Biodegradable and Biocompatible Adhesives for the Effective Stabilisation, Repair and Regeneration of Bone
Abstract
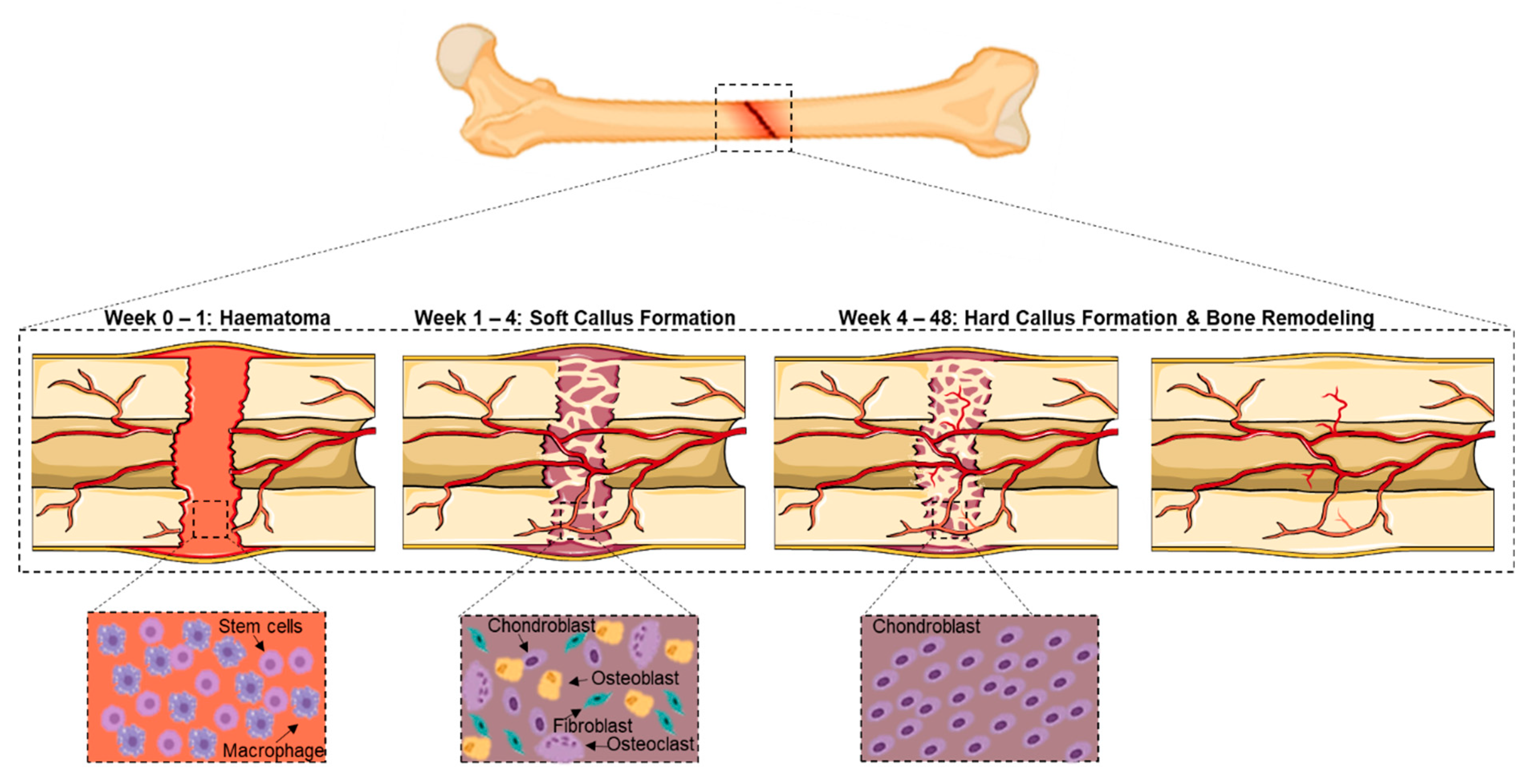
:1. Introduction
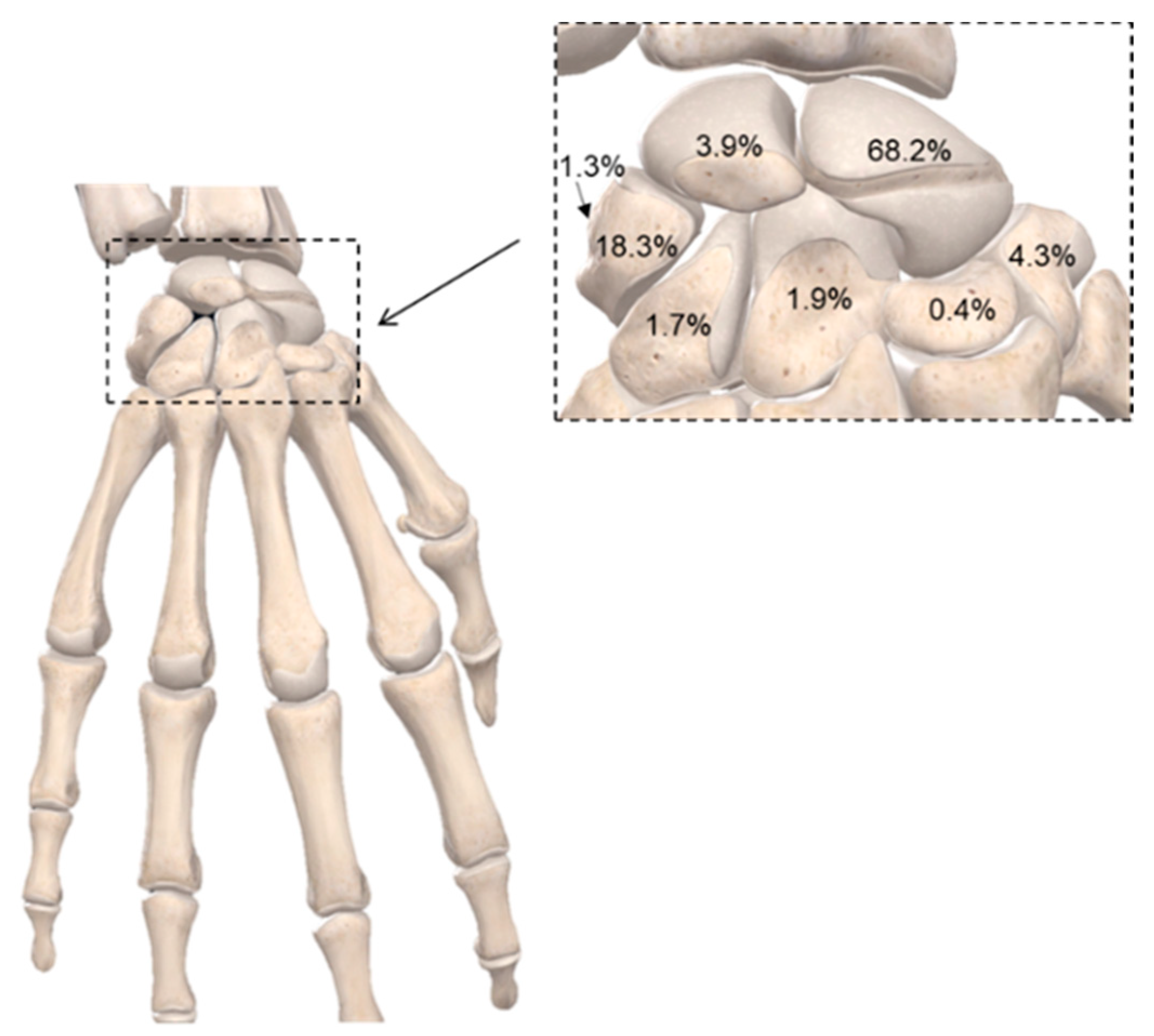
2. Complex Bone Fractures
3. Current Surgical Approaches for Fracture Repair
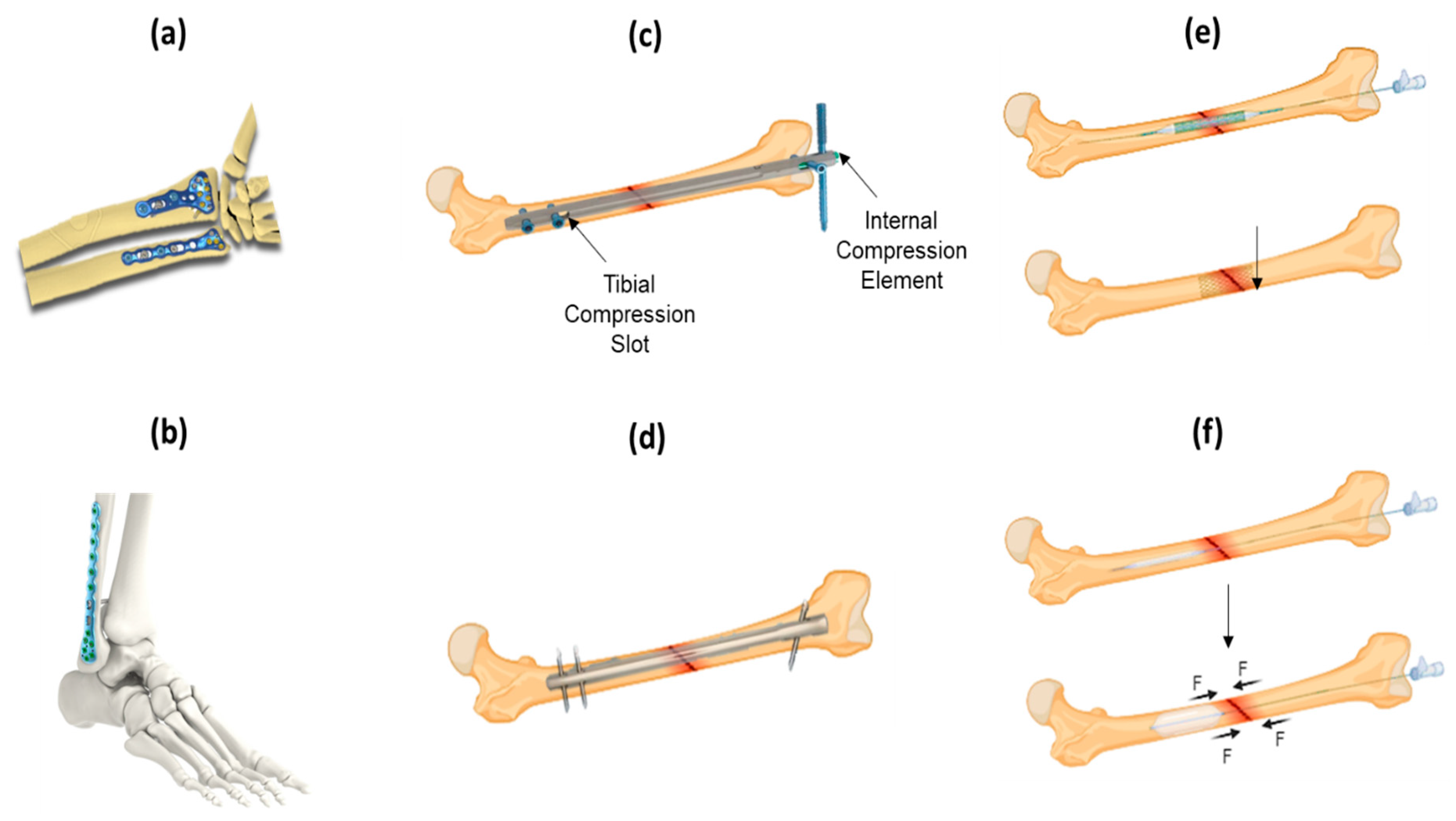
3.1. Open Reduction and Internal Fixation (ORIF)
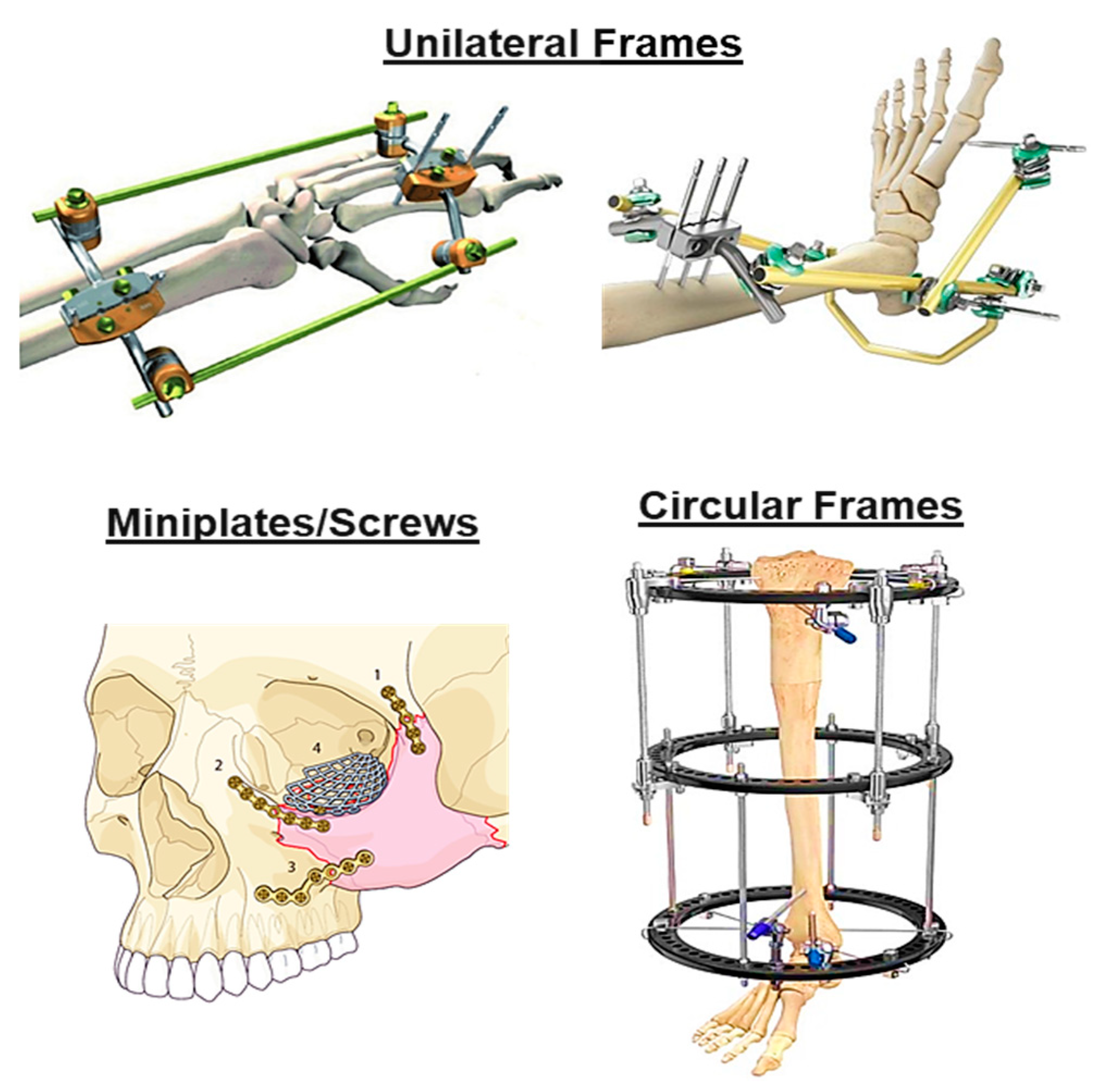
3.2. External Fixation
4. Bioadhesives
4.1. Synthetic Bioadhesives
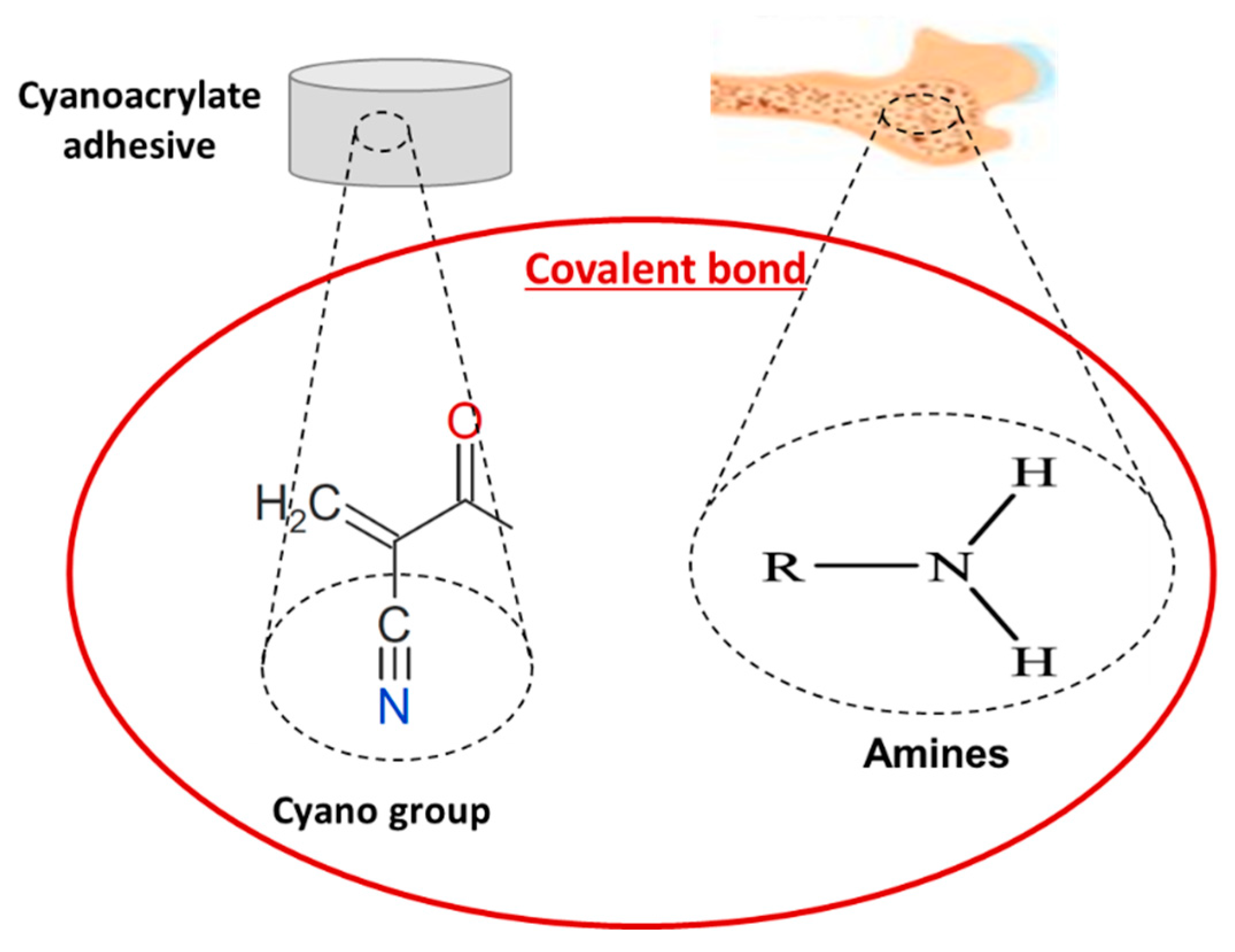
4.1.1. Cyanoacrylates
4.1.2. Polyurethanes
4.1.3. Polyesters
4.1.4. Poly-methyl Methacrylates (PMMA)
4.2. Naturally-Derived Bioadhesives
4.2.1. Fibrin
4.2.2. Gelatine–Resorcinol–Aldehydes
4.2.3. Polysaccharides
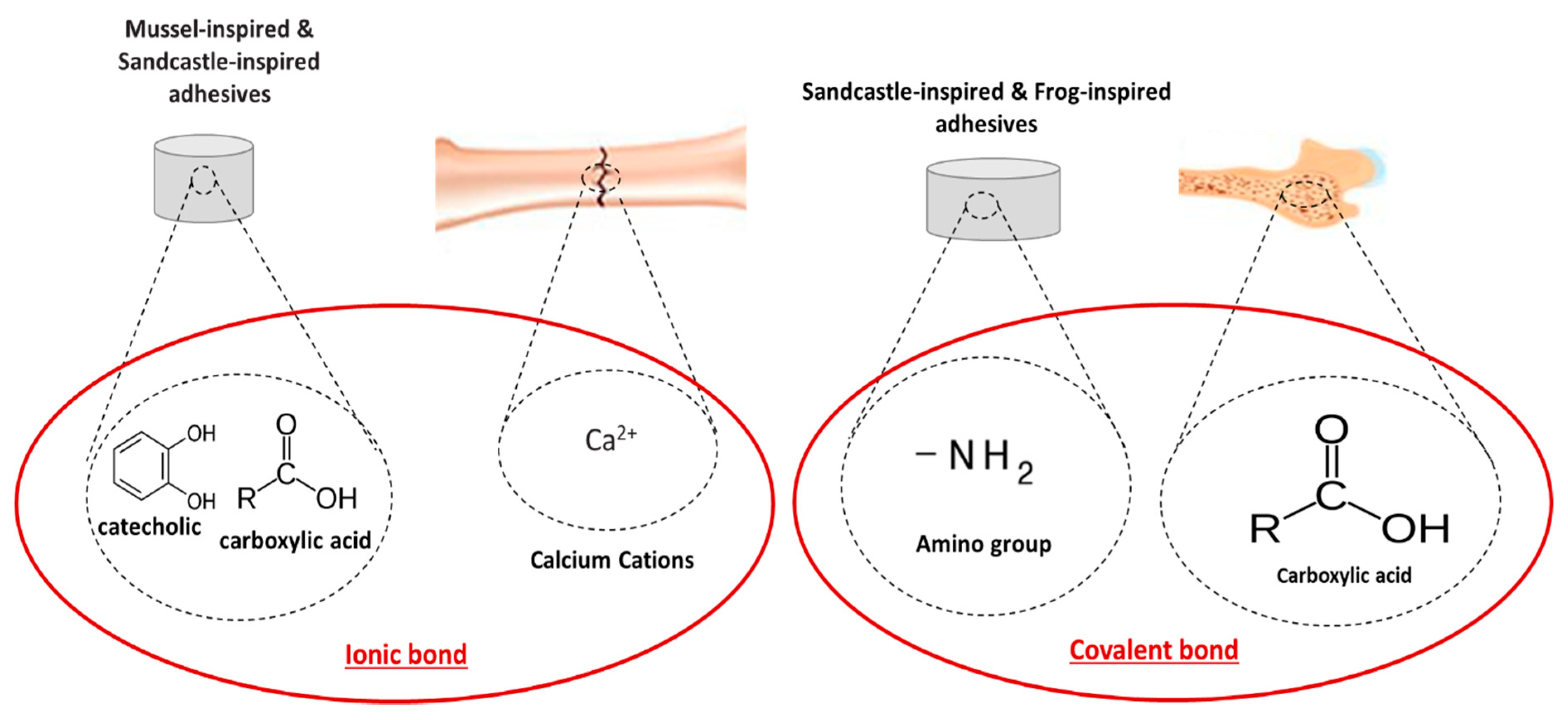
4.3. Biomimetic-Based Adhesives
4.3.1. Terrestrial Organisms-Inspired Adhesives
4.3.2. Marine Animals-Inspired Adhesives
| Biomimetic Adhesives | ||||
|---|---|---|---|---|
| Description | Application | Advantages | Disadvantages | |
| Notaden bennetti frog bioadhesives [81,108] | Protein-based elastic glue | Bone adhesion and fragments fixation (cartilage bone repair) Binding to biological tissues as well as other surfaces | Better biocompatibility and biodegradation than fibrin glues Function in moist environments | Lower adhesion strength than cyanoacrylates |
| Caddisfly silk bioadhesives [109,110,124] | Phosphate-functionalised and amino acid-based polyester copolymers | Bovine bone adhesion (orthopaedic) Scaffold materials for spinal cord injury Mesh grafts to treat hernias, ulcers and burns | Adhesion strength of 1.17 MPa Biodegradable in vitro and in vivo Higher interface compliance | Cohesive failure Low curing kinetics and adhesive properties on translationally relevant substrates |
| Balanus hameri barnacle bioadhesives [119,121,126] | Polyacrylamide-based copolymer with hydroxyl and hexyl groups | Repeatable and robust underwater adhesion to various substrates Material transfer, temporary fixation (orthopaedics) and material separation Bovine bone adhesion | Tensile shear strength of 2 MPa Enhanced toughness and cohesion strength Good elastic properties Rapid and reversible adhesion in water | Poor adhesion to bovine bone approx. 363 kPa Low mechanical strength |
| Mytilus edulis blue mussel bioadhesives [112,113,117,118] | Adhesives based on complex interaction between different proteins | Strong attachment to inorganic/organic surfaces at dry/wet environment Reliable crosslinking using oxidation agents, such as iron Suitable for joining titanium implants to a bone and/or bonding sternal bones | Non-immunogenicity and low cytotoxicity Greater adhesion on various substrates with adhesion strength of up to 10 MPa Good biodegradability Low exothermic reaction for the bonding of sternal bones | Difficulties relating to protein extraction resulting in high production costs, hampering the practical use Further research needed to determine the suitability of this adhesive as bone adhesive |
| Calfornica sandcastle worm bioadhesives [123,124,125,127] | Polyphenolic protein and phosphoserine-based adhesive | Strong attachment in a wet environment Reconstruction of craniofacial fractures Bonding of wet bone fragments Bond tissues to metallic and polymeric biomaterials | Maximum adhesion strength and hardness in <30 s Osteointegration, bone ingrowth and resorbability Small amount of adhesive needed to achieve the optimal properties Biodegradable and osteoconductive | Further in vitro and in vivo studies need to be conducted to verify the suitability to natural bone adhesion |
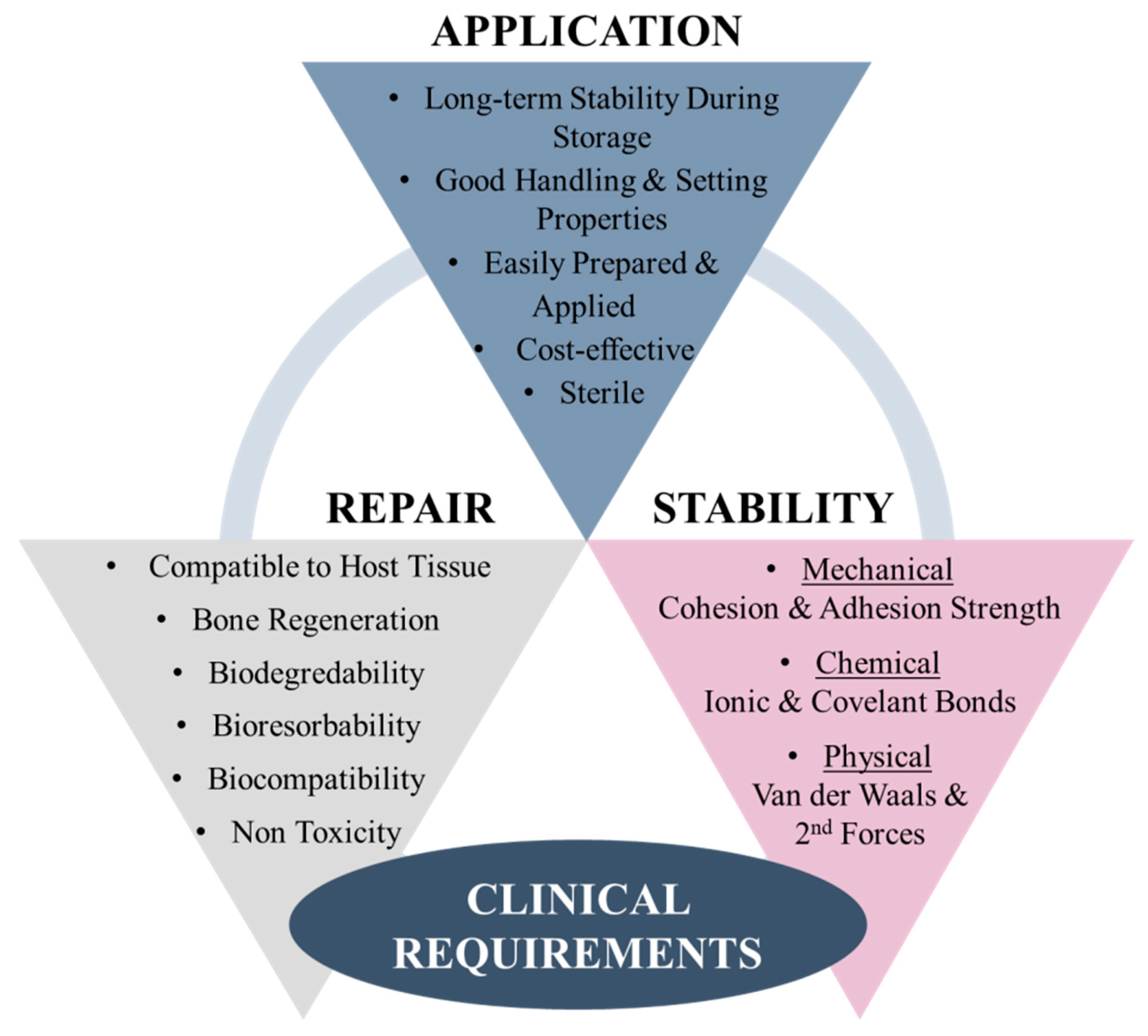
5. Clinical Requirements of Bioadhesive for Bone Fracture Repair
6. Bioadhesives for Bone Fracture Repair
Reinforced Bioactive Adhesives for Bone Fracture Repair
7. Conclusions and Future Research Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Augat, P.; Simon, U.; Liedert, A.; Claes, L. Mechanics and mechano-biology of fracture healing in normal and osteoporotic bone. Osteoporos. Int. 2005, 16 (Suppl. 2), 36–43. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.S.; Zioupos, P. Fracture of bone tissue: The ‘hows’ and the ‘whys’. Med. Eng. Phys. 2008, 30, 1209–1226. [Google Scholar] [CrossRef]
- Nellans, K.W.; Kowalski, E.; Chung, K.C. The Epidemiology of Distal Radius Fractures. Hand Clin. 2012, 28, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.-M.; Bisignano, C.; James, S.L.; Abady, G.G.; Abedi, A.; Abu-Gharbieh, E.; Alhassan, R.K.; Alipour, V.; Arabloo, J.; Asaad, M.; et al. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Health Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone physiological microenvironment and healing mechanism: Basis for future bone-tissue engineering scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [CrossRef] [PubMed]
- Kovach, T.K.; Dighe, A.S.; Lobo, P.I.; Cui, Q. Interactions between MSCs and immune cells: Implications for bone healing. J. Immunol. Res. 2015, 248. [Google Scholar] [CrossRef]
- Bahney, C.S.; Zondervan, R.L.; Allison, P.; Theologis, A.; Ashley, J.W.; Ahn, J.; Miclau, T.; Marcucio, R.S.; Hankenson, K.D. Cellular biology of fracture healing. J. Orthop. Res. 2019, 37, 35–50. [Google Scholar] [CrossRef] [Green Version]
- Elliott, D.S.; Newman, K.J.H.; Forward, D.P.; Hahn, D.M.; Ollivere, B.; Kojima, K.; Handley, R.; Rossiter, N.D.; Wixted, J.J.; Smith, R.M.; et al. A unified theory of bone healing and nonunion. Bone Jt. J. 2016, 98B, 884–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böker, K.O.; Richter, K.; Jäckle, K.; Taheri, S.; Grunwald, I.; Borcherding, K.; von Byern, J.; Hartwig, A.; Wildemann, B.; Schilling, A.F.; et al. Current state of bone adhesives-Necessities and hurdles. Materials 2019, 12, 3975. [Google Scholar] [CrossRef] [Green Version]
- Lewis, G. Properties of acrylic bone cement: State of the art review. J. Biomed. Mater. Res. 1997, 38, 155–182. [Google Scholar] [CrossRef]
- Lewis, G. Alternative acrylic bone cement formulations for cemented arthroplasties: Present status, key issues, and future prospects. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 84, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Jalil, M.A.A.; Shuid, A.N.; Muhammad, N. Role of medicinal plants and natural products on osteoporotic fracture healing. Evid.-Based Complement. Altern. Med. 2012, 2012, 714512. [Google Scholar] [CrossRef]
- Vriens, J.; Moos, K. Morbidity of the infraorbital nerve following orbitozygomatic complex fractures. J. Cranio-Maxillofac. Surg. 1995, 23, 363–368. [Google Scholar] [CrossRef]
- Muraoka, M.; Nakai, Y. Twenty years of statistics and observation of facial bone fracture. Acta Oto-Laryngol. Suppl. 1998, 118, 261–265. [Google Scholar] [CrossRef]
- Kasimova, G.; Yunusov, D.; Sakhibova, M.; Yaxudaev, E.; Batirova, B. Analysis of fracture of the foot bones in children according to the andijan region. Ann. Rom. Soc. Cell Biol. 2021, 25, 6186–6192. [Google Scholar]
- Meena, S.; Sharma, P.; Sambharia, A.K.; Dawar, A. Fractures of distal radius: An overview. J. Fam. Med. Prim. Care 2014, 3, 325–332. [Google Scholar] [CrossRef]
- Souyris, F.; Klersy, F.; Jammet, P.; Payrot, C. Malar bone fractures and their sequelae. J. Cranio-Maxillofac. Surg. 1989, 17, 64–68. [Google Scholar] [CrossRef]
- Welling, R.D.; Jacobson, J.A.; Jamadar, D.A.; Chong, S.; Caoili, E.M.; Jebson, P.J.L. MDCT and radiography of wrist fractures: Radiographic sensitivity and fracture patterns. Am. J. Roentgenol. 2008, 190, 10–16. [Google Scholar] [CrossRef]
- Jones, W.A.; Ghorbal, M.S. Fractures of the trapezium a report on three cases. J. Hand Surg. Am. 1985, 10, 227–230. [Google Scholar] [CrossRef]
- Erol, B.; Tanrikulu, R.; Görgün, B. Maxillofacial fractures. Analysis of demographic distribution and treatment in 2901 patients (25-year experience). J. Cranio-Maxillofac. Surg. 2004, 32, 308–313. [Google Scholar] [CrossRef]
- Hogg, N.J.V.; Stewart, T.C.; Armstrong, J.E.A.; Girotti, M.J. Epidemiology of maxillofacial injuries at trauma hospitals in Ontario, Canada, between 1992 and 1997. J. Trauma Inj. Infect. Crit. Care 2000, 49, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.; You, S.H. Analysis of facial bone fractures: An 11-year study of 2,094 patients. Indian J. Plast. Surg. 2010, 43, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17(12), 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Fisher, W.D.; Hamblen, D.L. Problems and pitfalls of compression fixation of long bone fractures: A review of results and complications. Injury 1979, 10, 99–107. [Google Scholar] [CrossRef]
- Larsson, S. Treatment of osteoporotic fractures. Scandinavian J. Surg. 2002, 91, 140–146. [Google Scholar] [CrossRef]
- Xue, X.H.; Yan, S.G.; Cai, X.Z.; Shi, M.M.; Lin, T. Intramedullary nailing versus plating for extra-articular distal tibial metaphyseal fracture: A systematic review and meta-analysis. Injury 2014, 45, 667–676. [Google Scholar] [CrossRef]
- Misra, A.; Kapur, R.; Maffulli, N. Complex proximal humeral fractures in adults- A systematic review of management. Injury 2001, 32, 363–372. [Google Scholar] [CrossRef]
- Gross, C.E.; Nunley, J.A. Navicular Stress Fractures. Foot Ankle Int. 2015, 36, 1117–1122. [Google Scholar] [CrossRef]
- Jackson, L.C.; Pacchiana, P.D. Common complications of fracture repair. Clin. Tech. Small Anim. Pract. 2004, 19, 168–179. [Google Scholar] [CrossRef]
- Uhthoff, H.K.; Poitras, P.; Backman, D.S. Internal plate fixation of fractures: Short history and recent developments. J. Orthop. Sci. 2006, 11, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Mucha, P.E.; Farnell, M.B. Analysis of Pelvic Fracture Management. J. Trauma Inj. Infect. Crit. 1984, 24, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Method for Subcutaneous Suprafascial Pedicular Internal Fixaton. U.S. Patent 5171279A, 15 December 1992.
- Mardam-Bey, S.W.; Bernholt, D.L.; Bogunovic, L.; Wright, R.W. Treatment of Tibial Eminence Fractures. In The Anterior Cruciate Ligament: Reconstruction and Basic Science, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 555–557. [Google Scholar]
- Gehr, J.; Friedl, W. Intramedullary locking compression nail for the treatment of an olecranon fracture. Oper Orthop Traumatol. 2006, 18, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.E.; Charles, P.; Ambrosia, D.; Robert, D. Complex Femur Fractures: Treatment with the Wagner External Device or the Grosse–Kempf Interlocking Nail. J. Trauma Inj. Infect. Crit. Care 1988, 28, 1553–1556. [Google Scholar] [CrossRef] [PubMed]
- Mische, H.A. Methods and Devices for Treatment of Bone Fractures. U.S. Patent 8007468B2, 30 August 2011. [Google Scholar]
- Schatzker, J. Principles of Stable Internal Fixation. Biomech. Dent. Implant. Handb. Res. 1980, 23, 232–235. [Google Scholar]
- Berger, J.L. ‘Methods and device for internal fixation of bone fractures’ United States Patent, 1996. Prog. Med. 2008, 28, 3077–3080. [Google Scholar]
- Fragomen, A.T.; Rozbruch, S.R. The mechanics of external fixation. HSS J. 2007, 3, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Collinge, C.; Sanders, R.; DiPasquale, T. Treatment of complex tibial periarticular fractures using percutaneous techniques. Clin. Orthop. Relat. Res. 2000, 375, 69–77. [Google Scholar] [CrossRef]
- External Fixation—Orthopedic Implants Industry. 2016. Available online: https://orthopedicimplantsindia.wordpress.com/2016/04/14/top-benefits-of-external-fixation/ (accessed on 24 May 2021).
- Hoffmann 3—Stryker External Fixation System. 2018. Available online: https://www.stryker.com/us/en/trauma-and-extremities/products/hoffmann-3-external-fixation-system.html (accessed on 24 May 2021).
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Petrie, E.M. Cyanoacrylate adhesives in surgical applications: A critical review. Rev. Adhes. Adhes. 2014, 2, 253–310. [Google Scholar] [CrossRef]
- Chen, C.F.; Shen, H.H.; Lin, T.Y.; Yu, Y.J.; Chen, W.C.; Chu, I.M. Studies on the preparation and characterization of mPEG-polyester biodegradable bioglue for bone defect repair. J. Med. Biol. Eng. 2011, 31, 13–17. [Google Scholar] [CrossRef]
- Arora, M.; Chan, E.K.S.; Gupta, S.; Diwan, A.D. Polymethylmethacrylate bone cements and additives: A review of the literature. World J. Orthop. 2013, 4, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Schlag, G.; Redl, H. Fibrin Sealant in Orthopedic Surgery. Clin. Orthop. Relat. Res. 1988, 227, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fernández, M.J.; Hammoudeh, H.; Lanao, R.P.F.; van Erk, M.; van Hest, J.C.M.; Leeuwenburgh, S.C.G. Bone-Adhesive Materials: Clinical Requirements, Mechanisms of Action, and Future Perspective. Adv. Mater. Interfaces 2019, 6, 1802021. [Google Scholar] [CrossRef]
- Dunne, N.J.; Orr, J.F. Thermal characteristics of curing acrylic bone cement. ITBM-RBM 2001, 22, 88–97. [Google Scholar] [CrossRef]
- Gosain, A.K. The current status of tissue glues: I. For bone fixation. Plast. Reconstr. Surg. 2002, 109, 2581–2583. [Google Scholar] [CrossRef] [PubMed]
- Kandalam, U.; Bouvier, A.; Casas, S.; Smith, R.; Gallego, A.; Rothrock, J.; Thompson, J.; Huang, C.-Y.; Stelnicki, E. Novel bone adhesives: A comparison of bond strengths in vitro. Int. J. Oral Maxillofac. Surg. 2013, 42, 1054–1059. [Google Scholar] [CrossRef]
- Kukleta, J.F.; Freytag, C.; Weber, M. Efficiency and safety of mesh fixation in laparoscopic inguinal hernia repair using n-butyl cyanoacrylate: Long-term biocompatibility in over 1, 300 mesh fixations. Hernia 2012, 16, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J.; Jung, G.B.; Choi, S.; Lee, G.; Kim, J.H.; Son, H.S.; Bae, H.; Park, H.-K. Biocompatibility of a novel cyanoacrylate based tissue adhesive: Cytotoxicity and biochemical property evaluation. PLoS ONE 2013, 8, e79761. [Google Scholar] [CrossRef] [Green Version]
- Pascual, G.; Sotomayor, S.; Rodríguez, M.; Pérez-Köhler, B.; Kühnhardt, A.; Fernández-Gutiérrez, M.; Román, J.S.; Bellón, J.M. Cytotoxicity of cyanoacrylate-based tissue adhesives and short-term preclinical in vivo biocompatibility in abdominal hernia repair. PLoS ONE 2016, 11, e0157920. [Google Scholar] [CrossRef] [Green Version]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive glasses: Where are we and where are we going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Liu, J.; Zhu, T.; Le, H.; Wang, X.; Guo, J.; Liu, G.; Ding, J. Functional Macromolecular Adhesives for Bone Fracture Healing. ACS Appl. Mater. Interfaces 2021, 14, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Gao, S.; Zhou, R.; Zhou, F.; Qiao, Y.; Qiu, D. Bioactive Pore-Forming Bone Adhesives Facilitating Cell Ingrowth for Fracture Healing. Adv. Mater. 2020, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, S.; Moztarzadeh, F.; Sadeghi, G.M.M.; Jafari, Y. In vitro study of a new biodegradable nanocomposite based on poly propylene fumarate as bone glue. Mater. Sci. Eng. C 2016, 69, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.; Henning, S.; Michler, G.; Hein, W.; Bernstein, A.; Schulz, M. Nanocrystalline hydroxyapatite for bone repair: An animal study. J. Mater. Sci. Mater. Med. 2009, 21, 283–294. [Google Scholar] [CrossRef]
- Schreader, K.J.; Bayer, I.S.; Milner, D.J.; Loth, E.; Jasiuk, I. A polyurethane-based nanocomposite biocompatible bone adhesive. J. Appl. Polym. Sci. 2012, 127, 4974–4982. [Google Scholar] [CrossRef]
- Bhagat, V.; Becker, M.L. Degradable Adhesives for Surgery and Tissue Engineering. Biomacromolecules 2017, 18, 3009–3039. [Google Scholar] [CrossRef] [Green Version]
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Brandeis, E.; Katz, D.; Silbermann, M.; Zinman, C. A new bioadhesive for in vivo bone adhesion. J. Mater. Sci. Mater. Med. 1993, 4, 543–546. [Google Scholar] [CrossRef]
- Jain, R.; Wairkar, S. Recent developments and clinical applications of surgical glues: An overview. Int. J. Biol. Macromol. 2019, 137, 95–106. [Google Scholar] [CrossRef]
- Idris, S.B.; Arvidson, K.; Plikk, P.; Ibrahim, S.; Finne-Wistrand, A.; Albertsson, A.-C.; Bolstad, A.I.; Mustafa, K. Polyester copolymer scaffolds enhance expression of bone markers in osteoblast-like cells. J. Biomed. Mater. Res. Part A 2010, 9999A, 631–639. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hyon, S.-H.; Ikada, Y. Water-curable and biodegradable prepolymers. J. Biomed. Mater. Res. 1991, 25, 1481–1494. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhou, P.; Jérôme, V.; Freitag, R.; Agarwal, S. Enzymatically Degradable Polyester-Based Adhesives. ACS Biomater. Sci. Eng. 2015, 1, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Yue, K.; Tamayol, A.; Khademhosseini, A. Elastic sealants for surgical applications. Eur. J. Pharm. Biopharm. 2015, 95, 27–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Ameer, G.A.; Sheppard, B.J.; Langer, R. A tough biodegradable elastomer. Nat. Biotechnol. 2002, 20, 602–606. [Google Scholar] [CrossRef]
- Vogt, L.; Ruther, F.; Salehi, S.; Boccaccini, A.R. Poly(Glycerol Sebacate) in Biomedical Applications—A Review of the Recent Literature. Adv. Healthc. Mater. 2021, 10, 2002026. [Google Scholar] [CrossRef]
- Ferrari, P.F.; Aliakbarian, B.; Lagazzo, A.; Tamayol, A.; Palombo, D.; Perego, P. Tailored electrospun small-diameter graft for vascular prosthesis. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 635–643. [Google Scholar] [CrossRef]
- Kalakonda, P.; Aldhahri, M.A.; Abdel-Wahab, M.S.; Tamayol, A.; Moghaddam, K.M.; Ben Rached, F.; Pain, A.; Khademhosseini, A.; Memic, A.; Chaieb, S. Microfibrous silver-coated polymeric scaffolds with tunable mechanical properties. RSC Adv. 2017, 7, 34331–34338. [Google Scholar] [CrossRef] [Green Version]
- Memic, A.; Aldhahri, M.; Tamayol, A.; Mostafalu, P.; Abdel-Wahab, M.S.; Samandari, M.; Moghaddam, K.M.; Annabi, N.; Bencherif, S.A.; Khademhosseini, A. Nanofibrous silver-coated polymeric scaffolds with tunable electrical properties. Nanomaterials 2017, 7, 63. [Google Scholar] [CrossRef]
- Saudi, A.; Rafienia, M.; Kharazi, A.Z.; Salehi, H.; Zarrabi, A.; Karevan, M. Design and fabrication of poly (glycerol sebacate)-based fibers for neural tissue engineering: Synthesis, electrospinning, and characterization. Polym. Adv. Technol. 2019, 30, 1427–1440. [Google Scholar] [CrossRef]
- Sha, D.; Wu, Z.; Zhang, J.; Ma, Y.; Yang, Z.; Yuan, Y. Development of modified and multifunctional poly(glycerol sebacate) (PGS)-based biomaterials for biomedical applications. Eur. Polym. J. 2021, 161, 110830. [Google Scholar] [CrossRef]
- Wu, T.; Frydrych, M.; Kelly, K.O.; Chen, B. Poly(glycerol sebacate urethane)—Cellulose Nanocomposites with Water-Active Shape-Memory Effects. Biomacromolecules 2014, 15, 2663–2671. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, Y.; Du, Y.; Chen, X.; Lei, B.; Xue, Y.; Ma, P.X. A highly bioactive and biodegradable poly(glycerol sebacate)-silica glass hybrid elastomer with tailored mechanical properties for bone tissue regeneration. J. Mater. Chem. B 2015, 3, 3222–3233. [Google Scholar] [CrossRef] [PubMed]
- Kerativitayanan, P.; Tatullo, M.; Khariton, M.; Joshi, P.; Perniconi, B.; Gaharwar, A.K. Gaharwar, Nanoengineered Osteoinductive and Elastomeric Scaffolds for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2017, 3, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Shi, J.; Liu, Y.; Si, J.; Yuan, Y.; Liu, C. A mechanically robust and flexible PEGylated poly(glycerol sebacate)/β-TCP nanoparticle composite membrane for guided bone regeneration. J. Mater. Chem. B 2019, 7, 3279–3290. [Google Scholar] [CrossRef]
- Hasandoost, L.; Rodriguez, O.; Alhalawani, A.; Zalzal, P.; Schemitsch, E.H.; Waldman, S.D.; Papini, M.; Towler, M.R. The role of poly(methyl methacrylate) in management of bone loss and infection in revision total knee arthroplasty: A review. J. Funct. Biomater. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Farrar, D.F. Bone adhesives for trauma surgery: A review of challenges and developments. Int. J. Adhes. Adhes. 2012, 33, 89–97. [Google Scholar] [CrossRef]
- Lu, J. Orthopedic bone cements. Biomechanics and Biomaterials in Orthopedics, 2nd ed.; Springer: London, UK, 2016; pp. 123–138. [Google Scholar]
- Mikkelsen, D.B.; Pedersen, C.; Højbjerg, T.; Schønheyder, H.C. Culture of multiple peroperative biopsies and diagnosis of infected knee arthroplasties. APMIS 2006, 114, 449–452. [Google Scholar] [CrossRef]
- Sa, Y.; Wang, M.; Deng, H.; Wang, Y.; Jiang, T. Beneficial effects of biomimetic nano-sized hydroxyapatite/antibiotic gentamicin enriched chitosan-glycerophosphate hydrogel on the performance of injectable polymethylmethacrylate. RSC Adv. 2015, 5, 91082–91092. [Google Scholar] [CrossRef]
- Grossterlinden, L.; Janssen, A.; Schmitz, N.; Priemel, M.; Pogoda, P.; Amling, M.; Rueger, J.M.; Linhart, W. Deleterious tissue reaction to an alkylene bis(dilactoyl)-methacrylate bone adhesive in long-term follow up after screw augmentation in an ovine model. Biomaterials 2006, 27, 3379–3386. [Google Scholar] [CrossRef]
- Meyer, G.; Muster, D.; Schmitt, D.; Jung, P.; Jaeger, J.H. Bone bonding through bioadhesives: Present status. Biomater. Med. Devices. Artif. Organs 1979, 7, 55–71. [Google Scholar] [CrossRef]
- Heiss, C.; Kraus, R.; Peters, F.; Henn, W.; Schnabelrauch, M.; Berg, A.; Pautzsch, T.; Weisser, J.; Schnettler, R. Development of a bioresorbable self-hardening bone adhesive based on a composite consisting of polylactide methacrylates and β-tricalcium phosphate. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90B, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.E.; Evans, A.J.; Mathis, J.M.; Kallmes, D.F.; Cloft, H.J.; Dion, J.E. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: Technical aspects. Am. J. Neuroradiol. 1997, 18, 1897–1904. [Google Scholar] [PubMed]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Shah, N.V.; Meislin, R. Current state and use of biological adhesives in orthopedic surgery. Orthopedics 2013, 36, 945–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbes, H.; Bösch, P.; Lintner, F.; Salzer, M. First clinical experience with heterologous cancellous bone grafting combined with the Fibrin Adhesive System (F.A.S.). Arch. Orthop. Trauma. Surg. 1981, 98, 183–188. [Google Scholar] [CrossRef]
- Yang, G.; Rothrauff, B.B.; Tuan, R.S. Tendon and ligament regeneration and repair: Clinical relevance and developmental paradigm. Birth Defects Res. Part C Embryo Today Rev. 2013, 99, 203–222. [Google Scholar] [CrossRef] [Green Version]
- Sierra, D.H.; Eberhardt, A.W.; Lemons, J.E. Failure characteristics of multiple-component fibrin-based adhesives. J. Biomed. Mater. Res. 2001, 59, 1–11. [Google Scholar] [CrossRef]
- Strausberg, R.L.; Link, R.P. Protein-based medical adhesives. Trends Biotechnol. 1990, 8, 53–57. [Google Scholar] [CrossRef]
- Wanasingha, N.; Dutta, N.K.; Choudhury, N.R. Emerging bioadhesives: From traditional bioactive and bioinert to a new biomimetic protein-based approach. Adv. Colloid Interface Sci. 2021, 296, 102521. [Google Scholar] [CrossRef]
- Tatooles, C.J.; Braunwald, N.S. The use of crosslinked gelatin as a tissue adhesive to control hemorrhage from liver and kidney. Surgery 1966, 60, 857–861. [Google Scholar] [CrossRef]
- Braunwald, N.S.; Gay, W.; Tatooles, C.J. Evaluation of crosslinked gelatin as a tissue adhesive and hemostatic agent: An experimental study. Surgery 1966, 59, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Nomori, H.; Horio, H.; Morinaga, S.; Suemasu, K. Gelatin-resorcinol-formaldehyde-glutaraldehyde glue for sealing pulmonary air leaks during thoracoscopic operation. Ann. Thorac. Surg. 1999, 67, 212–216. [Google Scholar] [CrossRef]
- Smith, D.C. Lutes, Glues, Cements and Adhesives in Medicine and Dentistry. Bio-Medical Eng. 1973, 8, 108–115. [Google Scholar]
- Kull, S.; Martinelli, I.; Briganti, E.; Losi, P.; Spiller, D.; Tonlorenzi, S.; Soldani, G. Glubran2 Surgical Glue: In Vitro Evaluation of Adhesive and Mechanical Properties. J. Surg. Res. 2009, 157, e15–e21. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ng, S.C.; Yu, J.; Tsai, W.-B. Modification and crosslinking of gelatin-based biomaterials as tissue adhesives. Colloids Surf. B Biointerfaces 2019, 174, 316–323. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.T.S.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Wang, D.-A.; Varghese, S.; Sharma, B.; Strehin, I.; Fermanian, S.; Gorham, J.; Fairbrother, D.H.; Cascio, B.; Elisseeff, J.H. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat. Mater. 2007, 6, 385–392. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Arthur, S.D.; Chenault, H.K.; Figuly, G.D.; Kodokian, G.K. Polysaccharide-based tissue adhesives for sealing corneal incisions. Curr. Eye Res. 2007, 32, 1045–1050. [Google Scholar] [CrossRef]
- Hoffmann, B.; Seitz, D.; Mencke, A.; Kokott, A.; Ziegler, G. Glutaraldehyde and oxidised dextran as crosslinker reagents for chitosan-based scaffolds for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2009, 20, 1495–1503. [Google Scholar] [CrossRef]
- Simson, J.; Crist, J.; Strehin, I.; Lu, Q.; Elisseeff, J.H. An orthopedic tissue adhesive for targeted delivery of intraoperative biologics. J. Orthop. Res. 2012, 31, 392–400. [Google Scholar] [CrossRef]
- Li, D.; Chen, J.; Wang, X.; Zhang, M.; Li, C.; Zhou, J. Recent Advances on Synthetic and Polysaccharide Adhesives for Biological Hemostatic Applications. Front. Bioeng. Biotechnol. 2020, 8, 926. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.L.; Bradley, T.A.; Walsh, N.A.; Appleyard, R.C.; Tyler, M.J.; Murrell, G.A.C. Frog glue enhances rotator cuff repair in a laboratory cadaveric model. J. Shoulder Elb. Surg. 2009, 18, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, V.; O’Brien, E.; Zhou, J.; Becker, M.L. Caddisfly Inspired Phosphorylated Poly(ester urea)-Based Degradable Bone Adhesives. Biomacromolecules 2016, 17, 3016–3024. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.P.; Coelho, J.F.; Bordado, J.C.; Cidade, M.T.; Gil, M.H. Surgical adhesives: Systematic review of the main types and development forecast. Prog. Polym. Sci. 2011, 37, 1031–1050. [Google Scholar] [CrossRef]
- Waite, J.H. Adhesion in Byssally Attached Bivalves. Biol. Rev. 1983, 58, 209–231. [Google Scholar] [CrossRef]
- Silverman, H.G.; Roberto, F.F. Understanding marine mussel adhesion. Mar. Biotechnol. 2007, 9, 661–681. [Google Scholar] [CrossRef] [Green Version]
- Flammang, P.; Lambert, A.; Bailly, P.; Hennebert, E. Polyphosphoprotein-containing marine adhesives. J. Adhes. 2009, 85, 447–464. [Google Scholar] [CrossRef]
- Cha, H.J.; Hwang, D.S.; Lim, S. Development of bioadhesives from marine mussels. Biotechnol. J. 2008, 3, 631–638. [Google Scholar] [CrossRef]
- Yamamoto, H. Bonding Strength of Synthetic Poly(amino acid)s on Metals. Nippon Kagaku Kaishi 1986, 1, 90–92. [Google Scholar] [CrossRef]
- Nagai, A.; Yamamoto, H. Insolubilizing Studies of Water-Soluble Poly(Lys Tyr) by Tyrosinase. Bull. Chem. Soc. Jpn. 1989, 62, 2410–2412. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, H.; Kuno, S.; Nagai, A.; Nishida, A.; Yamauchi, S.; Ikeda, K. Insolubilizing and adhesive studies of water-soluble synthetic model proteins. Int. J. Biol. Macromol. 1990, 12, 305–310. [Google Scholar] [CrossRef]
- Meredith, H.J.; Jenkins, C.L.; Wilker, J.J. Enhancing the adhesion of a biomimetic polymer yields performance rivaling commercial glues. Adv. Funct. Mater. 2014, 24, 3259–3267. [Google Scholar] [CrossRef]
- Yamamoto, H.; Nagai, A.; Okada, T.; Nishida, A. Synthesis and adhesive studies of barnacle model proteins. Mar. Chem. 1989, 26, 331–338. [Google Scholar] [CrossRef]
- Yamamoto, H.; Nagai, A. Polypeptide models of the arthropodin protein of the barnacle Balanus balanoides. Mar. Chem. 1992, 37, 131–143. [Google Scholar] [CrossRef]
- Nishida, J.; Higaki, Y.; Takahara, A. Synthesis and characterization of barnacle adhesive mimetic towards underwater adhesion. Chem. Lett. 2015, 44, 1047–1049. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Weerasekare, G.M.; Stewart, R.J. Multiphase adhesive coacervates inspired by the sandcastle worm. ACS Appl. Mater. Interfaces 2011, 3, 941–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.; Jia, M.; Mu, Y.; Jiang, W.; Wan, X. Humid bonding with a water-soluble adhesive inspired by mussels and sandcastle worms. Macromol. Chem. Phys. 2014, 216, 450–459. [Google Scholar] [CrossRef]
- Kirillova, A.; Kelly, C.; von Windheim, N.; Gall, K. Bioinspired Mineral-Organic Bioresorbable Bone Adhesive. Adv. Healthc. Mater. 2018, 7, e1800467. [Google Scholar] [CrossRef]
- Bhagat, V.; O’Brien, E.; Zhou, J.; Becker, M.L. A novel class of injectable bioceramics that glue tissues and biomaterials. Materials 2018, 11, 2492. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.; Wang, J.; Gong, J.P. Barnacle Cement Proteins-Inspired Tough Hydrogels with Robust, Long-Lasting, and Repeatable Underwater Adhesion. Adv. Funct. Mater. 2020, 31, 2009334. [Google Scholar] [CrossRef]
- Shao, H.; Bachus, K.N.; Stewart, R.J. A water-borne adhesive modeled after the sandcastle glue of P. californica. Macromol. Biosci. 2009, 9, 464–471. [Google Scholar] [CrossRef] [Green Version]
- Gardziella, A.; Mueller, R. Phenolic Resins. Kunstst. Ger. Plast. 1990, 80, 66–68. [Google Scholar] [CrossRef] [Green Version]
- Donkerwolcke, M.; Burny, F.; Muster, D. Tissues and bone adhesives historical aspects. Biomaterials 1998, 19, 1461–1466. [Google Scholar] [CrossRef]
- Ishihara, K.; Nakabayashi, N. Adhesive bone cement both to bone and metals: 4-META in MMA initiated with tri-n-butyl borane. J. Biomed. Mater. Res. 1989, 23, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Wistlich, L.; Rücker, A.; Schamel, M.; Kübler, A.C.; Gbureck, U.; Groll, J. A Bone Glue with Sustained Adhesion under Wet Conditions. Adv. Healthc. Mater. 2017, 6, 1600902. [Google Scholar] [CrossRef]
- Liu, X.; Pujari-Palmer, M.; Wenner, D.; Procter, P.; Insley, G.; Engqvist, H. Adhesive cements that bond soft tissue ex vivo. Materials 2019, 12, 2473. [Google Scholar] [CrossRef] [Green Version]
- Hulsart-Billström, G.; Stelzl, C.; Procter, P.; Pujari-Palmer, M.; Insley, G.; Engqvist, H.; Larsson, S. In vivo safety assessment of a bio-inspired bone adhesive. J. Mater. Sci. Mater. Med. 2020, 31, 24. [Google Scholar] [CrossRef] [Green Version]
- Le Nihouannen, D.; Saffarzadeh, A.; Gauthier, O.; Moreau, F.; Pilet, P.; Spaethe, R.; Layrolle, P.; Daculsi, G. Bone tissue formation in sheep muscles induced by a biphasic calcium phosphate ceramic and fibrin glue composite. J. Mater. Sci. Mater. Med. 2008, 19, 667–675. [Google Scholar] [CrossRef]
- Cassaro, C.V.; Justulin, L.A., Jr.; De Lima, P.R.; Golim, M.D.A.; Biscola, N.P.; de Castro, M.V.; De Oliveira, A.L.R.; Doiche, D.P.; Pereira, E.J.; Ferreira, R.S., Jr.; et al. Fibrin biopolymer as scaffold candidate to treat bone defects in rats. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e20190027. [Google Scholar] [CrossRef] [Green Version]
- Kumbar, S.G.; Toti, U.S.; Deng, M.; James, R.; Laurencin, C.T.; Aravamudhan, A.; Harmon, M.; Ramos, D.M. Novel mechanically competent polysaccharide scaffolds for bone tissue engineering. Biomed. Mater. 2011, 6, 065005. [Google Scholar] [CrossRef]
- Hoffmann, B.; Volkmer, E.; Kokott, A.; Augat, P.; Ohnmacht, M.; Sedlmayr, N.; Schieker, M.; Claes, L.; Mutschler, W.; Ziegler, G. Characterisation of a new bioadhesive system based on polysaccharides with the potential to be used as bone glue. J. Mater. Sci. Mater. Med. 2009, 20, 2001–2009. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-H.; Cheong, H.; Ahn, J.-S.; Zhou, C.; Kwon, J.J.; Cha, H.J.; Jun, S.H. Engineered mussel bioglue as a functional osteoinductive binder for grafting of bone substitute particles to accelerate in vivo bone regeneration. J. Mater. Chem. B 2015, 3, 546–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.; Kay, G.W.; Cochran, D.; Fiorellini, J.; Hess, B. From bench-to-bedside: Licensing and development of a mineral-organic bone adhesive for bone repair. In Society for Biomaterials Annual Meeting and Exposition 42nd Annual Meeting; Society for Biomaterials: Mount Laurel, NJ, USA, 2019; Volume 40, 169p. [Google Scholar]
- Bystrom, J.L.; Pujari-Palmer, M. Phosphoserine functionalized cements preserve metastable phases, and reprecipitate octacalcium phosphate, hydroxyapatite, dicalcium phosphate, and amorphous calcium phosphate, during degradation, in vitro. J. Funct. Biomater. 2019, 10, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lillie, M.A.; David, G.J.; Gosline, J.M. Mechanical role of elastin-associated microfibrils in pig aortic elastic tissue. Connect. Tissue Res. 1998, 37, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Norton, M.R.; Kay, G.W.; Brown, M.C.; Cochran, D.L. Bone glue—The final frontier for fracture repair and implantable device stabilization. Int. J. Adhes. Adhes. 2020, 102, 102647. [Google Scholar] [CrossRef]
- Kirillova, A.; Nillissen, O.; Liu, S.; Kelly, C.; Gall, K. Reinforcement and Fatigue of a Bioinspired Mineral–Organic Bioresorbable Bone Adhesive. Adv. Healthc. Mater. 2021, 10, 2001058. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Zhou, D.; Dai, Z.; Zhao, W.; Yang, Q.; Xu, Y.; Li, X.; Wu, J.; Guo, S.; Jiang, D. Bone Plate Composed of a Ternary Nanohydroxyapatite/Polyamide 66/Glass Fiber Composite: Biocompatibility In Vivo and Internal Fixation for Canine Femur Fractures. Adv. Funct. Mater. 2019, 29, 1808738. [Google Scholar] [CrossRef]
- Nordberg, A.; Antoni, P.; Montañez, M.I.; Hult, A.; von Holst, H.; Malkoch, M. Highly adhesive phenolic compounds as interfacial primers for bone fracture fixations. ACS Appl. Mater. Interfaces 2010, 2, 654–657. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Lode, A.; Richter, R.F.; Pradel, W.; Franke, A.; Rauner, M.; Stadlinger, B.; Lauer, G.; Gelinsky, M.; Korn, P. Toward biofabrication of resorbable implants consisting of a calcium phosphate cement and fibrin-a characterization in vitro and in vivo. Int. J. Mol. Sci. 2021, 22, 1218. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Z.; Mo, J.; Lyu, Y.; Tang, X.; Shen, X. Effects of guar gum on adhesion properties of soybean protein isolate onto porcine bones. Int. J. Adhes. Adhes. 2017, 75, 124–131. [Google Scholar] [CrossRef]
- Liu, J.; Scherman, O.A. Cucurbit[n]uril Supramolecular Hydrogel Networks as Tough and Healable Adhesives. Adv. Funct. Mater. 2018, 28, 1800848. [Google Scholar] [CrossRef]
- Liu, H.; Liu, B.; Gao, C.; Meng, B.; Yang, H.; Yu, H.; Yang, L. Injectable, biomechanically robust, biodegradable and osseointegrative bone cement for percutaneous kyphoplasty and vertebroplasty. Int. Orthop. 2018, 42, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Granskog, V.; García-Gallego, S.; Von Kieseritzky, J.; Rosendahl, J.; Stenlund, P.; Zhang, Y.; Petronis, S.; Lyvén, B.; Arner, M.; Håkansson, J.; et al. High-Performance Thiol–Ene Composites Unveil a New Era of Adhesives Suited for Bone Repair. Adv. Funct. Mater. 2018, 28, 1800372. [Google Scholar] [CrossRef] [Green Version]
- Erken, M.; Tevlek, A.; Hosseinian, P.; Topuz, B.; Aydin, H.M. Effects of ceramic particle size on cell attachment and viability in polyurethane-based bone adhesive composites. J. Compos. Mater. 2020, 54, 2013–2022. [Google Scholar] [CrossRef]
- Lei, K.; Zhu, Q.; Wang, X.; Xiao, H.; Zheng, Z. In Vitro and in Vivo Characterization of a Foam-Like Polyurethane Bone Adhesive for Promoting Bone Tissue Growth. ACS Biomater. Sci. Eng. 2019, 5, 5489–5497. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, X.; Lv, X.; Zhang, M.; Huang, X.; Shi, Y.; Lu, C.; Song, J.; Yang, H. Bioinspired Mineral–Organic Bone Adhesives for Stable Fracture Fixation and Accelerated Bone Regeneration. Adv. Funct. Mater. 2020, 30, 1908381. [Google Scholar] [CrossRef]
- Mikai, A.; Ono, M.; Tosa, I.; Nguyen, H.T.T.; Hara, E.S.; Nosho, S.; Kimura-Ono, A.; Nawachi, K.; Takarada, T.; Kuboki, T.; et al. BMP-2/Β-TCP local delivery for bone regeneration in MRONJ-like mouse model. Int. J. Mol. Sci. 2020, 21, 7028. [Google Scholar] [CrossRef]
- Tran, R.T.; Wang, L.; Zhang, C.; Huang, M.; Tang, W.; Zhang, C.; Zhang, Z.; Jin, D.; Banik, B.; Brown, J.L.; et al. Synthesis and characterization of biomimetic citrate-based biodegradable composites. J. Biomed. Mater. Res. Part A 2014, 102, 2521–2532. [Google Scholar] [CrossRef]
- Ma, C.; Tian, X.; Kim, J.P.; Xie, D.; Ao, X.; Shan, D.; Lin, Q.; Hudock, M.R.; Bai, X.; Yang, J. Citrate-based materials fuel human stem cells by metabonegenic regulation. Proc. Natl. Acad. Sci. USA 2018, 115, E11741–E11750. [Google Scholar] [CrossRef] [Green Version]
- Xie, D.; Guo, J.; Mehdizadeh, M.R.; Tran, R.T.; Chen, R.; Sun, D.; Qian, G.; Jin, D.; Bai, X.; Yang, J. Development of injectable citrate-based bioadhesive bone implants. J. Mater. Chem. B 2015, 3, 387–398. [Google Scholar] [CrossRef]
- Zhang, K.; Jia, Z.; Yang, B.; Feng, Q.; Xu, X.; Yuan, W.; Li, X.; Chen, X.; Duan, L.; Wang, D.; et al. Adaptable Hydrogels Mediate Cofactor-Assisted Activation of Biomarker-Responsive Drug Delivery via Positive Feedback for Enhanced Tissue Regeneration. Adv. Sci. 2018, 5, 1800875. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; He, X.; Chen, Z.; He, L.; Lu, M.; Ge, J.; Weng, J.; Mu, Y.; Duan, K. Effect of magnesium particle fraction on osteoinduction of hydroxyapatite sphere-based scaffolds. J. Mater. Chem. B 2019, 7, 5648–5660. [Google Scholar] [CrossRef] [PubMed]
- Brückner, T.; Meininger, M.; Groll, J.; Kübler, A.C.; Gbureck, U. Magnesium phosphate cement as mineral bone adhesive. Materials 2019, 12, 3819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, e435-17. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Tammam, S.N.; Azzazy, H.M.E.; Lamprecht, A. Biodegradable particulate carrier formulation and tuning for targeted drug delivery. J. Biomed. Nanotechnol. 2015, 11, 555–577. [Google Scholar] [CrossRef]
- Levingstone, T.J.; Herbaj, S.; Dunne, N.J. Calcium phosphate nanoparticles for therapeutic applications in bone regeneration. Nanomaterials 2019, 9, 1570. [Google Scholar] [CrossRef] [Green Version]







| Scheme | |||
|---|---|---|---|
| Application | Advantages | Disadvantages | |
| Cyanoacrylates [45,49,50,51] | Craniofacial, osteochondral and trabecular fractures Bone formation and fragments fixation Enhancement or replacement of screws/plates | Max adhesive strength of 9 MPa Enhanced tensile and shear bond in wet and dry environment Higher shear strength (1–2 MPa) than screws and plates | Partial bone formation Less efficient than screws with low adhesive and mechanical properties Chronic inflammatory response and tissue necrosis Cytotoxicity to cells in vitro and dermatitis in vivo |
| Polyurethane [53,54,55,56] | Bone formation and fragments fixation Bone to bone adhesion Closure of fractures | High adhesive or/and cohesive strength Osteogenic, non-toxic and biocompatible Degradation in wet environment | Bond failure between bone and adhesive Low biodegradability Infection Tissue necrosis |
| Polyester [58,59,69] | Scaffold in bone regeneration Tissue adhesion | Faster degradation in wet environment than polyurethane-based High mechanical & adhesion strength | Mechanical stability during degradation Osteogenic capacities (osteoconduction and osteoinduction) Inflammation at the application site Low yield strength Significant cytotoxicity |
| Poly-methyl methacrylate (PMMA) [70,71] | Bone fragment and implant fixation Adhesives in dentistry Bone formation | Hydrophobic behaviour Increased bonding to wet bone Easy application Cytocompatibility | Low adhesive strength Thermal necrosis of bone tissue Lack of biodegradability |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzagiollari, A.; McCarthy, H.O.; Levingstone, T.J.; Dunne, N.J. Biodegradable and Biocompatible Adhesives for the Effective Stabilisation, Repair and Regeneration of Bone. Bioengineering 2022, 9, 250. https://doi.org/10.3390/bioengineering9060250
Tzagiollari A, McCarthy HO, Levingstone TJ, Dunne NJ. Biodegradable and Biocompatible Adhesives for the Effective Stabilisation, Repair and Regeneration of Bone. Bioengineering. 2022; 9(6):250. https://doi.org/10.3390/bioengineering9060250
Chicago/Turabian StyleTzagiollari, Antzela, Helen O. McCarthy, Tanya J. Levingstone, and Nicholas J. Dunne. 2022. "Biodegradable and Biocompatible Adhesives for the Effective Stabilisation, Repair and Regeneration of Bone" Bioengineering 9, no. 6: 250. https://doi.org/10.3390/bioengineering9060250
APA StyleTzagiollari, A., McCarthy, H. O., Levingstone, T. J., & Dunne, N. J. (2022). Biodegradable and Biocompatible Adhesives for the Effective Stabilisation, Repair and Regeneration of Bone. Bioengineering, 9(6), 250. https://doi.org/10.3390/bioengineering9060250









