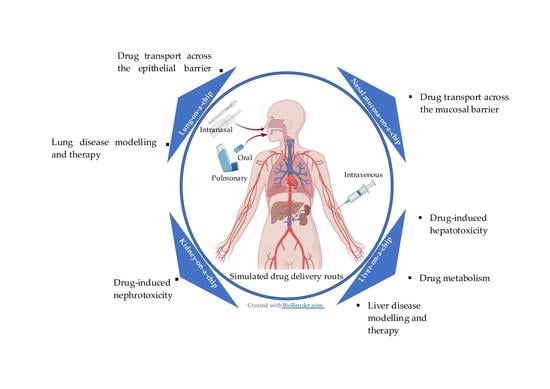
Application of Micro-Engineered Kidney, Liver, and Respiratory System Models to Accelerate Preclinical Drug Testing and Development
Abstract
:1. Introduction
2. Drug Testing Capabilities Using Liver-, Kidney-, and Lung-on-Chip Models
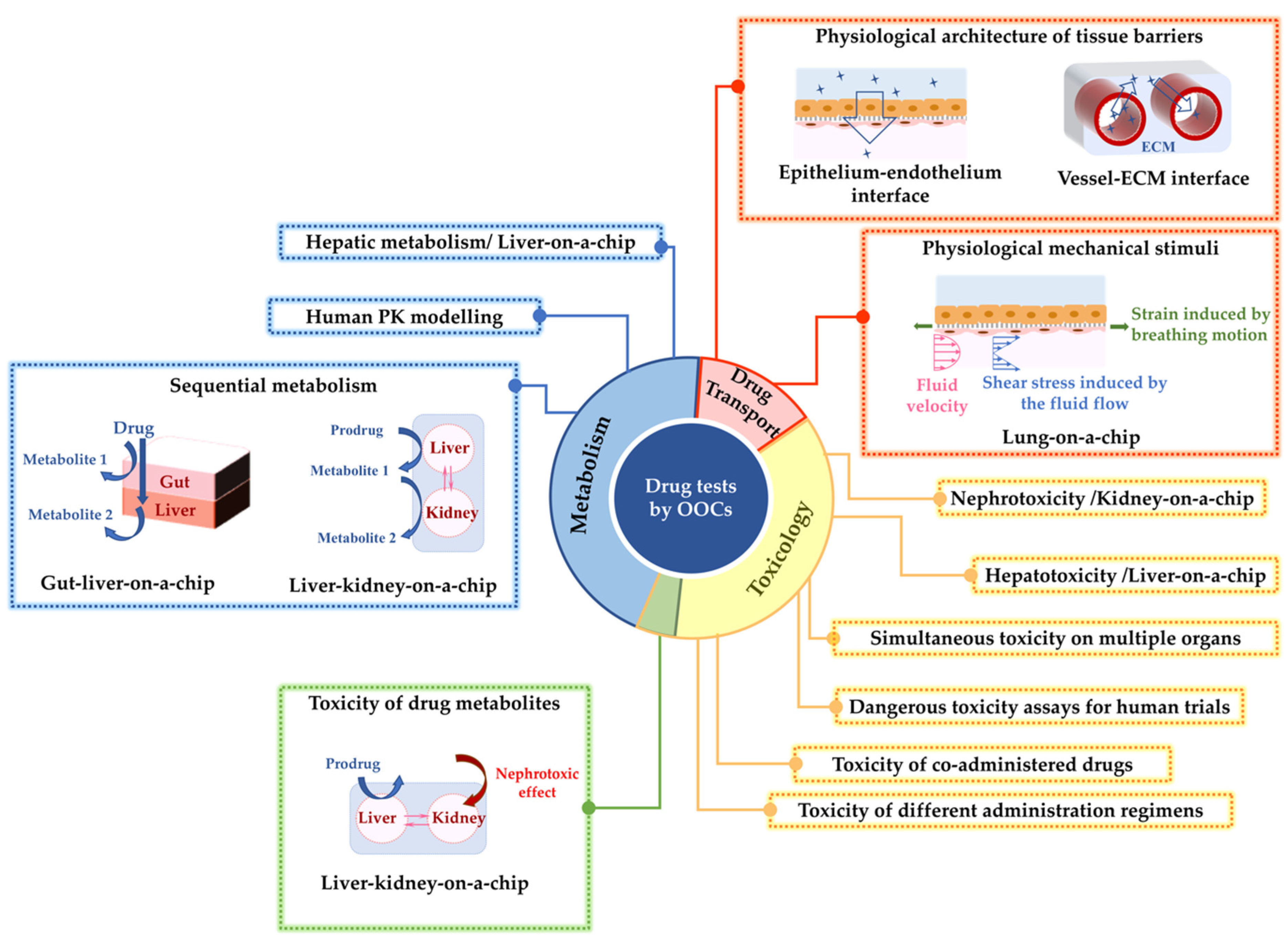
2.1. Drug Metabolism Studies
| Drug | Toxicology | Metabolism | Tissue(s) | Reference |
|---|---|---|---|---|
| diclofenac acetaminophen | ✓ | liver | [18] | |
| troglitazone | ✓ | liver | [19] | |
| acetaminophen | ✓ | liver | [32,33,34,35,36] | |
| acetaminophen | ✓ | ✓ | liver | [37,38] |
| acetaminophen isoniazid rifampicin | ✓ | ✓ | liver | [39] |
| rifampin ketoconzazole acetaminophen | ✓ | ✓ | liver | [40] |
| bupropion tolbutamide omeprazole testosterone | ✓ | liver | [24] | |
| 7-ethoxy-4-trifluoromethyl coumarin | ✓ | liver | [25] | |
| acetaminophen chlorpromazine tacrine | ✓ | liver | [41] | |
| ccetaminophen fialuridine | ✓ | ✓ | liver | [42] |
| diclofenac | ✓ | ✓ | liver | [43] |
| cadmium aspirin caffein troglitazone rosiglitazone pioglitazone acetaminophen | ✓ | liver | [2] | |
| cisplatin | ✓ | kidney | [14] | |
| adriamycin | ✓ | kidney | [44] | |
| gentamicin | ✓ | kidney | [45] | |
| polymyxin B | ✓ | kidney | [46] | |
| carboxylated polystyrene nanoparticles | ✓ | GI tract–liver | [47] | |
| troglitazone | ✓ | ✓ | liver–intestine liver–skin | [48] |
| apigenin | ✓ | gut–liver | [28] | |
| epirubicine irinotecan cyclophosphamide | ✓ | small intestine–liver–lung | [29] | |
| ifosfamide verapamil | ✓ | ✓ | liver–kidney | [49] |
| paracetamol | ✓ | liver–gut | [31] | |
| mannitol propranolol caffeine | ✓ | GI–liver | [50] | |
| combination of genistein and dacarbazine | ✓ | intestine–liver | [51] | |
| 5-fluorouracil | ✓ | liver–tumor–marrow | [52] | |
| paracetamol | ✓ | ✓ | liver–kidney | [53] |
| diclofenac ketoconazole hydrocortisone acetaminophen | ✓ | liver–heart–skin | [54] | |
| luteolin | ✓ | liver–tumor | [30] | |
| capecitabine tegafur | ✓ | liver–cancer intestine–liver–cancer–connective tissue | [27] | |
| digoxin | ✓ | intestine–kidney | [55] | |
| ifosfamide | ✓ | ✓ | liver–kidney | [56] |
| vitamin D | ✓ | liver–kidney | [26] |
2.2. Toxicology
2.2.1. Toxicology Studies by the Kidney- or Liver-on-Chip Models
2.2.2. Toxicology Studies by the Kidney- and Liver-on-Chip Models Interconnected with Other Organs
2.3. Drug Delivery/Transport
2.3.1. Simulation of In Vivo-Level Barrier Functions On-Chip
2.3.2. Simulation of Multiple Drug-Delivery Routes On-Chip
2.3.3. Drug Delivery Tests under In Vivo-Inspired Dynamic Conditions On-Chip
2.3.4. Further Improvements of Drug Delivery Studies by Organ-on-Chips
3. Advantages of Organ-on-Chips for Drug Testing
3.1. Organ-on-Chips Offer Engineered In Vivo-Inspired Microenvironments
3.1.1. Cellular Coculture and Organ–Organ Crosstalk
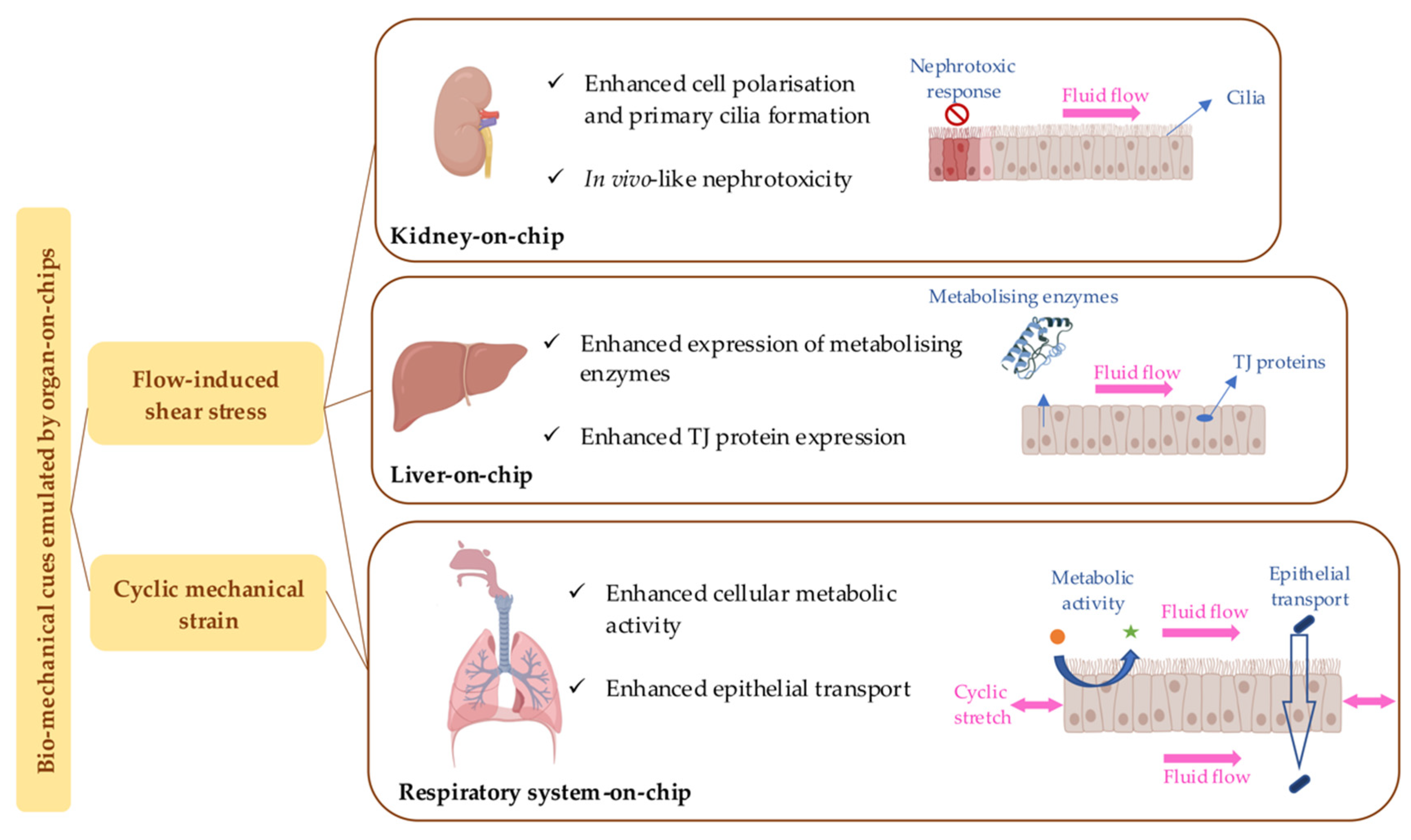
3.1.2. Simulation of Biomechanical Cues
3.1.3. Drug Testing Using Modelled Human Diseases On-Chip
3.2. Integrated Sensing Tools in Organ-on-Chips for In Situ Drug Testing
3.3. Organ-on-Chips Enable Personalized Drug Testing
4. Disadvantages of Organ-on-Chips in Drug Testing
5. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- McGonigle, P.; Ruggeri, B. Animal models of human disease: Challenges in enabling translation. Biochem. Pharmacol. 2014, 87, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Khetani, S.R.; Bhatia, S.N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2008, 26, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Esch, E.W.; Bahinski, A.; Huh, D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discoov. 2015, 14, 248–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-chip: Recent breakthroughs and future prospects. Biomed. Eng. Online 2020, 19, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Kimura, H.; Sakai, Y.; Fujii, T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab. Pharmacok. 2018, 33, 43–48. [Google Scholar] [CrossRef]
- Deng, J.; Qu, Y.; Liu, T.; Jing, B.; Zhang, X.; Chen, Z.; Luo, Y.; Zhao, W.; Lu, Y.; Lin, B. Recent organ-on-a-chip advances toward drug toxicity testing. Development 2018, 19, 20. [Google Scholar] [CrossRef]
- Cong, Y.; Han, X.; Wang, Y.; Chen, Z.; Lu, Y.; Liu, T.; Wu, Z.; Jin, Y.; Luo, Y.; Zhang, X. Drug toxicity evaluation based on organ-on-a-chip technology: A review. Micromachines 2020, 11, 381. [Google Scholar] [CrossRef] [Green Version]
- Bhise, N.S.; Ribas, J.; Manoharan, V.; Zhang, Y.S.; Polini, A.; Massa, S.; Dokmeci, M.R.; Khademhosseini, A. Organ-on-a-chip platforms for studying drug delivery systems. J. Control. Release 2014, 190, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Peng, Y.; Li, H.; Chen, W. Organ-on-a-Chip: A new paradigm for drug development. Trends Pharmacol. Sci. 2020, 42, 119–133. [Google Scholar] [CrossRef]
- Vulto, P.; Joore, J. Adoption of organ-on-chip platforms by the pharmaceutical industry. Nat. Rev. Drug Discov. 2021, 20, 961–962. [Google Scholar] [CrossRef]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-chips: Into the next decade. Nat. Rev. Drug Discov. 2021, 20, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stucki, A.O.; Stucki, J.D.; Hall, S.R.; Felder, M.; Mermoud, Y.; Schmid, R.A.; Geiser, T.; Guenat, O.T. A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab Chip 2015, 15, 1302–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, K.-J.; Mehr, A.P.; Hamilton, G.A.; McPartlin, L.A.; Chung, S.; Suh, K.Y.; Ingber, D.E. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr. Biol. 2013, 5, 1119–1129. [Google Scholar] [CrossRef]
- Abaci, H.E.; Shuler, M.L. Human-on-a-chip design strategies and principles for physiologically based pharmacokinetics/pharmacodynamics modeling. Integr. Biol. 2015, 7, 383–391. [Google Scholar] [CrossRef]
- Sung, J.H.; Wang, Y.I.; Narasimhan Sriram, N.; Jackson, M.; Long, C.; Hickman, J.J.; Shuler, M.L. Recent advances in body-on-a-chip systems. Anal. Chem. 2018, 91, 330–351. [Google Scholar] [CrossRef]
- Van Den Berg, A.; Mummery, C.L.; Passier, R.; Van der Meer, A.D. Personalised organs-on-chips: Functional testing for precision medicine. Lab Chip 2019, 19, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Deng, R.; Tong, W.H.; Huan, L.; Chan Way, N.; IslamBadhan, A.; Iliescu, C.; Yu, H. A perfusion incubator liver chip for 3D cell culture with application on chronic hepatotoxicity testing. Sci. Rep. 2017, 7, 14528. [Google Scholar] [CrossRef] [Green Version]
- Bavli, D.; Prill, S.; Ezra, E.; Levy, G.; Cohen, M.; Vinken, M.; Vanfleteren, J.; Jaeger, M.; Nahmias, Y. Real-time monitoring of metabolic function in liver-on-chip microdevices tracks the dynamics of mitochondrial dysfunction. Proc. Natl. Acad. Sci. USA 2016, 113, E2231–E2240. [Google Scholar] [CrossRef] [Green Version]
- Hornberg, J.J.; Laursen, M.; Brenden, N.; Persson, M.; Thougaard, A.V.; Toft, D.B.; Mow, T. Exploratory toxicology as an integrated part of drug discovery. Part I: Why and how. Drug Discov. Today 2014, 19, 1131–1136. [Google Scholar] [CrossRef]
- Kumar, G.N.; Surapaneni, S. Role of drug metabolism in drug discovery and development. Med. Res. Rev. 2001, 21, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Tscheik, C.; Blasig, I.E.; Winkler, L. Trends in drug delivery through tissue barriers containing tight junctions. Tissue Barriers 2013, 1, e24565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, Y.S.; Chang, S.F.; Shih, M.C.; Tseng, S.H.; Lai, C.H. Scaffold-free liver-on-a-chip with multiscale organotypic cultures. Adv. Mater. 2017, 29, 1701545. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [Green Version]
- Chang, R.; Emami, K.; Wu, H.; Sun, W. Biofabrication of a three-dimensional liver micro-organ as an in vitro drug metabolism model. Biofabrication 2010, 2, 045004. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Liang, X.; Roberts, M.S. Hepatic Metabolism in Liver Health and Disease. In Liver Pathophysiology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 391–400. [Google Scholar]
- Satoh, T.; Sugiura, S.; Shin, K.; Onuki-Nagasaki, R.; Ishida, S.; Kikuchi, K.; Kakiki, M.; Kanamori, T. A multi-throughput multi-organ-on-a-chip system on a plate formatted pneumatic pressure-driven medium circulation platform. Lab Chip 2018, 18, 115–125. [Google Scholar] [CrossRef]
- Bhise, N.S.; Manoharan, V.; Massa, S.; Tamayol, A.; Ghaderi, M.; Miscuglio, M.; Lang, Q.; Zhang, Y.S.; Shin, S.R.; Calzone, G.; et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 2016, 8, 014101. [Google Scholar] [CrossRef]
- Lee, H.; Kim, D.S.; Ha, S.K.; Choi, I.; Lee, J.M.; Sung, J.H. A pumpless multi-organ-on-a-chip (MOC) combined with a pharmacokinetic–pharmacodynamic (PK–PD) model. Biotechnol. Bioeng. 2017, 114, 432–443. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Deng, P.; Chen, W.; Guo, Y.; Tao, T.; Qin, J. In situ differentiation and generation of functional liver organoids from human iPSCs in a 3D perfusable chip system. Lab Chip 2018, 18, 3606–3616. [Google Scholar] [CrossRef]
- Theobald, J.; Ghanem, A.; Wallisch, P.; Banaeiyan, A.A.; Andrade-Navarro, M.A.; Taškova, K.; Haltmeier, M.; Kurtz, A.; Becker, H.; Reuter, S.; et al. Liver-kidney-on-chip to study toxicity of drug metabolites. ACS Biomater. Sci. Eng. 2018, 4, 78–89. [Google Scholar] [CrossRef]
- Jalili-Firoozinezhad, S.; Prantil-Baun, R.; Jiang, A.; Potla, R.; Mammoto, T.; Weaver, J.C.; Ferrante, T.C.; Kim, H.J.; Cabral, J.; Levy, O.; et al. Modeling radiation injury-induced cell death and countermeasure drug responses in a human Gut-on-a-Chip. Cell Death Dis. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.; Hamid, Q.; Wang, C.; Chang, R.; Emami, K.; Wu, H.; Sun, W. Bioprinting cell-laden matrigel for radioprotection study of liver by pro-drug conversion in a dual-tissue microfluidic chip. Biofabrication 2011, 3, 034112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuler, M.L. Organ-, body-and disease-on-a-chip systems. Lab Chip 2017, 17, 2345–2346. [Google Scholar] [CrossRef] [PubMed]
- Hongmao, S. (Ed.) Chapter 7—In Silico ADMET Profiling: Predictive Models for CYP450, P-gp, PAMPA, and hERG. In A Practical Guide to Rational Drug Design; Woodhead Publishing: Cambridge, UK, 2016; pp. 225–268. [Google Scholar]
- Larrey, D.; Ursic-Bedoya, J.; Meunier, L. Drug-induced Hepatotoxicity. Schiff Dis. Liver 2017, 32, 740–773. [Google Scholar]
- Theobald, J.; el Maaty, M.A.A.; Kusterer, N.; Wetterauer, B.; Wink, M.; Cheng, X.; Wölfl, S. In vitro metabolic activation of vitamin D3 by using a multi-compartment microfluidic liver-kidney organ on chip platform. Sci. Rep. 2019, 9, 4616. [Google Scholar] [CrossRef]
- Jie, M.; Lin, H.; He, Z.; Liu, H.; Li, H.; Lin, J.M. An on-chip intestine-liver model for multiple drugs absorption and metabolism behavior simulation. Sci. China Chem. 2018, 61, 236–242. [Google Scholar] [CrossRef]
- Kim, S.; LesherPerez, S.C.; Yamanishi, C.; Labuz, J.M.; Leung, B.; Takayama, S. Pharmacokinetic profile that reduces nephrotoxicity of gentamicin in a perfused kidney-on-a-chip. Biofabrication 2016, 8, 015021. [Google Scholar] [CrossRef]
- Mi, S.; Yi, X.; Du, Z.; Xu, Y.; Sun, W. Construction of a liver sinusoid based on the laminar flow on chip and self-assembly of endothelial cells. Biofabrication 2018, 10, 025010. [Google Scholar] [CrossRef]
- Jang, M.; Neuzil, P.; Volk, T.; Manz, A.; Kleber, A. On-chip three-dimensional cell culture in phaseguides improves hepatocyte functions in vitro. Biomicrofluidics 2015, 9, 034113. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Chae, S.; Kim, J.Y.; Han, W.; Kim, J.; Choi, Y.; Cho, D.W. Cell-printed 3D liver-on-a-chip possessing a liver microenvironment and biliary system. Biofabrication 2019, 11, 025001. [Google Scholar] [CrossRef]
- Ozkan, A.; Ghousifam, N.; Hoopes, P.J.; Yankeelov, T.E.; Rylander, M.N. In vitro vascularized liver and tumor tissue microenvironments on a chip for dynamic determination of nanoparticle transport and toxicity. Biotechnol. Bioeng. 2019, 116, 1201–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musah, S.; Mammoto, A.; Ferrante, T.C.; Jeanty, S.S.; Hirano-Kobayashi, M.; Mammoto, T.; Roberts, K.; Chung, S.; Novak, R.; Ingram, M.; et al. Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat. Biomed. 2017, 1, 69. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Miller, P.; Shuler, M.L. A pumpless body-on-a-chip model using a primary culture of human intestinal cells and a 3D culture of liver cells. Lab Chip 2018, 18, 2036–2046. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.J.; Lidberg, K.A.; Wang, L.; Bammler, T.K.; MacDonald, J.W.; Li, M.J.; Redhair, M.; Atkins, W.M.; Tran, C.; Hines, K.M.; et al. Human kidney on a chip assessment of polymyxin antibiotic nephrotoxicity. JCI Insight 2018, 3, 123673. [Google Scholar] [CrossRef] [Green Version]
- Delalat, B.; Cozzi, C.; Rasi Ghaemi, S.; Polito, G.; Kriel, F.H.; Michl, T.D.; Harding, F.J.; Priest, C.; Barillaro, G.; Voelcker, N.H. Microengineered bioartificial liver chip for drug toxicity screening. Adv. Funct. Mater. 2018, 28, 1801825. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, L.; Zhou, E.M.; Xu, J.; Shen, S.; Wang, J. On-chip construction of liver lobule-like microtissue and its application for adverse drug reaction assay. Anal. Chem. 2016, 88, 1719–1727. [Google Scholar] [CrossRef]
- Choucha-Snouber, L.; Aninat, C.; Grsicom, L.; Madalinski, G.; Brochot, C.; Poleni, P.E.; Razan, F.; Guillouzo, C.G.; Legallais, C.; Corlu, A.; et al. Investigation of ifosfamide nephrotoxicity induced in a liver–kidney co-culture biochip. Biotechnol. Bioeng. 2013, 110, 597–608. [Google Scholar] [CrossRef]
- Choe, A.; Ha, S.K.; Choi, I.; Choi, N.; Sung, J.H. Microfluidic Gut-liver chip for reproducing the first pass metabolism. Biomed. Microdevices 2017, 19, 4. [Google Scholar] [CrossRef]
- Lee, D.W.; Ha, S.K.; Choi, I.; Sung, J.H. 3D gut-liver chip with a PK model for prediction of first-pass metabolism. Biomed. Microdevices 2017, 19, 100. [Google Scholar] [CrossRef]
- Zakhariants, A.; Burmistrova, O.; Shkurnikov, M.Y.; Poloznikov, A.A.; Sakharov, D.A. Development of a Specific Substrate—Inhibitor Panel (Liver-on-a-Chip) for Evaluation of Cytochrome P450 Activity. Bull. Exp. Biol. Med. 2016, 162, 170–174. [Google Scholar] [CrossRef]
- Kimura, H.; Ikeda, T.; Nakayama, H.; Sakai, Y.; Fujii, T. An on-chip small intestine–liver model for pharmacokinetic studies. J. Lab. Autom. 2015, 20, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Gao, D.; Liu, W.; Wei, H.; Lin, J.M. Imitation of drug metabolism in human liver and cytotoxicity assay using a microfluidic device coupled to mass spectrometric detection. Lab Chip 2012, 12, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Prot, J.-M.; Bunescu, A.; Elena-Herrmann, B.; Aninat, C.; Snouber, L.C.; Griscom, L.; Razan, F.; Bois, F.Y.; Legallais, C.; Brochot, C. Predictive toxicology using systemic biology and liver microfluidic “on chip” approaches: Application to acetaminophen injury. Toxicol. Appl. Pharmacol. 2012, 259, 270–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, A.J.; Chouhan, B.; Regan, S.L.; Rollison, H.; Amberntsson, S.; Andersson, L.C.; Srivastava, A.; Darnell, M.; Cairns, J.; Lazic, S.E.; et al. Integrated in vitro models for hepatic safety and metabolism: Evaluation of a human Liver-Chip and liver spheroid. Arch. Toxicol. 2019, 93, 1021–1037. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.J.; Hung, P.J.; Lee, L.P. An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnol. Bioeng. 2007, 97, 1340–1346. [Google Scholar] [CrossRef]
- Esch, M.B.; Mahler, G.J.; Stokol, T.; Shuler, M.L. Body-on-a-chip simulation with gastrointestinal tract and liver tissues suggests that ingested nanoparticles have the potential to cause liver injury. Lab Chip 2014, 14, 3081–3092. [Google Scholar] [CrossRef]
- Maschmeyer, I.; Hasenberg, T.; Jaenicke, A.; Lindner, M.; Lorenz, A.K.; Zech, J.; Garbe, L.A.; Sonntag, F.; Hayden, P.; Ayehunie, S. Chip-based human liver–intestine and liver–skin co-cultures–A first step toward systemic repeated dose substance testing in vitro. Eur. J. Pharm. Biopharm. 2015, 95, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Jiang, L.; Zhu, Y.; Su, W.; Xu, C.; Tao, T.; Shi, Y.; Qin, J. Assessment of hepatic metabolism-dependent nephrotoxicity on an organs-on-a-chip microdevice. Toxicol. In Vitro 2018, 46, 1–8. [Google Scholar] [CrossRef]
- Sung, J.H.; Kam, C.; Shuler, M.L. A microfluidic device for a pharmacokinetic–pharmacodynamic (PK–PD) model on a chip. Lab Chip 2010, 10, 446–455. [Google Scholar] [CrossRef]
- Shintu, L.; Baudoin, R.; Navratil, V.; Prot, J.M.; Pontoizeau, C.; Defernez, M.; Blaise, B.J.; Domange, C.; Péry, A.R.; Toulhoat, P.; et al. Metabolomics-on-a-chip and predictive systems toxicology in microfluidic bioartificial organs. Anal. Chem. 2012, 84, 1840–1848. [Google Scholar] [CrossRef]
- De Mello, C.P.P.; Carmona-Moran, C.; McAleer, C.W.; Perez, J.; Coln, E.A.; Long, C.J.; Oleaga, C.; Riu, A.; Note, R.; Teissier, S.; et al. Microphysiological heart–liver body-on-a-chip system with a skin mimic for evaluating topical drug delivery. Lab Chip 2020, 20, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Su, W.; Zhu, Y.; Tao, T.; Li, D.; Peng, X.; Qin, J. Drug absorption related nephrotoxicity assessment on an intestine-kidney chip. Biomicrofluidics 2017, 11, 034114. [Google Scholar] [CrossRef] [PubMed]
- Liebler, D.C.; Guengerich, F.P. Elucidating mechanisms of drug-induced toxicity. Nat. Rev. Drug Discoov. 2005, 4, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.-J.; Otieno, M.A.; Ronxhi, J.; Lim, H.K.; Ewart, L.; Kodella, K.R.; Petropolis, D.B.; Kulkarni, G.; Rubins, J.E.; Conegliano, D.; et al. Reproducing human and cross-species drug toxicities using a Liver-Chip. Sci. Transl. Med. 2019, 11, eaax5516. [Google Scholar] [CrossRef] [PubMed]
- Bovard, D.; Sandoz, A.; Luettich, K.; Frentzel, S.; Iskandar, A.; Marescotti, D.; Trivedi, K.; Guedj, E.; Dutertre, Q.; Peitsch, M.C.; et al. A lung/liver-on-a-chip platform for acute and chronic toxicity studies. Lab Chip 2018, 18, 3814–3829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, A. Drug Absorption and Bioavailability. In Introduction to Basics of Pharmacology and Toxicology: Volume 1: General and Molecular Pharmacology: Principles of Drug Action; Raj, G.M., Raveendran, R., Eds.; Springer: Singapore, 2019; pp. 81–88. [Google Scholar]
- Haste, P.; Tann, M.; Persohn, S.; LaRoche, T.; Aaron, V.; Mauxion, T.; Chauhan, N.; Dreher, M.R.; Johnson, M.S. Correlation of technetium-99m macroaggregated albumin and Yttrium-90 glass microsphere biodistribution in hepatocellular carcinoma: A retrospective review of pretreatment single photon emission CT and posttreatment positron emission tomography/CT. J. Vasc. Interv. Radiol. 2017, 28, 722–730. [Google Scholar] [CrossRef]
- NDong, C.; Tate, J.A.; Kett, W.C.; Batra, J.; Demidenko, E.; Lewis, L.D.; Hoopes, P.J.; Gerngross, T.U.; Griswold, K.E. Tumor cell targeting by iron oxide nanoparticles is dominated by different factors in vitro versus in vivo. PLoS ONE 2015, 10, e0115636. [Google Scholar] [CrossRef]
- Petryk, A.A.; Giustini, A.J.; Gottesman, R.E.; Kaufman, P.A.; Hoopes, P.J. Magnetic nanoparticle hyperthermia enhancement of cisplatin chemotherapy cancer treatment. Int. J. Hyperth. 2013, 29, 845–851. [Google Scholar] [CrossRef] [Green Version]
- Terentyuk, G.S.; Maslyakova, G.N.; Suleymanova, L.V.; Khlebtsov, B.N.; Kogan, B.Y.; Akchurin, G.G.; Shantrocha, A.V.; Maksimova, I.L.; Khlebtsov, N.G.; Tuchin, V.V. Circulation and distribution of gold nanoparticles and induced alterations of tissue morphology at intravenous particle delivery. J. Biophotonics 2009, 2, 292–302. [Google Scholar] [CrossRef]
- Gholizadeh, H.; Ong, H.X.; Bradbury, P.; Kourmatzis, A.; Traini, D.; Young, P.; Li, M.; Cheng, S. Real-time quantitative monitoring of in vitro nasal drug delivery by a nasal epithelial mucosa-on-a-chip model. Expert Opin. Drug Del. 2021, 18, 803–818. [Google Scholar] [CrossRef]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Kourmatzis, A.; Mekonnen, T.; Gholizadeh, H.; Raco, J.; Chen, L.; Tang, P.; Chan, H.K. Does upper airway deformation affect drug deposition? Int. J. Pharm. 2019, 572, 118773. [Google Scholar] [CrossRef] [PubMed]
- Benam, K.H.; Villenave, R.; Lucchesi, C.; Varone, A.; Hubeau, C.; Lee, H.H.; Alves, S.E.; Salmon, M.; Ferrante, T.C.; Weaver, J.C.; et al. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat. Methods 2016, 13, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Hassell, B.A.; Goyal, G.; Lee, E.; Sontheimer-Phelps, A.; Levy, O.; Chen, C.S.; Ingber, D.E. Human organ chip models recapitulate orthotopic lung cancer growth, therapeutic responses, and tumor dormancy in vitro. Cell Rep. 2017, 21, 508–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, A.; Barrile, R.; van der Meer, A.D.; Mammoto, A.; Mammoto, T.; De Ceunynck, K.; Aisiku, O.; Otieno, M.A.; Louden, C.S.; Hamilton, G.A.; et al. Primary human lung alveolus-on-a-chip model of intravascular thrombosis for assessment of therapeutics. Clin. Pharmacol. Ther. 2018, 103, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Khosa, A.; Saha, R.N.; Singhvi, G. Chapter 16—Drug delivery to the brain. In Nanomaterials for Drug Delivery and Therapy; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 461–514. [Google Scholar]
- Choucha Snouber, L.; Bunescu, A.; Naudot, M.; Legallais, C.; Brochot, C.; Dumas, M.E.; Elena-Herrmann, B.; Leclerc, E. Metabolomics-on-a-chip of hepatotoxicity induced by anticancer drug flutamide and its active metabolite hydroxyflutamide using HepG2/C3a microfluidic biochips. Toxicol. Sci. 2013, 132, 8–20. [Google Scholar] [CrossRef]
- Humerickhouse, R.; Lohrbach, K.; Li, L.; Bosron, W.F.; Dolan, M.E. Characterization of CPT-11 hydrolysis by human liver carboxylesterase isoforms hCE-1 and hCE-2. Cancer Res. 2000, 60, 1189–1192. [Google Scholar]
- Zhao, X.; Chen, L.; Lu, J. A similarity-based method for prediction of drug side effects with heterogeneous information. Math. Biosci. 2018, 306, 136–144. [Google Scholar] [CrossRef]
- Testart-Paillet, D.; Girard, P.; You, B.; Freyer, G.; Pobel, C.; Tranchand, B. Contribution of modelling chemotherapy-induced hematological toxicity for clinical practice. Crit. Rev. Oncol. Hematol. 2007, 63, 1–11. [Google Scholar] [CrossRef]
- Van Kuilenburg, A.B. Dihydropyrimidine dehydrogenase and the efficacy and toxicity of 5-fluorouracil. Eur. J. Cancer 2004, 40, 939–950. [Google Scholar] [CrossRef]
- Maschmeyer, I.; Lorenz, A.K.; Schimek, K.; Hasenberg, T.; Ramme, A.P.; Hübner, J.; Lindner, M.; Drewell, C.; Bauer, S.; Thomas, A.; et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip 2015, 15, 2688–2699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.Y.; Sung, J.H. Gut–liver on a chip toward an in vitro model of hepatic steatosis. Biotechnol. Bioeng. 2018, 115, 2817–2827. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Siddharthan, V.; Kim, Y.V.; Liu, S.; Kim, K.S. Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res. 2007, 1147, 39–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, R.; Kim, H. Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB). Lab Chip 2012, 12, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.; Yi, B.; Oh, S.; Park, D.J.; Sung, J.H.; Park, S. A microfluidic cell culture device (μFCCD) to culture epithelial cells with physiological and morphological properties that mimic those of the human intestine. Biomed. Microdevices 2015, 17, 58. [Google Scholar] [CrossRef]
- Gholizadeh, H.; Cheng, S.; Pozzoli, M.; Messerotti, E.; Traini, D.; Young, P.; Kourmatzis, A.; Ong, H.X. Smart thermosensitive chitosan hydrogel for nasal delivery of ibuprofen to treat neurological disorders. Expert Opin. Drug Del. 2019, 16, 453–466. [Google Scholar] [CrossRef]
- Ong, H.X.; Jackson, C.L.; Cole, J.L.; Lackie, P.M.; Traini, D.; Young, P.M.; Lucas, J.; Conway, J. Primary air–liquid interface culture of nasal epithelium for nasal drug delivery. Mol. Pharm. 2016, 13, 2242–2252. [Google Scholar] [CrossRef]
- Thomson, A.; Dietschy, J. The role of the unstirred water layer in intestinal permeation. In Pharmacology of Intestinal Permeation II; Springer: Berlin/Heidelberg, Germany, 1984; pp. 165–269. [Google Scholar]
- Cotton, C.U.; Reuss, L. Measurement of the effective thickness of the mucosal unstirred layer in Necturus gallbladder epithelium. J. Gen. Physiol. 1989, 93, 631–647. [Google Scholar] [CrossRef] [Green Version]
- Pohl, P.; Saparov, S.M.; Antonenko, Y.N. Size of the unstirred layer as a function of the solute diffusion coefficient. Biophys. J. 1998, 75, 1403–1409. [Google Scholar] [CrossRef] [Green Version]
- Naruhashi, K.; Tamai, I.; Li, Q.; Sai, Y.; Tsuji, A. Experimental demonstration of the unstirred water layer effect on drug transport in Caco-2 cells. J. Pharm. Sci. 2003, 92, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.D.; Fordtran, J.S. Effect of perfusion rate on absorption, surface area, unstirred water layer thickness, permeability, and intraluminal pressure in the rat ileum in vivo. Gastroenterology 1975, 68, 1509–1516. [Google Scholar] [CrossRef]
- Guenat, O.T.; Berthiaume, F. Incorporating mechanical strain in organs-on-a-chip: Lung and skin. Biomicrofluidics 2018, 12, 042207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, G.; Peters, A.; Bagdades, E.; Myers, M.J.; Snook, D.; Hughes, J.M.B. Evaluation of pulmonary alveolar epithelial integrity by the detection of restriction to diffusion of hydrophilic solutes of different molecular sizes. Clin. Sci. 2001, 100, 231–236. [Google Scholar] [CrossRef]
- Marks, J.D.; Luce, J.M.; Lazar, N.M.; Wu, J.N.; Lipavsky, A.; Murray, J.F. Effect of increases in lung volume on clearance of aerosolized solute from human lungs. J. Appl. Physiol. 1985, 59, 1242–1248. [Google Scholar] [CrossRef]
- Kim, H.J.; Huh, D.; Hamilton, G.; Ingber, D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 2012, 12, 2165–2174. [Google Scholar] [CrossRef]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in translation: Animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 2014, 6, 114. [Google Scholar]
- Zhou, Q.; Patel, D.; Kwa, T.; Haque, A.; Matharu, Z.; Stybayeva, G.; Gao, Y.; Diehl, A.M.; Revzin, A. Liver injury-on-a-chip: Microfluidic co-cultures with integrated biosensors for monitoring liver cell signaling during injury. Lab Chip 2015, 15, 4467–4478. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Li, K.; Zhang, X.; Liu, C.; Guo, B.; Wen, W.; Gao, X. Nanofiber membrane supported lung-on-a-chip microdevice for anti-cancer drug testing. Lab Chip 2018, 18, 486–495. [Google Scholar] [CrossRef]
- Huh, D.; Leslie, D.C.; Matthews, B.D.; Fraser, J.P.; Jurek, S.; Hamilton, G.A.; Thorneloe, K.S.; McAlexander, M.A.; Ingber, D.E. A human disease model of drug toxicity–induced pulmonary edema in a lung-on-a-chip microdevice. Sci. Transl. Med. 2012, 4, 159ra147. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, E.; Palma, C.; Vesentini, S.; Occhetta, P.; Rasponi, M. Integrating biosensors in organs-on-chip devices: A perspective on current strategies to monitor microphysiological systems. Biosensors 2020, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mandal, K.; Hernandez, A.L.; Kawakita, S.; Huang, W.; Bandaru, P.; Ahadian, S.; Kim, H.J.; Jucaud, V.; Dokmeci, M.R.; et al. State of the art in integrated biosensors for organ-on-a-chip applications. Curr. Opin. Biomed. Eng. 2021, 19, 100–309. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Aleman, J.; Shin, S.R.; Kilic, T.; Kim, D.; Shaegh, S.A.M.; Massa, S.; Riahi, R.; Chae, S.; Hu, N.; et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl. Acad. Sci. USA 2017, 114, E2293–E2302. [Google Scholar] [CrossRef] [Green Version]
- Van der Helm, M.W.; Odijk, M.; Frimat, J.-P.; van der Meer, A.D.; Eijkel, J.C.; van den Berg, A.; Segerink, L.I. Direct quantification of transendothelial electrical resistance in organs-on-chips. Biosens. Bioelectron. 2016, 85, 924–929. [Google Scholar] [CrossRef] [Green Version]
- Maoz, B.M.; Herland, A.; Henry, O.Y.; Leineweber, W.D.; Yadid, M.; Doyle, J.; Mannix, R.; Kujala, V.J.; FitzGerald, E.A.; Parker, K.K.; et al. Organs-on-Chips with combined multi-electrode array and transepithelial electrical resistance measurement capabilities. Lab Chip 2017, 17, 2294–2302. [Google Scholar] [CrossRef]
- Henry, O.Y.; Villenave, R.; Cronce, M.J.; Leineweber, W.D.; Benz, M.A.; Ingber, D.E. Organs-on-chips with integrated electrodes for trans-epithelial electrical resistance (TEER) measurements of human epithelial barrier function. Lab Chip 2017, 17, 2264–2271. [Google Scholar] [CrossRef]
- Van der Helm, M.W.; Henry, O.Y.; Bein, A.; Hamkins-Indik, T.; Cronce, M.J.; Leineweber, W.D.; Odijk, M.; van der Meer, A.D.; Eijkel, J.C.; Ingber, D.E.; et al. Non-invasive sensing of transepithelial barrier function and tissue differentiation in organs-on-chips using impedance spectroscopy. Lab Chip 2019, 19, 452–463. [Google Scholar] [CrossRef] [Green Version]
- Uslu, B.; Ozkan, S.A. Electroanalytical Application of Carbon Based Electrodes to the Pharmaceuticals. Anal. Lett. 2007, 40, 817–853. [Google Scholar] [CrossRef]
- Dolberg, A.M.; Reichl, S. Expression of P-glycoprotein in excised human nasal mucosa and optimized models of RPMI 2650 cells. Int. J. Pharm. 2016, 508, 22–33. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, Y.; Hao, Y.; Li, E.; Wang, Y.; Zhang, J.; Wang, W.; Gao, Z.; Wang, Q. Application of a microfluidic chip-based 3D co-culture to test drug sensitivity for individualized treatment of lung cancer. Biomaterials 2013, 34, 4109–4117. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Z.; Fan, S.; Meng, Q.; Li, B.; Shao, S.; Wang, Q. Chemotherapy resistance research of lung cancer based on micro-fluidic chip system with flow medium. Biomed. Microdevices 2010, 12, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Humayun, M.; Chow, C.-W.; Young, E.W. Microfluidic lung airway-on-a-chip with arrayable suspended gels for studying epithelial and smooth muscle cell interactions. Lab Chip 2018, 18, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Punde, T.H.; Wu, W.-H.; Lien, P.C.; Chang, Y.L.; Kuo, P.H.; Chang, M.D.T.; Lee, K.Y.; Huang, C.D.; Kuo, H.P.; Chan, Y.F. A biologically inspired lung-on-a-chip device for the study of protein-induced lung inflammation. Integr. Biol. 2015, 7, 162–169. [Google Scholar] [CrossRef]
- Islam, N.; Cleary, M.J. Developing an efficient and reliable dry powder inhaler for pulmonary drug delivery—A review for multidisciplinary researchers. Med. Eng. Phys. 2012, 34, 409–427. [Google Scholar] [CrossRef]
- Dos Reis, L.G.; Chaugule, V.; Fletcher, D.F.; Young, P.M.; Traini, D.; Soria, J. In-vitro and particle image velocimetry studies of dry powder inhalers. Int. J. Pharm. 2021, 592, 119966. [Google Scholar] [CrossRef]
- Kourmatzis, A.; Cheng, S.; Chan, H.K. Airway geometry, airway flow, and particle measurement methods: Implications on pulmonary drug delivery. Expert Opin. Drug Del. 2018, 15, 271–282. [Google Scholar] [CrossRef]
| Refs. | Focus of the Review |
|---|---|
| [3,4,5] |
|
| [3,9,10,11] |
|
| [6,7] |
|
| [8] |
|
| This review |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gholizadeh, H.; Cheng, S.; Kourmatzis, A.; Xing, H.; Traini, D.; Young, P.M.; Ong, H.X. Application of Micro-Engineered Kidney, Liver, and Respiratory System Models to Accelerate Preclinical Drug Testing and Development. Bioengineering 2022, 9, 150. https://doi.org/10.3390/bioengineering9040150
Gholizadeh H, Cheng S, Kourmatzis A, Xing H, Traini D, Young PM, Ong HX. Application of Micro-Engineered Kidney, Liver, and Respiratory System Models to Accelerate Preclinical Drug Testing and Development. Bioengineering. 2022; 9(4):150. https://doi.org/10.3390/bioengineering9040150
Chicago/Turabian StyleGholizadeh, Hanieh, Shaokoon Cheng, Agisilaos Kourmatzis, Hanwen Xing, Daniela Traini, Paul M. Young, and Hui Xin Ong. 2022. "Application of Micro-Engineered Kidney, Liver, and Respiratory System Models to Accelerate Preclinical Drug Testing and Development" Bioengineering 9, no. 4: 150. https://doi.org/10.3390/bioengineering9040150
APA StyleGholizadeh, H., Cheng, S., Kourmatzis, A., Xing, H., Traini, D., Young, P. M., & Ong, H. X. (2022). Application of Micro-Engineered Kidney, Liver, and Respiratory System Models to Accelerate Preclinical Drug Testing and Development. Bioengineering, 9(4), 150. https://doi.org/10.3390/bioengineering9040150