic Hydrolysis
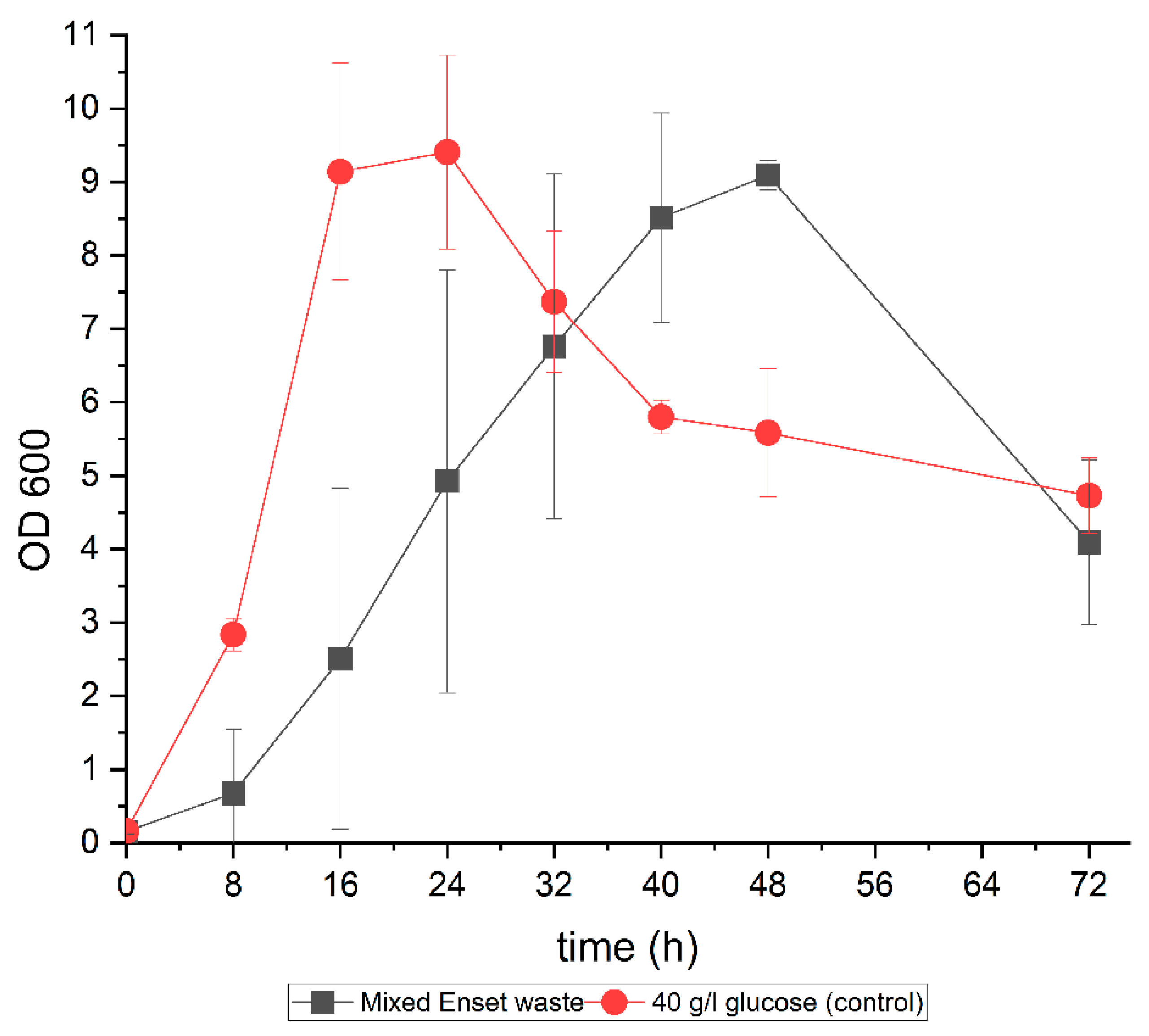
3.3. ABE Fermentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afolalu, S.A.; Yusuf, O.O.; Abioye, A.A.; Emetere, M.E.; Ongbali, S.O.; Samuel, O.D. Biofuel; A Sustainable Renewable Source of Energy—A Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 665, 012040. [Google Scholar] [CrossRef]
- Malode, S.J.; Prabhu, K.K.; Mascarenhas, R.J.; Shetti, N.P.; Aminabhavi, T.M. Recent advances and viability in biofuel production. Energy Convers. Manag. X 2021, 10, 100070. [Google Scholar] [CrossRef]
- Negash, M.; Swinnen, J.F.M. Biofuels and food security: Micro-evidence from Ethiopia. Energy Policy 2013, 61, 963–976. [Google Scholar] [CrossRef] [Green Version]
- Guta, D.D. Assessment of Biomass Fuel Resource Potential and Utilization in Ethiopia: Sourcing Strategies for Renewable Energies. Int. J. Renew. Energy Res. 2012, 2, 131–139. [Google Scholar]
- Berhanu, M.; Jabasingh, S.A.; Kifile, Z. Expanding sustenance in Ethiopia based on renewable energy resources—A comprehensive review. Renew. Sustain. Energy Rev. 2017, 75, 1035–1045. [Google Scholar] [CrossRef]
- Chandel, A.K.; Garlapati, V.K.; Jeevan Kumar, S.P.; Hans, M.; Singh, A.K.; Kumar, S. The role of renewable chemicals and biofuels in building a bioeconomy. Biofuels Bioprod. Biorefin. 2020, 14, 830–844. [Google Scholar] [CrossRef]
- Li, H.G.; Luo, W.; Gu, Q.Y.; Wang, Q.; Hu, W.J.; Yu, X. Bin Acetone, butanol, and ethanol production from cane molasses using Clostridium beijerinckii mutant obtained by combined low-energy ion beam implantation and N-methyl-N-nitro-N-nitrosoguanidine induction. Bioresour. Technol. 2013, 137, 254–260. [Google Scholar] [CrossRef]
- Savaliya, M.L.; Dhorajiya, B.D.; Dholakiya, B.Z. Recent advancement in production of liquid biofuels from renewable resources: A review. Res. Chem. Intermed. 2015, 41, 475–509. [Google Scholar] [CrossRef]
- Hönig, V.; Kotek, M.; Mařík, J. Use of butanol as a fuel for internal combustion engines. Agron. Res. 2014, 12, 333–340. [Google Scholar]
- Zhu, L.D.; Hiltunen, E. Application of livestock waste compost to cultivate microalgae for bioproducts production: A feasible framework. Renew. Sustain. Energy Rev. 2016, 54, 1285–1290. [Google Scholar] [CrossRef]
- Cheng, H.H.; Whang, L.M.; Chan, K.C.; Chung, M.C.; Wu, S.H.; Liu, C.P.; Tien, S.Y.; Chen, S.Y.; Chang, J.S.; Lee, W.J. Biological butanol production from microalgae-based biodiesel residues by Clostridium acetobutylicum. Bioresour. Technol. 2015, 184, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.B.; Ballesteros, I.; Ballesteros, M. The potential of agricultural banana waste for bioethanol production. Fuel 2018, 213, 176–185. [Google Scholar] [CrossRef]
- Qureshi, N.; Saha, B.C.; Dien, B.; Hector, R.E.; Cotta, M.A. Production of butanol (a biofuel) from agricultural residues: Part I—Use of barley straw hydrolysate. Biomass Bioenergy 2010, 34, 559–565. [Google Scholar] [CrossRef]
- Ruan, Z.; Zanotti, M.; Archer, S.; Liao, W.; Liu, Y. Oleaginous fungal lipid fermentation on combined acid- and alkali-pretreated corn stover hydrolysate for advanced biofuel production. Bioresour. Technol. 2014, 163, 12–17. [Google Scholar] [CrossRef]
- Singhania, R.R.; Saini, J.K.; Saini, R.; Adsul, M.; Mathur, A.; Gupta, R.; Tuli, D.K. Bioethanol production from wheat straw via enzymatic route employing penicillium janthinellum cellulases. Bioresour. Technol. 2014, 169, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Dagnino, E.P.; Chamorro, E.R.; Romano, S.D.; Felissia, F.E.; Area, M.C. Optimization of the acid pretreatment of rice hulls to obtain fermentable sugars for bioethanol production. Ind. Crops Prod. 2013, 42, 363–368. [Google Scholar] [CrossRef]
- Laser, M.; Schulman, D.; Allen, S.G.; Lichwa, J.; Antal, M.J.; Lynd, L.R. A comparison of liquid hot water and steam pretreatments of sugar cane bagasse for bioconversion to ethanol. Bioresour. Technol. 2002, 81, 33–44. [Google Scholar] [CrossRef]
- Baral, N.R.; Shah, A. Microbial inhibitors: Formation and effects on acetone-butanol-ethanol fermentation of lignocellulosic biomass. Appl. Microbiol. Biotechnol. 2014, 98, 9151–9172. [Google Scholar] [CrossRef]
- Shahzadi, T.; Mehmood, S.; Irshad, M.; Anwar, Z.; Afroz, A.; Zeeshan, N.; Rashid, U.; Sughra, K. Advances in lignocellulosic biotechnology: A brief review on lignocellulosic biomass and cellulases. Adv. Biosci. Biotechnol. 2014, 5, 246–251. [Google Scholar] [CrossRef] [Green Version]
- Berhanu, H.; Kiflie, Z.; Miranda, I.; Lourenço, A.; Ferreira, J.; Feleke, S.; Yimam, A.; Pereira, H. Characterization of crop residues from false banana /Ensete ventricosum/ in Ethiopia in view of a full-resource valorization. PLoS ONE 2018, 13, e0199422. [Google Scholar] [CrossRef] [PubMed]
- Tsegaye, A.; Struik, P.C. Enset (Ensete ventricosum (Welw.) Cheesman) kocho yield under different crop establishment methods as compared to yields of other carbohydrate-rich food crops. Neth. J. Agric. Sci. 2001, 49, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, B.; Martin, G.; Laila, M.K. Nutritive values of the drought tolerant food and fodder crop enset. Afr. J. Agric. Res. 2013, 8, 2326–2333. [Google Scholar] [CrossRef]
- Brandt, S.A.; Spring, A.; Hiebsch, C.; McCabe, J.T.; Tabogie, E.; Diro, M.; Wolde-Michael, G.; Yntiso, G.; Shigeta, M.; Tesfaye, S. Tree Against Hunger: Enset-Based Agricultural Systems in Ethiopia; American Association for the Advancement of Science: Washington, DC, USA, 1997. [Google Scholar]
- Birmeta, G.; Nybom, H.; Bekele, E. Distinction between wild and cultivated enset (Ensete ventricosum) gene pools in Ethiopia using RAPD markers. Hereditas 2004, 148, 139–148. [Google Scholar] [CrossRef]
- Nurfeta, A.; Eik, L.O.; Tolera, A.; Sundstøl, F. Chemical composition and in sacco dry matter degradability of different morphological fractions of 10 enset (Ensete ventricosum) varieties. Anim. Feed. Sci. Technol. 2008, 146, 55–73. [Google Scholar] [CrossRef]
- Borrell, J.S.; Biswas, M.K.; Goodwin, M.; Blomme, G.; Schwarzacher, T.; Heslop-Harrison, J.S.; Wendawek, A.M.; Berhanu, A.; Kallow, S.; Janssens, S.; et al. Enset in Ethiopia: A poorly characterized but resilient starch staple. Ann. Bot. 2019, 123, 747–766. [Google Scholar] [CrossRef] [Green Version]
- Acero, M.; Mukasa, S.B.; Baguma, Y. Morphotypes, distribution and uses of false banana in Uganda. Afr. Crop Sci. J. 2018, 26, 575–585. [Google Scholar] [CrossRef]
- Koch, O.; Mengesha, W.A.; Pironon, S.; Pagella, T.; Ondo, I.; Rosa, I.; Wilkin, P.; Borrell, J.S. Modelling potential range expansion of an underutilised food security crop in Sub-Saharan Africa. Environ. Res. Lett. 2022, 17, 014022. [Google Scholar] [CrossRef]
- Hunduma, T.; Mogessie, A. Traditional Enset (Ensete ventricosum) Processing Techniques in Some Parts of West Shewa Zone, Ethiopia. J. Agric. Dev. 2011, 2, 37–57. [Google Scholar]
- Erebo, D.L. Evaluation of Bioethanol Production from Enset (Ensete ventricosum (Welw.) Cheesman) Processing Waste and Leaf Using Saccharomyces cerevisiae. Sci. Publ. Gr. 2021, 5, 49–55. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Wang, Y.; Wang, Y. Improvement of acetone-butanol-ethanol (ABE) production from switchgrass pretreated with a radio frequency-assisted heating process. Fuel 2016, 182, 166–173. [Google Scholar] [CrossRef]
- ANKOM Technology. Determining Acid Detergent Lignin in Beakers; ANKOM Technologies: Macedon, NY, USA, 2020. [Google Scholar]
- Ankom Technology. Neutral Detergent Fiber in Feeds—Filter Bag Technique (for A2000 and A2000I); ANKOM Technologies: Macedon, NY, USA, 2011. [Google Scholar]
- ANKOM Technology. Acid Detergent Fiber in Feeds—Filter Bag Technique (for A200 and A200I); ANKOM Technologies: Macedon, NY, USA, 2017. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Hindrichsen, I.K.; Kreuzer, M.; Madsen, J.; Bach Knudsen, K.E. Fiber and lignin analysis in concentrate, forage, and feces: Detergent versus enzymatic-chemical method. J. Dairy Sci. 2006, 89, 2168–2176. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Gao, K.; Boiano, S.; Marzocchella, A.; Rehmann, L. Cellulosic butanol production from alkali-pretreated switchgrass (Panicum virgatum) and phragmites (Phragmites australis). Bioresour. Technol. 2014, 174, 176–181. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Wolfe, J. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Procentese, A.; Raganati, F.; Olivieri, G.; Elena Russo, M.; Marzocchella, A. Pre-treatment and enzymatic hydrolysis of lettuce residues as feedstock for bio-butanol production. Biomass Bioenergy 2017, 96, 172–179. [Google Scholar] [CrossRef]
- Wang, P.; Chen, Y.M.; Wang, Y.; Lee, Y.Y.; Zong, W.; Taylor, S.; McDonald, T.; Wang, Y. Towards comprehensive lignocellulosic biomass utilization for bioenergy production: Efficient biobutanol production from acetic acid pretreated switchgrass with Clostridium saccharoperbutylacetonicum N1-4. Appl. Energy 2019, 236, 551–559. [Google Scholar] [CrossRef]
- Infantes, A.; Kugel, M.; Neumann, A. Evaluation of Media Components and Process Parameters in a Sensitive and Robust Fed-Batch Syngas Fermentation System with Clostridium ljungdahlii. Fermentation 2020, 6, 61. [Google Scholar] [CrossRef]
- Ujor, V.; Bharathidasan, A.K.; Cornish, K.; Ezeji, T.C. Evaluation of industrial dairy waste (milk dust powder) for acetone-butanol-ethanol production by solventogenic Clostridium species. SpringerPlus 2014, 3, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, D.; Dong, S.; Wang, P.; Chen, T.; Wang, J.; Yue, Z.B.; Wang, Y. Robustness of Clostridium saccharoperbutylacetonicum for acetone-butanol-ethanol production: Effects of lignocellulosic sugars and inhibitors. Fuel 2017, 208, 549–557. [Google Scholar] [CrossRef]
- Stabel, M.; Schweitzer, T.; Haack, K.; Gorenflo, P.; Aliyu, H. Isolation and Biochemical Characterization of Six Anaerobic Fungal Strains from Zoo Animal Feces. Microorganisms 2021, 9, 1655. [Google Scholar] [CrossRef]
- Baleeiro, F.C.F.; Kleinsteuber, S.; Sträuber, H. Hydrogen as a Co-electron Donor for Chain Elongation with Complex Communities. Front. Bioeng. Biotechnol. 2021, 9, 650631. [Google Scholar] [CrossRef]
- Ochsenreither, K.; Fischer, C.; Neumann, A.; Syldatk, C. Process characterization and influence of alternative carbon sources and carbon-to-nitrogen ratio on organic acid production by Aspergillus oryzae DSM1863. Appl. Microbiol. Biotechnol. 2014, 98, 5449–5460. [Google Scholar] [CrossRef]
- Neumann, A.; Scholz-Muramatsu, H.; Diekert, G. Tetrachloroethene metabolism of Dehalospirillum multivorans. Arch. Microbiol. 1994, 162, 295–301. [Google Scholar] [CrossRef] [PubMed]
- García, R.; Pizarro, C.; Lavín, A.G.; Bueno, J.L. Characterization of Spanish biomass wastes for energy use. Bioresour. Technol. 2012, 103, 249–258. [Google Scholar] [CrossRef]
- García, R.; Pizarro, C.; Lavín, A.G.; Bueno, J.L. Spanish biofuels heating value estimation. Part I: Ultimate analysis data. Fuel 2014, 117, 1130–1138. [Google Scholar] [CrossRef]
- Naik, S.; Goud, V.V.; Rout, P.K.; Jacobson, K.; Dalai, A.K. Characterization of Canadian biomass for alternative renewable biofuel. Renew. Energy 2010, 35, 1624–1631. [Google Scholar] [CrossRef]
- Ribas, E.; Fernandes, K.; Marangoni, C.; Souza, O.; Sellin, N. Thermochemical characterization of banana leaves as a potential energy source. Energy Convers. Manag. 2013, 75, 603–608. [Google Scholar] [CrossRef]
- Raj, T.; Kapoor, M.; Gaur, R.; Christopher, J.; Lamba, B.; Tuli, D.K.; Kumar, R. Physical and Chemical Characterization of Various Indian Agriculture Residues for Biofuels Production. Energy Fuels 2015, 29, 3111–3131. [Google Scholar] [CrossRef]
- Librenti, E.; Ceotto, E.; Di Candilo, M. Biomass Characteristics and Energy Contents of Dedicated Lignocellulosic Crops; CRA-CIN, Research Center for Industrial Crops: Bologna, Italy, 2018; 9p. [Google Scholar]
- Sohni, S.; Norulaini, N.A.N.; Hashim, R.; Khan, S.B.; Fadhullah, W.; Mohd Omar, A.K. Physicochemical characterization of Malaysian crop and agro-industrial biomass residues as renewable energy resources. Ind. Crops Prod. 2018, 111, 642–650. [Google Scholar] [CrossRef]
- Diyana, Z.N.; Jumaidin, R.; Selamat, M.Z.; Alamjuri, R.H.; Yusof, F.A. Extraction and Characterization of Natural Cellulosic Fiber from Pandanus amaryllifolius Leaves. Polymers 2021, 13, 4171. [Google Scholar] [CrossRef]
- Adapa, P.; Tabil, L.; Schoenau, G. Compaction characteristics of barley, canola, oat and wheat straw. Biosyst. Eng. 2009, 104, 335–344. [Google Scholar] [CrossRef]
- Mani, S.; Tabil, L.G.; Sokhansanj, S. Effects of compressive force, particle size and moisture content on mechanical properties of biomass pellets from grasses. Biomass Bioenergy 2008, 30, 648–654. [Google Scholar] [CrossRef]
- Guimarães, J.L.; Frollini, E.; Silva, C.G.; Wypych, F.; Satyanarayana, K.G. Characterization of banana, sugarcane bagasse and sponge gourd fibers of Brazil. Ind. Crops Prod. 2009, 30, 407–415. [Google Scholar] [CrossRef]
- Bhatnagar, R.; Gupta, G.; Yadav, S. A Review on Composition and Properties of Banana Fibers. Int. J. Sci. Eng. Res. 2015, 6, 49–52. [Google Scholar]
- Mahardika, M.; Abral, H.; Kasim, A.; Arief, S.; Asrofi, M. Production of Nanocellulose from Pineapple Leaf Fibers via High-Shear Homogenization and Ultrasonication. Fibers 2018, 6, 28. [Google Scholar] [CrossRef] [Green Version]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Raud, M.; Kikas, T.; Sippula, O.; Shurpali, N.J. Potentials and challenges in lignocellulosic biofuel production technology. Renew. Sustain. Energy Rev. 2019, 111, 44–56. [Google Scholar] [CrossRef]
- Nanda, S.; Mohanty, P.; Pant, K.K.; Naik, S.; Kozinski, J.A.; Dalai, A.K. Characterization of North American Lignocellulosic Biomass and Biochars in Terms of their Candidacy for Alternate Renewable Fuels. Bioenergy Res. 2013, 6, 663–677. [Google Scholar] [CrossRef]
- Rasmussen, H.; Sørensen, H.R.; Meyer, A.S. Formation of degradation compounds from lignocellulosic biomass in the biorefinery: Sugar reaction mechanisms. Carbohydr. Res. 2014, 385, 45–57. [Google Scholar] [CrossRef]
- Haldar, D.; Sen, D.; Gayen, K. A review on the production of fermentable sugars from lignocellulosic biomass through conventional and enzymatic route—A comparison. Int. J. Green Energy 2016, 13, 1232–1253. [Google Scholar] [CrossRef]
- Zhu, L.; O’Dwyer, J.P.; Chang, V.S.; Granda, C.B.; Holtzapple, M.T. Structural features affecting biomass enzymatic digestibility. Bioresour. Technol. 2008, 99, 3817–3828. [Google Scholar] [CrossRef]
- Lorenci, A.; José, C.; Neto, D.; Porto, L.; Vandenberghe, D.S.; Pedro, D.; Neto, D.C.; Cristine, A.; Sydney, N.; Alberto, L.; et al. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance—Conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Prakash, J. Sustainable bio-ethanol production from agro-residues: A review. Renew. Sustain. Energy Rev. 2015, 41, 550–567. [Google Scholar] [CrossRef]
- Gao, K.; Rehmann, L. ABE fermentation from enzymatic hydrolysate of NaOH-pretreated corncobs. Biomass Bioenergy 2014, 66, 110–115. [Google Scholar] [CrossRef]
- Cho, D.H.; Shin, S.J.; Kim, Y.H. Effects of acetic and formic acid on ABE production by Clostridium acetobutylicum and Clostridium beijerinckii. Biotechnol. Bioprocess Eng. 2012, 17, 270–275. [Google Scholar] [CrossRef]
- Antranikian, G.; Friese, C.; Quentmeier, A.; Hippe, H.; Gottschalk, G. Distribution of the ability for citrate utilization amongst Clostridia. Arch. Microbiol. 1984, 138, 179–182. [Google Scholar] [CrossRef]
- Kihara, T.; Noguchi, T.; Tashiro, Y.; Sakai, K.; Sonomoto, K. Highly efficient continuous acetone-butanol-ethanol production from mixed sugars without carbon catabolite repression. Bioresour. Technol. Reports 2019, 7, 100185. [Google Scholar] [CrossRef]
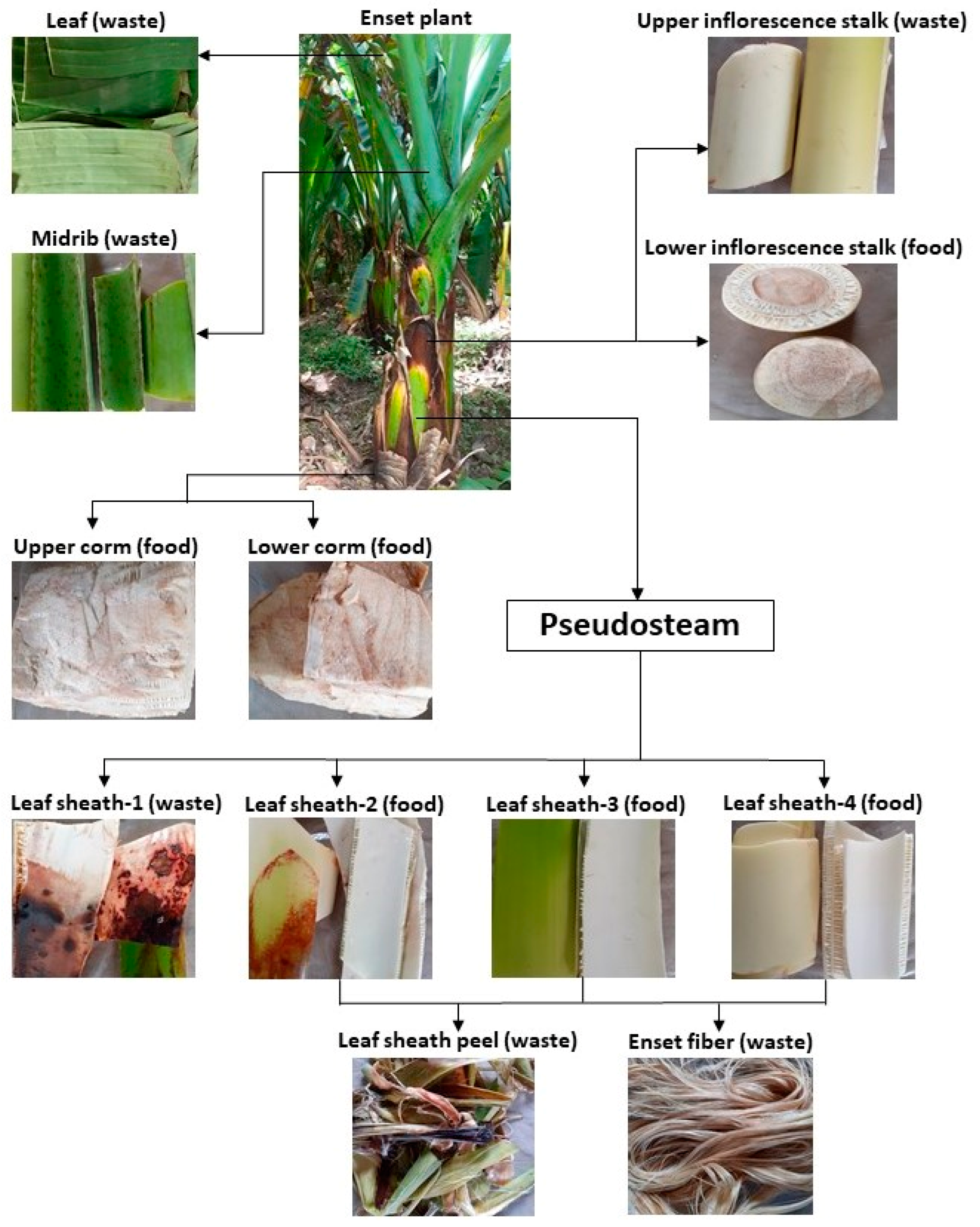



| Analysis | LS1 | LS2 | LS3 | LS4 | UIS | LIS | UC | LC | LSP | EF | M | L |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proximate analysis (% dry weight, w/w) | ||||||||||||
| Ash | 12.9 | 7.4 | 10.8 | 8.8 | 19.1 | 8.5 | 7.1 | 4.8 | 14.4 | 4.7 | 15.4 | 13.7 |
| Moisture | 7.4 | 9.7 | 9.4 | 10.3 | 7.8 | 9 | 10.2 | 10.6 | 7.7 | 7.3 | 6.5 | 5.7 |
| Elemental analysis (% dry weight, w/w) | ||||||||||||
| C | 37.8 | 38.5 | 37.9 | 38.2 | 37.3 | 39.1 | 38.4 | 38.4 | 37.9 | 41.2 | 37.1 | 42.8 |
| H | 5.5 | 6.1 | 6 | 6.2 | 5.2 | 5.9 | 6.2 | 6.2 | 5.5 | 6.1 | 5.2 | 5.7 |
| N | 0.5 | 0.5 | 0.8 | 0.6 | 2.5 | 1.2 | 0.6 | 0.6 | 0.7 | 0.3 | 1.2 | 3.3 |
| O a | 43.3 | 47.5 | 44.5 | 46.2 | 35.9 | 45.3 | 47.7 | 50 | 41.5 | 47.7 | 41.1 | 34.5 |
| Calorific value (CV) (MJ/kg) | ||||||||||||
| CV | 14.4 | 15.02 | 14.7 | 14.8 | 14.7 | 15.3 | 14.9 | 14.98 | 14.62 | 16.14 | 14.28 | 17.4 |
| Analysis (% Dry Matter) | LS1 | LS2 | LS3 | LS4 | UIS | LIS | UC | LC | LSP | EF | M | L |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cellulose | 26.4 | 5.6 | 8 | 6.1 | 32 | 6.7 | 2.2 | 3.8 a | 34.1 | 67.1 | 40 | 20 |
| Hemicellulose | 18.6 | 10.2 | 9.5 | 6.6 | 20 | 20 | 6.2 | 11.4 | 15.7 | 15.6 | 19.7 | 27 |
| Lignin | 6.8 | 0.3 | 0.6 | 0.5 | 6.5 | 1.7 | 0.4 | 0.7 | 6.3 | 5.1 | 3.1 | 3.8 |
| Compounds [% (w/w)] | Leaf Sheath Peel | Enset Fiber | Midrib | Mixed Enset Waste |
|---|---|---|---|---|
| Cellobiose | 0.8 ± 0.3 | 2.5 ± 0.4 | 1.1 ± 0.2 | 1.3 ± 0.4 |
| Glucose | 56.4 ± 0.56 | 65.5 ± 4.73 | 39.1 ± 2.22 | 45.1 ± 0.21 |
| Arabinose | 2.4 ± 0.42 | 0.9 ± 0.16 | 3.1 ± 0.65 | 3.3 ± 0.04 |
| Other sugar (xylose, mannose, and galactose) | 11.4 ± 0.61 | 12.8 ± 0.68 | 10.5 ± 1.27 | 10.5 ± 0.84 |
| Formic acid | 2.0 ± 0.08 | 2.2 ± 0.23 | 2.1 ± 0.13 | 1.9 ± 0.12 |
| Acetic acid | 5.0 ± 0.46 | 6.7± 0.57 | 7.4 ± 0.55 | 9.0 ± 0.7 |
| Furfural | 0.8 ± 0.02 | 1.0 ± 0.04 | 0.4 ± 0.03 | 0.7 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seid, N.; Griesheimer, P.; Neumann, A. Investigating the Processing Potential of Ethiopian Agricultural Residue Enset/Ensete ventricosum for Biobutanol Production. Bioengineering 2022, 9, 133. https://doi.org/10.3390/bioengineering9040133
Seid N, Griesheimer P, Neumann A. Investigating the Processing Potential of Ethiopian Agricultural Residue Enset/Ensete ventricosum for Biobutanol Production. Bioengineering. 2022; 9(4):133. https://doi.org/10.3390/bioengineering9040133
Chicago/Turabian StyleSeid, Nebyat, Pia Griesheimer, and Anke Neumann. 2022. "Investigating the Processing Potential of Ethiopian Agricultural Residue Enset/Ensete ventricosum for Biobutanol Production" Bioengineering 9, no. 4: 133. https://doi.org/10.3390/bioengineering9040133
APA StyleSeid, N., Griesheimer, P., & Neumann, A. (2022). Investigating the Processing Potential of Ethiopian Agricultural Residue Enset/Ensete ventricosum for Biobutanol Production. Bioengineering, 9(4), 133. https://doi.org/10.3390/bioengineering9040133