Automatic Programmable Bioreactor with pH Monitoring System for Tissue Engineering Application
Abstract
:1. Introduction
2. Materials and Methods
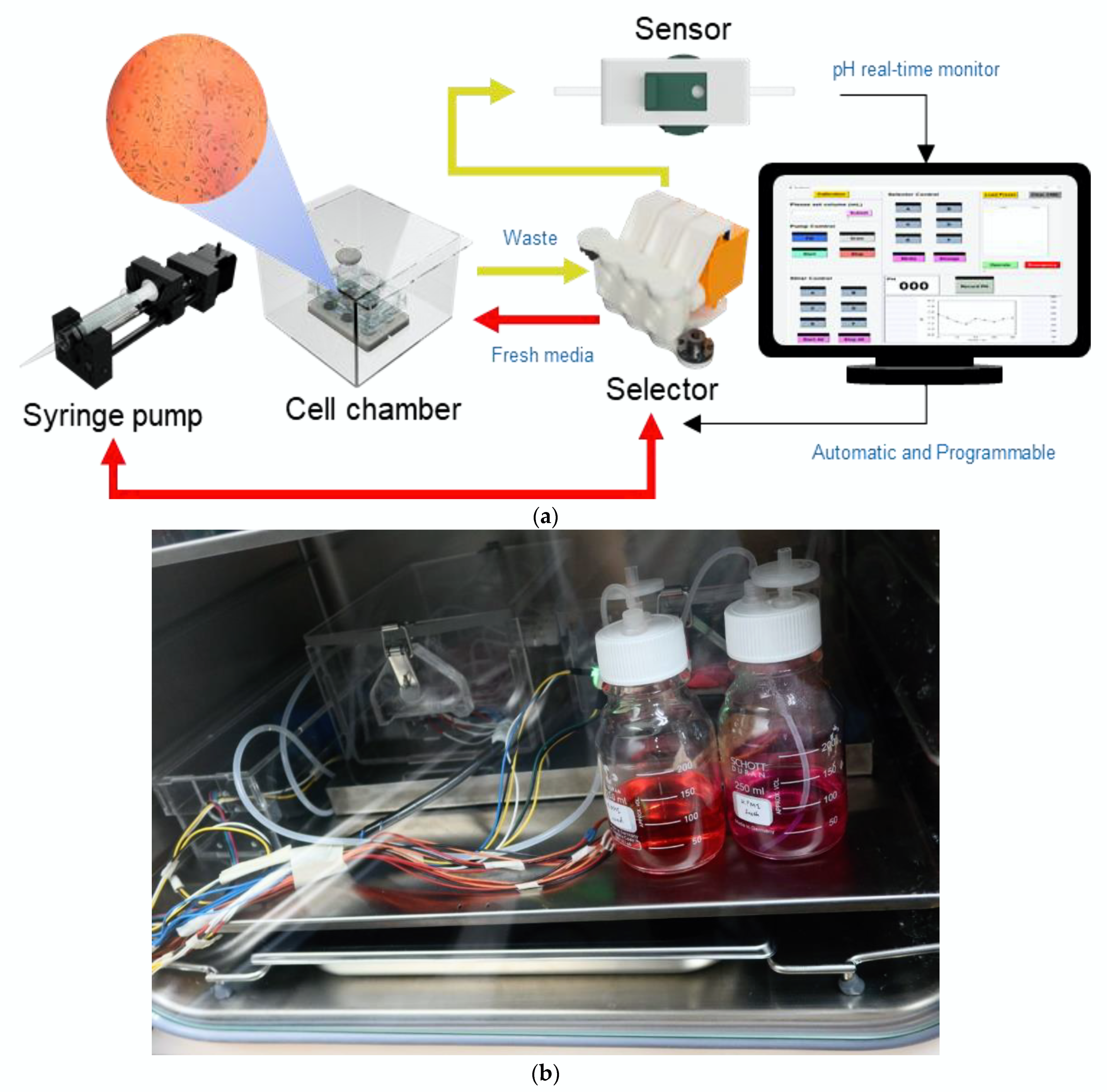
2.1. Design and Construction of Bioreactor
2.1.1. Cell Culture Chamber
2.1.2. Syringe Pump
2.1.3. Selector
2.1.4. pH Monitoring System
2.1.5. Controlling Program
2.2. Bioreactor Setup
2.3. Bioreactor Performance Test
2.3.1. Cell Viability Testing
2.3.2. Cell Proliferation Assay
2.4. pH Monitoring System Test
3. Results
3.1. Syringe Pump Calibration
3.2. pH Calibration
3.3. Cell Viability—MTT Assay
3.4. Cell Proliferation Assay
3.5. pH Monitoring Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandra, P.K.; Soker, S.; Atala, A. Chapter 1—Tissue engineering: Current status and future perspectives. In Principles of Tissue Engineering; Lanza, R., Langer, R., Vacanti, J.P., Atala, A., Eds.; Academic Press: London, UK, 2020; pp. 1–35. ISBN 978-0-12-818422-6. [Google Scholar]
- Khademhosseini, A.; Langer, R. A decade of progress in tissue engineering. Nat. Protoc. 2016, 11, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- O’Mara, P.; Farrell, A.; Bones, J.; Twomey, K. Staying alive! Sensors used for monitoring cell health in bioreactors. Talanta 2018, 176, 130–139. [Google Scholar] [CrossRef]
- Kropp, C.; Kempf, H.; Halloin, C.; Robles-Diaz, D.; Franke, A.; Scheper, T.; Kinast, K.; Knorpp, T.; Joos, T.O.; Haverich, A.; et al. Impact of Feeding Strategies on the Scalable Expansion of Human Pluripotent Stem Cells in Single-Use Stirred Tank Bioreactors. Stem Cells Transl. Med. 2016, 5, 1289–1301. [Google Scholar] [CrossRef] [Green Version]
- Abbasalizadeh, S.; Larijani, M.R.; Samadian, A.; Baharvand, H. Bioprocess Development for Mass Production of Size-Controlled Human Pluripotent Stem Cell Aggregates in Stirred Suspension Bioreactor. Tissue Eng. Part C Methods 2012, 18, 831–851. [Google Scholar] [CrossRef] [PubMed]
- Gaydhane, M.K.; Mahanta, U.; Sharma, C.S.; Khandelwal, M.; Ramakrishna, S. Cultured meat: State of the art and future. Biomanufacturing Rev. 2018, 3, 1. [Google Scholar] [CrossRef]
- Choi, J.; Mathew, S.; Oerter, S.; Appelt-Menzel, A.; Hansmann, J.; Schmitz, T. Online Measurement System for Dynamic Flow Bioreactors to Study Barrier Integrity of hiPSC-Based Blood–Brain Barrier In Vitro Models. Bioengineering 2022, 9, 39. [Google Scholar] [CrossRef]
- Lee, B.; Jung, S.; Hashimura, Y.; Lee, M.; Borys, B.S.; Dang, T.; Kallos, M.S.; Rodrigues, C.A.; Silva, T.P.; Cabral, J.M. Cell Culture Process Scale-Up Challenges for Commercial-Scale Manufacturing of Allogeneic Pluripotent Stem Cell Products. Bioengineering 2022, 9, 92. [Google Scholar] [CrossRef]
- Alexovič, M.; Dotsikas, Y.; Bober, P.; Sabo, J. Achievements in robotic automation of solvent extraction and related approaches for bioanalysis of pharmaceuticals. J. Chromatogr. B 2018, 1092, 402–421. [Google Scholar] [CrossRef]
- Elpa, D.P.; Prabhu, G.R.D.; Wu, S.-P.; Tay, K.S.; Urban, P.L. Automation of mass spectrometric detection of analytes and related workflows: A review. Talanta 2020, 208, 120304. [Google Scholar] [CrossRef]
- Alexovič, M.; Urban, P.L.; Tabani, H.; Sabo, J. Recent advances in robotic protein sample preparation for clinical analysis and other biomedical applications. Clin. Chim. Acta 2020, 507, 104–116. [Google Scholar] [CrossRef]
- Djisalov, M.; Knežić, T.; Podunavac, I.; Živojević, K.; Radonic, V.; Knežević, N.Ž.; Bobrinetskiy, I.; Gadjanski, I. Cultivating Multidisciplinarity: Manufacturing and Sensing Challenges in Cultured Meat Production. Biology 2021, 10, 204. [Google Scholar] [CrossRef]
- Tric, M.; Lederle, M.; Neuner, L.; Dolgowjasow, I.; Wiedemann, P.; Wölfl, S.; Werner, T. Optical biosensor optimized for continuous in-line glucose monitoring in animal cell culture. Anal. Bioanal. Chem. 2017, 409, 5711–5721. [Google Scholar] [CrossRef] [PubMed]
- Mross, S.; Zimmermann, T.; Winkin, N.; Kraft, M.; Vogt, H. Integrated Multi-sensor System for Parallel In-situ Monitoring of Cell Nutrients, Metabolites and Cell Mass in Biotechnological Processes. Procedia Eng. 2015, 120, 372–375. [Google Scholar] [CrossRef]
- Weltin, A.; Slotwinski, K.; Kieninger, J.; Moser, I.; Jobst, G.; Wego, M.; Ehret, R.; Urban, G.A. Cell culture monitoring for drug screening and cancer research: A transparent, microfluidic, multi-sensor microsystem. Lab Chip 2014, 14, 138–146. [Google Scholar] [CrossRef]
- Park, D.; Lee, J.; Chung, J.J.; Jung, Y.; Kim, S.H. Integrating Organs-on-Chips: Multiplexing, Scaling, Vascularization, and Innervation. Trends Biotechnol. 2020, 38, 99–112. [Google Scholar] [CrossRef]
- Xue, Y.; Seiler, M.J.; Tang, W.C.; Wang, J.Y.; Delgado, J.; McLelland, B.T.; Nistor, G.; Keirstead, H.S.; Browne, A.W. Retinal organoids on-a-chip: A micro-millifluidic bioreactor for long-term organoid maintenance. Lab Chip 2021, 21, 3361–3377. [Google Scholar] [CrossRef]
- Moya, A.; Ortega-Ribera, M.; Guimerà, X.; Sowade, E.; Zea, M.; Illa, X.; Ramon, E.; Villa, R.; Gracia-Sancho, J.; Gabriel, G. Online oxygen monitoring using integrated inkjet-printed sensors in a liver-on-a-chip system. Lab Chip 2018, 18, 2023–2035. [Google Scholar] [CrossRef] [Green Version]
- Rojas, D.; Hernández-Rodríguez, J.F.; Della Pelle, F.; Escarpa, A.; Compagnone, D. New trends in enzyme-free electrochemical sensing of ROS/RNS. Application to live cell analysis. Microchim. Acta 2022, 189, 102. [Google Scholar] [CrossRef]
- Coluccio, M.L.; Perozziello, G.; Malara, N.; Parrotta, E.; Zhang, P.; Gentile, F.; Limongi, T.; Raj, P.M.; Cuda, G.; Candeloro, P.; et al. Microfluidic platforms for cell cultures and investigations. Microelectron. Eng. 2019, 208, 14–28. [Google Scholar] [CrossRef]
- Huang, J.; Qin, Q.; Wang, J. A Review of Stereolithography: Processes and Systems. Processes 2020, 8, 1138. [Google Scholar] [CrossRef]
- International Standardization Organization. ISO 10993-5:2009, Geneva. Available online: https://www.iso.org/standard/36406.html. (accessed on 10 March 2022).
- Freshney, R.I. Defined Media and Supplements. In Culture of Animal Cells: A Manual of Basic Technique, 5th ed.; Wiley-Liss: New York, USA, 2005. [Google Scholar] [CrossRef]
- Treloar, N.J.; Fedorec, A.J.H.; Ingalls, B.; Barnes, C.P. Deep reinforcement learning for the control of microbial co-cultures in bioreactors. PLoS Comput. Biol. 2020, 16, e1007783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, W.L.; Chan, A.; Ong, Y.S.; Chua, C.K. Deep learning for fabrication and maturation of 3D bioprinted tissues and organs. Virtual Phys. Prototyp. 2020, 15, 340–358. [Google Scholar] [CrossRef]











| Command | Value | Description |
|---|---|---|
| Loop | number | Set number of scripts to repeat |
| Vol | number | Set volume of syringe pump to fill or drain (mL) |
| Goto | number | Jump to a line number that set of the script |
| Wait | hh:mm:ss | Set delay time |
| Dir | F or B | Set syringe pump direction (Forward or Backward) |
| Ch | Selector channel (A or B or C or else) | Select selector channel |
| Stir | Stirrer channel (A or B or C or else) | ON/OFF selected stirrer |
| Start | Starting syringe pump | |
| Msgbox | Text | Displays the specified text in the message box |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udomsom, S.; Budwong, A.; Wongsa, C.; Sangngam, P.; Baipaywad, P.; Manaspon, C.; Auephanwiriyakul, S.; Theera-Umpon, N.; Paengnakorn, P. Automatic Programmable Bioreactor with pH Monitoring System for Tissue Engineering Application. Bioengineering 2022, 9, 187. https://doi.org/10.3390/bioengineering9050187
Udomsom S, Budwong A, Wongsa C, Sangngam P, Baipaywad P, Manaspon C, Auephanwiriyakul S, Theera-Umpon N, Paengnakorn P. Automatic Programmable Bioreactor with pH Monitoring System for Tissue Engineering Application. Bioengineering. 2022; 9(5):187. https://doi.org/10.3390/bioengineering9050187
Chicago/Turabian StyleUdomsom, Suruk, Apiwat Budwong, Chanyanut Wongsa, Pakorn Sangngam, Phornsawat Baipaywad, Chawan Manaspon, Sansanee Auephanwiriyakul, Nipon Theera-Umpon, and Pathinan Paengnakorn. 2022. "Automatic Programmable Bioreactor with pH Monitoring System for Tissue Engineering Application" Bioengineering 9, no. 5: 187. https://doi.org/10.3390/bioengineering9050187
APA StyleUdomsom, S., Budwong, A., Wongsa, C., Sangngam, P., Baipaywad, P., Manaspon, C., Auephanwiriyakul, S., Theera-Umpon, N., & Paengnakorn, P. (2022). Automatic Programmable Bioreactor with pH Monitoring System for Tissue Engineering Application. Bioengineering, 9(5), 187. https://doi.org/10.3390/bioengineering9050187







