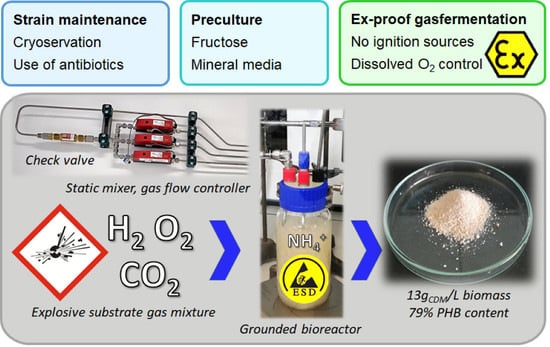
Lab-Scale Cultivation of Cupriavidus necator on Explosive Gas Mixtures: Carbon Dioxide Fixation into Polyhydroxybutyrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Enzymatic Assays and Strains
2.2. Growth Media
2.3. Heterotrophic Cultivations
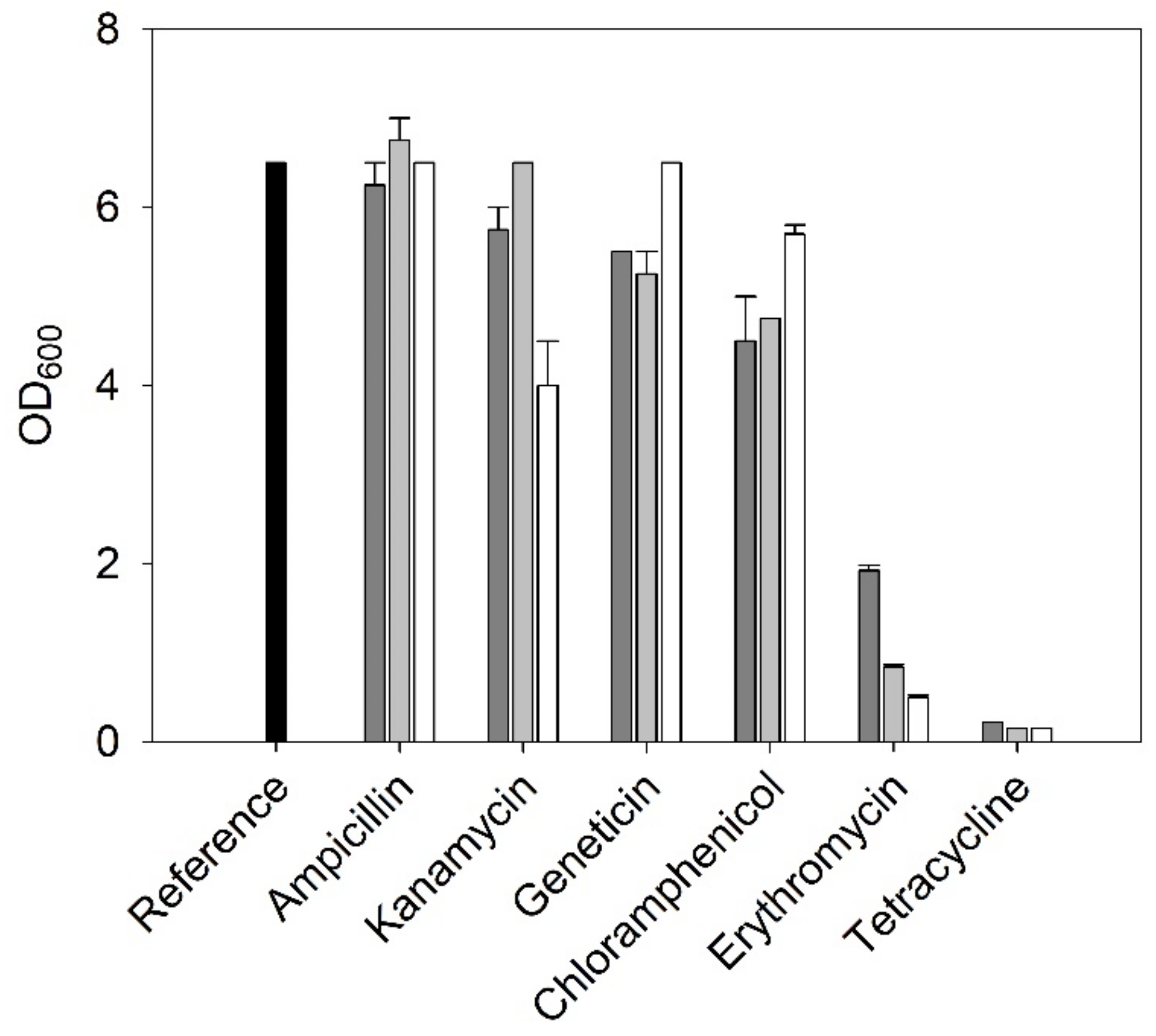
2.3.1. Antibiotic Resistances
2.3.2. Comparison of TSB and MM
2.3.3. Effect of Cultivation Temperature on Growth
2.3.4. Determination of Optical Density (OD600) and Cell Dry Mass (CDM)
2.4. Preservation of C. necator H16
2.5. Chemolithotrophic Cultivation (Oxyhydrogen Cultivation)
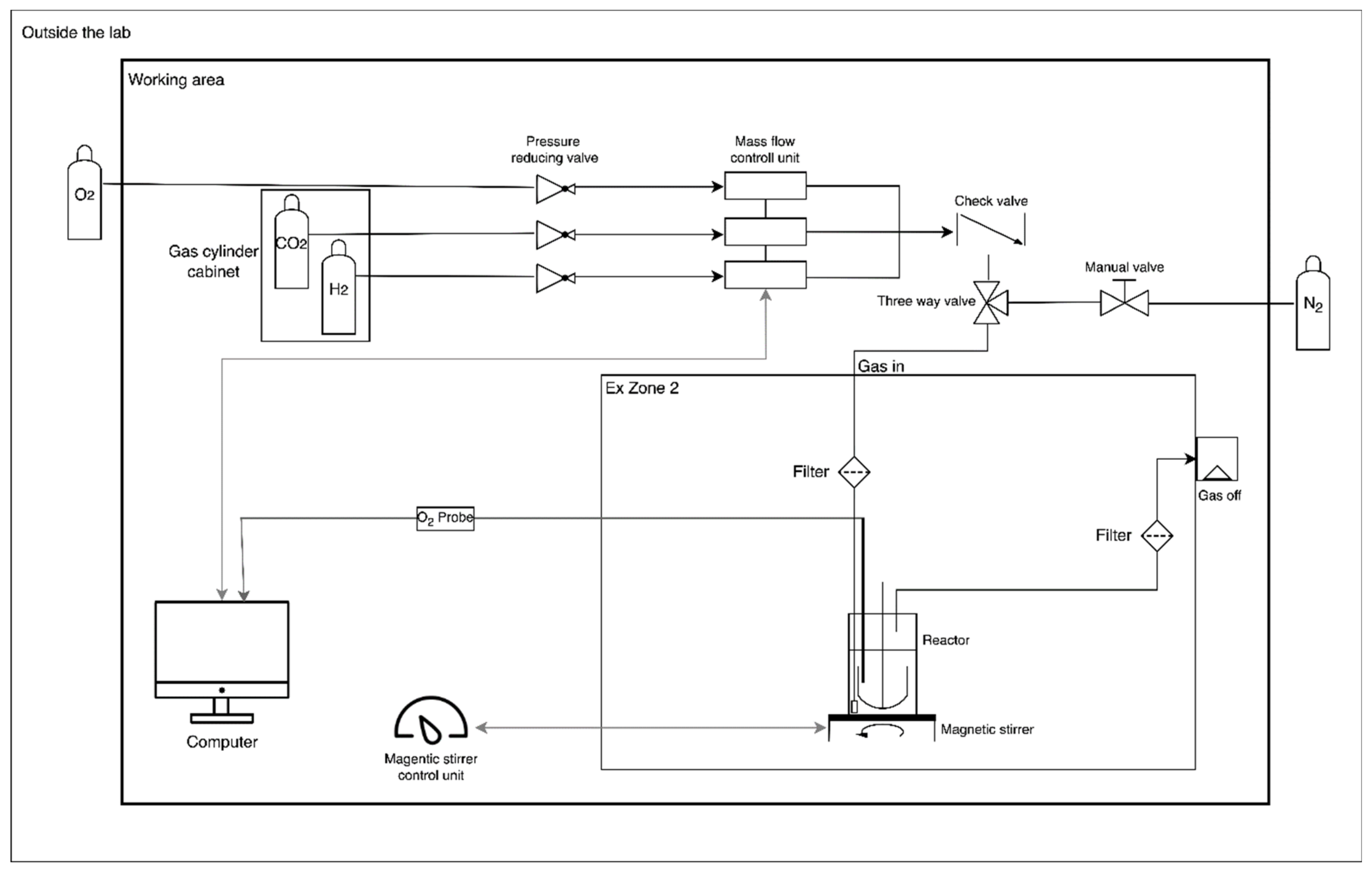
2.5.1. Installations and Equipment
2.5.2. Determination of the Volumetric Oxygen Transfer Coefficient (kLa)
2.5.3. Calculation of Henry’s Law Constant Hcp and Oxygen Transfer Theory
2.5.4. Gas Cultivations
2.5.5. Sample Analysis with PHB Quantification
3. Results
3.1. Heterotrophic cultivation
3.1.1. Antibiotic Resistances of C. necator H16
3.1.2. Heterotrophic Cultivations Using Mineral Media (MM)
3.1.3. Effect of Temperature on Growth Rate
3.1.4. Strain Maintenance
3.2. Chemolithotrophic Cultivation
3.2.1. Chemolithotrophic Cultivations with Constant Gas Flow
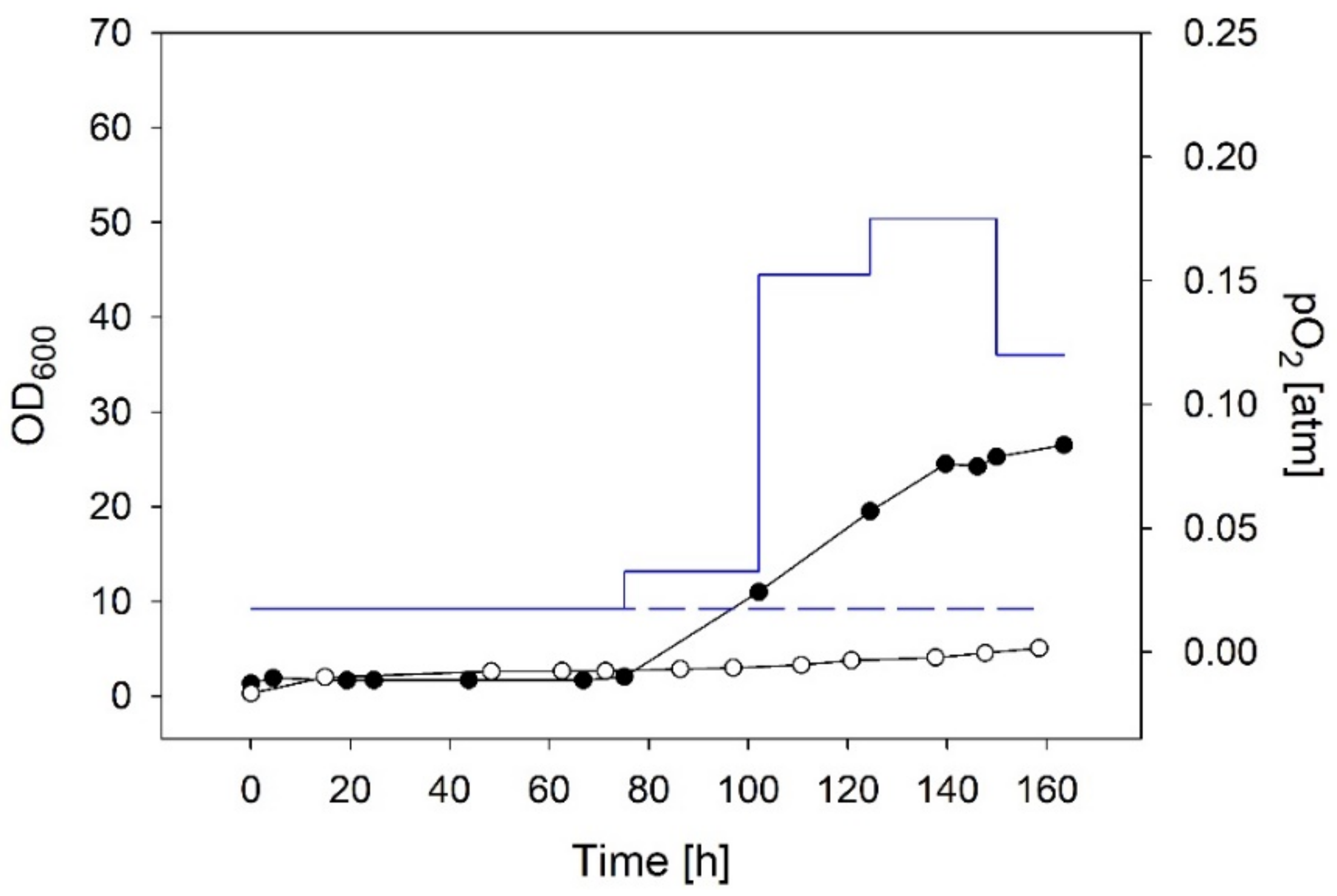
3.2.2. Chemolithotrophic Cultivations with Stepwise Increased Gas Flow Guided by the Biomass Concentration
3.2.3. O2 Supply Guided by an O2 Sensor
3.2.4. pH Development in Chemolithotrophic Cultivations
4. Discussion
4.1. Chemolithotrophic Cultivation
4.1.1. Biological Limitations of DO Concentration
4.1.2. Technological Limitations in Oxyhydrogen Cultivations
4.1.3. Online Analytics in Chemolithotrophic Cultivations
4.2. Polyhydroxyalkanoate from CO2
4.3. Development of the Mineral Medium for Autotrophic Cultivations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jajesniak, P.; Ali, H.E.M.O.; Wong, T.S. Carbon dioxide capture and utilization using biological systems: Opportunities and challenges. J. Bioprocess. Biotech. 2014, 4, 155. [Google Scholar] [CrossRef]
- Olajire, A.A. Valorization of greenhouse carbon dioxide emissions into value-added products by catalytic processes. J CO2 Util. 2013, 3–4, 74–92. [Google Scholar] [CrossRef]
- Pander, B.; Mortimer, Z.; Woods, C.; McGregor, C.; Dempster, A.; Thomas, L.; Maliepaard, J.; Mansfield, R.; Rowe, P.; Krabben, P. Hydrogen oxidising bacteria for production of single-cell protein and other food and feed ingredients. Eng. Biol. 2020, 4, 21–24. [Google Scholar] [CrossRef]
- Sillman, J.; Nygren, L.; Kahiluoto, H.; Ruuskanen, V.; Tamminen, A.; Bajamundi, C.; Nappa, M.; Wuokko, M.; Lindh, T.; Vainikka, P.; et al. Bacterial protein for food and feed generated via renewable energy and direct air capture of CO2: Can it reduce land and water use? Glob. Food Sec. 2019, 22, 25–32. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, J. Comparison analysis on the energy efficiencies and biomass yields in microbial CO2 fixation. Proc. Biochem. 2017, 62, 151–160. [Google Scholar] [CrossRef]
- Mozumder, S.I.; Garcia-Gonzalez, L.; DeWever, H.; Volcke, E.I.P. Poly(3-hydroxybutyrate) (PHB) production from CO2: Model development and process optimization. Biochem. Eng. J. 2015, 98, 107–116. [Google Scholar] [CrossRef]
- Taga, N.; Tanaka, K.; Ishizaki, A. Effects of rheological change by addition of carboxymethylcellulose in culture media of an air-lift fermentor on poly-D-3-hydroxybutyric acid productivity in autotrophic culture of hydrogen-oxidizing bacterium, Alcaligenes eutrophus. Biotechnol. Bioeng. 1997, 53, 529–533. [Google Scholar] [CrossRef]
- Nangle, S.N.; Ziesack, M.; Buckley, S.; Trivedi, D.; Lohe, D.M.; Nocera, D.G.; Silver, P.A. Valorization of CO2 through lithoautotrophic production of sustainable chemicals in Cupriavidus necator. Metab. Eng. 2020, 62, 207–220. [Google Scholar] [CrossRef]
- Tanaka, K.; Yoshida, K.; Orita, I.; Fukui, T. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from CO2 by a recombinant Cupriavidus necator. Bioengineering 2021, 8, 179. [Google Scholar] [CrossRef]
- Garrigues, L.; Maignien, L.; Lombard, E.; Singh, J.; Guillouet, S.E. Isopropanol production from carbon dioxide in Cupriavidus necator in a pressurized bioreactor. N. Biotechnol. 2020, 56, 16–20. [Google Scholar] [CrossRef]
- Müller, J.; MacEachran, D.; Burd, H.; Sathitsuksanoh, N.; Bi, C.; Yeh, Y.-C.; Lee, T.S.; Hillson, N.J.; Chhabra, S.R.; Singer, S.W.; et al. Engineering of Ralstonia eutropha H16 for autotrophic and heterotrophic production of methyl ketones. Appl. Environ. Microbiol. 2013, 79, 4433–4439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Repaske, R.; Mayer, R. Dense autotrophic cultures of Alcaligenes eutrophus. Appl. Environ. Microbiol. 1976, 32, 592–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bommareddy, R.R.; Wang, Y.; Pearcy, N.; Hayes, M.; Lester, E.; Minton, N.P.; Conradie, A.V. A sustainable chemicals manufacturing paradigm using CO2 and renewable H2. iScience 2020, 23, 101218. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.H.; Lan, C.-W. Enhanced polyhydroxybutyrate production through incorporation of a hydrogen fuel cell and electro-fermentation system. Int. J. Hydrog. Energy 2021, 46, 16787–16800. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, J. Biomass yield, reversal respiratory quotient, stoichiometric equations and bioenergetics. Biochem. Eng. J. 2019, 152, 107369. [Google Scholar] [CrossRef]
- Milker, S.; Sydow, A.; Torres-Monroy, I.; Jach, G.; Faust, F.; Kranz, L.; Tkatschuk, L.; Holtmann, D. Gram-scale production of the sesquiterpene α-humulene with Cupriavidus necator. Biotechnol. Bioeng. 2021, 118, 2694–2702. [Google Scholar] [CrossRef]
- Nyyssölä, A.; Ojala, L.S.; Wuokko, M.; Peddinti, G.; Tamminen, A.; Tsitko, I.; Nordlund, E.; Lienemann, M. Production of endotoxin-free microbial biomass for food applications by gas fermentation of gram-positive H2-oxidizing bacteria. ACS Food Sci. Technol. 2021, 1, 470–479. [Google Scholar] [CrossRef]
- Lütte, S.; Pohlmann, A.; Zaychikov, E.; Schwartz, E.; Becher, J.R.; Heumann, H.; Friedrich, B. Autotrophic production of stable-isotope-labeled arginine in Ralstonia eutropha strain H16. Appl. Environ. Microbiol. 2012, 78, 7884–7890. [Google Scholar] [CrossRef] [Green Version]
- Assil-Companioni, L.; Schmidt, S.; Heidinger, P.; Schwab, H.; Kourist, R. Hydrogen-driven cofactor regeneration for stereoselective whole-cell C=C bond reduction in Cupriavidus necator. ChemSusChem 2019, 12, 2361–2365. [Google Scholar] [CrossRef]
- Sonnleitner, B.; Heinzle, E.; Braunegg, G.; Lafferty, R.M. Formal kinetics of poly-β-hydroxybutyric acid (PHB) production in Alcaligenes eutrophus H 16 and Mycoplana rubra R 14 with respect to the dissolved oxygen tension in ammonium-limited batch cultures. Eur. J. Appl. Microbiol. Biotechnol. 1979, 7, 1–10. [Google Scholar] [CrossRef]
- Atlić, A.; Koller, M.; Scherzer, D.; Kutschera, C.; Grillo-Fernandes, E.; Horvat, P.; Chiellini, E.; Braunegg, G. Continuous production of poly([R]-3-hydroxybutyrate) by Cupriavidus necator in a multistage bioreactor cascade. Appl. Microbiol. Biotechnol. 2011, 91, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Riet, K.V. Review of measuring methods and results in nonviscous gas-liquid mass transfer in stirred vessel. Ind. Eng. Chem. Process Des. Dev. 1979, 18, 357–364. [Google Scholar] [CrossRef]
- Schumpe, A.; Adler, I.; Deckwer, W.D. Solubility of oxygen in electrolyte solutions. Biotechnol. Bioeng. 1978, 20, 145–150. [Google Scholar] [CrossRef]
- Juengert, J.R.; Bresan, S.; Jendrossek, D. Determination of polyhydroxybutyrate content in Ralstonia eutropha using gas chromatography and nile red staining. Bio Protoc. 2018, 8, e2748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florentino, L.A.; Jaramillo, P.M.D.; Silva, K.; da Silva, J.S.; de Oliveira, S.M.; de Souza Moreira, F.M. Physiological and symbiotic diversity of Cupriavidus necator strains isolated from nodules of Leguminosae species. Sci. Agric. 2012, 69, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Gruber, S.; Schwendenwein, D.; Magomedo, Z.; Thaler, E.; Hagen, J.; Schwab, H.; Heidinger, P. Design of inducible expression vectors for improved protein production in Ralstonia eutropha H16 derived host strains. J. Biotechnol. 2016, 235, 92–99. [Google Scholar] [CrossRef]
- Massip, C.; Coullaud-Gamel, M.; Gaudru, C.; Amoureux, L.; Doleans-Jordheim, A.; Hery-Arnaud, G.; Marchandin, H.; Oswald, E.; Segonds, C.; Guet-Revillet, H. In vitro activity of 20 antibiotics against Cupriavidus clinical strains. J. Antimicrob. Chemother. 2020, 75, 1654–1658. [Google Scholar] [CrossRef]
- González-Villanueva, M.; Galaiya, H.; Staniland, P.; Staniland, J.; Savill, I.; Wong, T.S.; Tee, K.L. Adaptive laboratory evolution of Cupriavidus necator H16 for carbon co-utilization with glycerol. Int. J. Mol. Sci. 2019, 20, 5737. [Google Scholar] [CrossRef] [Green Version]
- Novackova, I.; Kucera, D.; Porizka, J.; Pernicova, I.; Sedlacek, P.; Koller, M.; Kovalcik, A.; Obruca, S. Adaptation of Cupriavidus necator to levulinic acid for enhanced production of P(3HB-co-3HV) copolyesters. Biochem. Eng. J. 2019, 151, 107350. [Google Scholar] [CrossRef]
- Cramm, R. Genomic view of energy metabolism in Ralstonia eutropha H16. J. Mol. Microbiol. Biotechnol. 2009, 16, 38–52. [Google Scholar] [CrossRef]
- Pohlmann, A.; Fricke, W.; Reinecke, F.; Kusian, B.; Liesegang, H.; Cramm, R.; Eitinger, T.; Ewering, C.; Pötter, M.; Schwartz, E.; et al. Genome sequence of the bioplastic-producing “Knallgas” bacterium Ralstonia eutropha H16. Nat. Biotechnol. 2006, 24, 1257–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, J.F.; Litchfield, J.H. A continuous culture apparatus for the microbial utilization of hydrogen produced by electrolysis of water in closed-cycle space systems. Biotechnol. Bioeng. 1964, 6, 441–456. [Google Scholar] [CrossRef]
- Ishizaki, A.; Tanaka, K.; Taga, N. Microbial production of poly-D-3-hydroxybutyrate from CO2. Appl. Microbiol. Biotechnol. 2001, 57, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, L.; Mozumder, S.I.; Dubreuil, M.; Volcke, E.I.P.; DeWevera, H. Sustainable autotrophic production of polyhydroxybutyrate (PHB) from CO2 using a two-stage cultivation system. Catal. Today 2015, 257, 237–245. [Google Scholar] [CrossRef]
- Tanaka, K.; Ishizaki, A.; Kanamaru, T.; Kawano, T. Production of poly(D-3-hydroxybutyrate) from CO2, H2, and O2 by high cell density autotrophic cultivation of Alcaligenes eutrophus. Biotechnol. Bioeng. 1995, 45, 268–275. [Google Scholar] [CrossRef]
- Crépin, L.; Lombard, E.; Guillouet, S.E. Metabolic engineering of Cupriavidus necator for heterotrophic and autotrophic alka(e)ne production. Metab. Eng. 2016, 37, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Ishizaki, A.; Tanaka, K. Production of poly-β-hydroxybutyric acid from carbon dioxide by Alcaligenes eutrophus ATCC 17697T. J. Ferment. Bioeng. 1991, 71, 254–257. [Google Scholar] [CrossRef]
- Ishizaki, A.; Tanaka, K. Batch culture of Alcaligenes eutrophus ATCC 17697 T using recycled gas closed circuit culture system. J. Ferment. Bioeng. 1990, 69, 170–174. [Google Scholar] [CrossRef]
- Siegel, R.S.; Ollis, D.F. Kinetics of growth of the hydrogen-oxidizing bacterium Alcaligenes eutrophus (ATCC 17707) in chemostat culture. Biotechnol. Bioeng. 1984, 26, 764–770. [Google Scholar] [CrossRef]
- Burgdorf, T.; Lenz, O.; Buhrke, T.; van der Linden, E.; Jones, A.K.; Albracht, S.P.J.; Friedrich, B. [NiFe]-Hydrogenases of Ralstonia eutropha H16: Modular enzymes for oxygen-tolerant biological hydrogen oxidation. J. Mol. Microbiol. 2005, 10, 181–196. [Google Scholar] [CrossRef]
- Li, Z.; Li, G.; Chen, X.; Xia, Z.; Yao, J.; Yang, B.; Lei, L.; Hou, Y. Water splitting–biosynthetic hybrid system for CO2 conversion using nickel nanoparticles embedded in N-doped carbon nanotubes. Chemsuschem 2018, 11, 2382–2387. [Google Scholar] [CrossRef] [PubMed]
- Krieg, T.; Sydow, A.; Faust, S.; Huth, I.; Holtmann, D. CO2 to terpenes: Autotrophic and electroautotrophic α-humulene production with Cupriavidus necator. Angew. Chem. Int. Ed. 2017, 57, 1879–1882. [Google Scholar] [CrossRef] [PubMed]
- Schuster, E.; Schlegel, H.G. Chemolithotrophic growth of Hydrogenomonas H16 using electrolytic production of hydrogen and oxygen in a chemostat. Arch. Mikrobiol. 1967, 58, 380–409. [Google Scholar] [CrossRef] [PubMed]
- Torella, J.P.; Gagliardi, C.J.; Chen, J.S.; Bediako, D.K.; Colon, B.; Way, J.C.; Silver, P.A.; Nocera, D.G. Efficient solar-to-fuels production from a hybrid microbial–water-splitting catalyst system. Proc. Natl. Acad. Sci. USA 2015, 112, 2337–2342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiemeyer, A.; Link, H.; Weuster-Botz, D. Kinetic studies on autohydrogenotrophic growth of Ralstonia eutropha with nitrate as terminal electron acceptor. Appl. Microbiol. Biotechnol. 2007, 76, 75–81. [Google Scholar] [CrossRef]
- Schröder, V. Explosionsgrenzen von Wasserstoff und Wasserstoff/Methan-Gemischen. In Forschungsbericht Nr. 253; Bundesanstalt für Materialforschung und -prüfun: Berlin, Germany, 2003; pp. 1–40. ISSN 0938-5533. [Google Scholar]
- Miyahara, Y.; Yamamoto, M.; Thorbecke, R.; Mizuno, S.; Tsuge, T. Autotrophic biosynthesis of polyhydroxyalkanoate by Ralstonia eutropha from non-combustible gas mixture with low hydrogen content. Biotechnol. Lett. 2020, 42, 1655–1662. [Google Scholar] [CrossRef]
- Aragao, G.M.F.; Lindley, N.D.; Uribelarrea, J.L.; Pareilleux, A. Maintaining a controlled residual growth capacity increases the production of polyhydroxyalkanoate copolymers by Alcaligenes eutrophus. Biotechnol. Lett. 1996, 18, 937–942. [Google Scholar] [CrossRef]
- Ramsay, B.A.; Lomaliza, K.; Chavarie, C.; Dubé, B.; Bataille, P.; Ramsay, J.A. Production of poly-(β-hydroxybutyric-co-β-hydroxyvaleric) acids. Appl. Environ. Microbiol. 1990, 56, 2093–2098. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Yu, J. Gas mass transfer with microbial CO2 fixation and poly(3-hydroxybutyrate) synthesis in a packed bed bioreactor. Biochem. Eng. J. 2017, 122, 13–21. [Google Scholar] [CrossRef]
- Grousseau, E.; Lu, J.; Gorret, N.; Guillouet, S.E.; Sinskey, A.J. Isopropanol production with engineered Cupriavidus necator as bioproduction platform. Appl. Microbiol. Biotechnol. 2014, 98, 4277–4290. [Google Scholar] [CrossRef] [Green Version]
- Boy, C.; Lesage, J.; Alfenore, S.; Gorret, N.; Guillouet, S.E. Plasmid expression level heterogeneity monitoring via heterologous eGFP production at the single-cell level in Cupriavidus necator. Appl. Microbiol. Biotechnol. 2020, 104, 5899–5914. [Google Scholar] [CrossRef] [PubMed]
- Claassens, N.J.; Bordanaba-Florit, G.; Cotton, C.A.; De Maria, A.; Finger-Bou, M.; Friedeheim, L.; Giner-Laguarda, N.; Munar-Palmer, M.; Newell, W.; Scarinci, G.; et al. Replacing the Calvin cycle with the reductive glycine pathway in Cupriavidus necator. Metab. Eng. 2020, 62, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Sydow, A.; Pannek, A.; Krieg, T.; Huth, I.; Guillouet, S.E.; Holtmann, D. Expanding the genetic tool box for Cupriavidus necator by a stabilized L-rhamnose inducible plasmid system. J. Biotechnol. 2017, 263, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wahl, A.; Schuth, N.; Pfeiffer, D.; Nussberger, S.; Jendrossek, D. PHB granules are attached to the nucleoid via PhaM in Ralstonia eutropha. BMC Microbiol. 2012, 12, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Windhorst, C.; Gescher, J. Efficient biochemical production of acetoin from carbon dioxide using Cupriavidus necator H16. Biotechnol. Biofuels 2019, 12, 163. [Google Scholar] [CrossRef] [PubMed]




| Substance | g/L | Part |
|---|---|---|
| KH2PO4 | 1.5 | 1 |
| Na2HPO4·2H2O | 4.5 | |
| (NH4)2SO4 | 1.5 | 2 |
| MgSO4·7H2O | 0.2 | |
| NH4Fe(III) citrate | 0.05 | 3 |
| CaCl2·2H2O | 0.02 | |
| Tungsten solution | 1 mL | 4 |
| Fructose | 20 | 5 |
| Trace element solution | 1 mL | 6 |
| Tungsten solution | ||
| Na2WO4·2H2O | 0.06 | |
| Trace element solution | ||
| H3BO3 | 0.6 | |
| CoCl2·6H2O | 0.4 | |
| ZnSO4·7H2O | 0.2 | |
| MnCl2·4H2O | 0.06 | |
| NaMoO4·2H2O | 0.06 | |
| NiCl2·6H2O | 0.4 | |
| CuSO4·7H2O | 0.02 |
| Cultivation | pH2:pCO2:pO2 | Total Flow Rate (NmL/min) | Comment |
|---|---|---|---|
| Constant low O2 supply | |||
| Constant 90:8:2 | 400 | - | |
| Constant high O2 supply | |||
| Constant 85:10:5 | 100 | - | |
| Stepwise increase of O2 guided by the biomass | |||
| start 90:8:2 end 80:8:12 | 400 | Intermittent gas compositions are in the Supplementary Data file. | |
| Stepwise increase of O2 guided by the DO probe | |||
| Cultivation 1 | start 85:10:2 end 71:7:21 | start 97 end 140 | All intermittent gas compositions are in the Supplementary Data file. |
| Cultivation 2 | start 85:10:2 end 80:8:12 | start 97 end 250 | |
| Cultivation 3 | start 85:10:2 end 81:8:11 | start 97 end 250 | |
| Cultivation 4 | start 85:10:2 end 81:8:11 | start 97 end 250 |
| Cultivation | pO2 (atm) | μmax (h−1) | Final OD600 | Comment |
|---|---|---|---|---|
| Constant low O2 supply | constant at 0.02 | 0.008 | 5.0 | Figure 6 |
| Constant high O2 supply | constant at 0.05 | not determined | 1.3 | Figure S7 |
| Stepwise increase of O2 guided by the biomass | start 0.02 end 0.12 | 0.055 | 26.5 | Figure 6; biomass conc. measured offline, O2 supply increased manually |
| Stepwise increase of O2 guided by the DO probe (cultivations 3, 4) * | start 0.02 ± 0.00 highest 0.19 ± 0.02 end 0.11 ± 0.00 | 0.095 ± 0.01 | 53.5 ± 1.5 | Figure 7; DO measured online, O2 supply increased manually |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambauer, V.; Kratzer, R. Lab-Scale Cultivation of Cupriavidus necator on Explosive Gas Mixtures: Carbon Dioxide Fixation into Polyhydroxybutyrate. Bioengineering 2022, 9, 204. https://doi.org/10.3390/bioengineering9050204
Lambauer V, Kratzer R. Lab-Scale Cultivation of Cupriavidus necator on Explosive Gas Mixtures: Carbon Dioxide Fixation into Polyhydroxybutyrate. Bioengineering. 2022; 9(5):204. https://doi.org/10.3390/bioengineering9050204
Chicago/Turabian StyleLambauer, Vera, and Regina Kratzer. 2022. "Lab-Scale Cultivation of Cupriavidus necator on Explosive Gas Mixtures: Carbon Dioxide Fixation into Polyhydroxybutyrate" Bioengineering 9, no. 5: 204. https://doi.org/10.3390/bioengineering9050204
APA StyleLambauer, V., & Kratzer, R. (2022). Lab-Scale Cultivation of Cupriavidus necator on Explosive Gas Mixtures: Carbon Dioxide Fixation into Polyhydroxybutyrate. Bioengineering, 9(5), 204. https://doi.org/10.3390/bioengineering9050204