Simulating In Vitro the Bone Healing Potential of a Degradable and Tailored Multifunctional Mg-Based Alloy Platform
Abstract
:1. Introduction
2. Materials and Methods
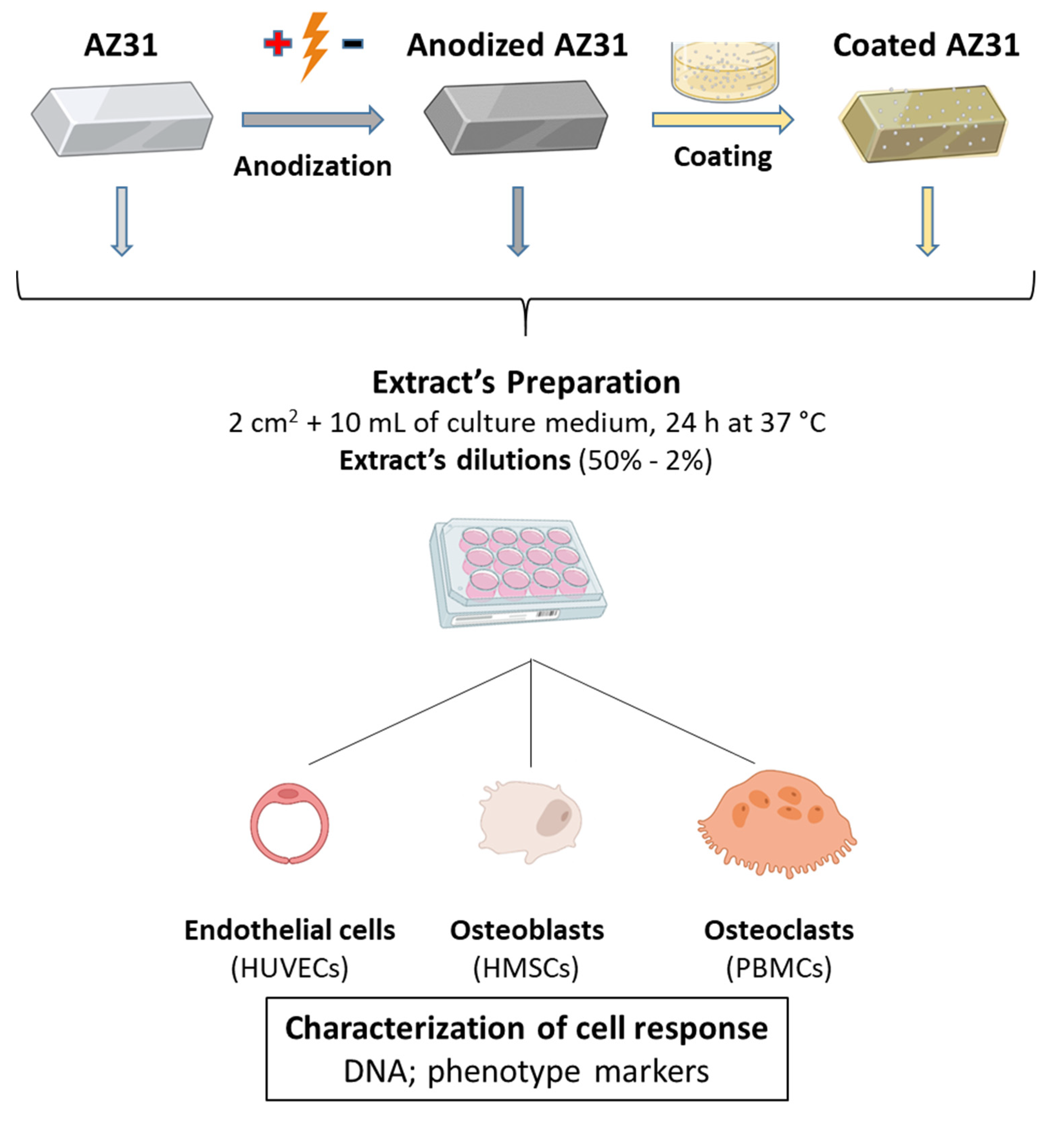
2.1. Materials’ Preparation
2.2. Surface Characterization of Material Samples
2.3. Extract Preparation
2.4. Cell Cultures
2.5. Characterization of the Cellular Response
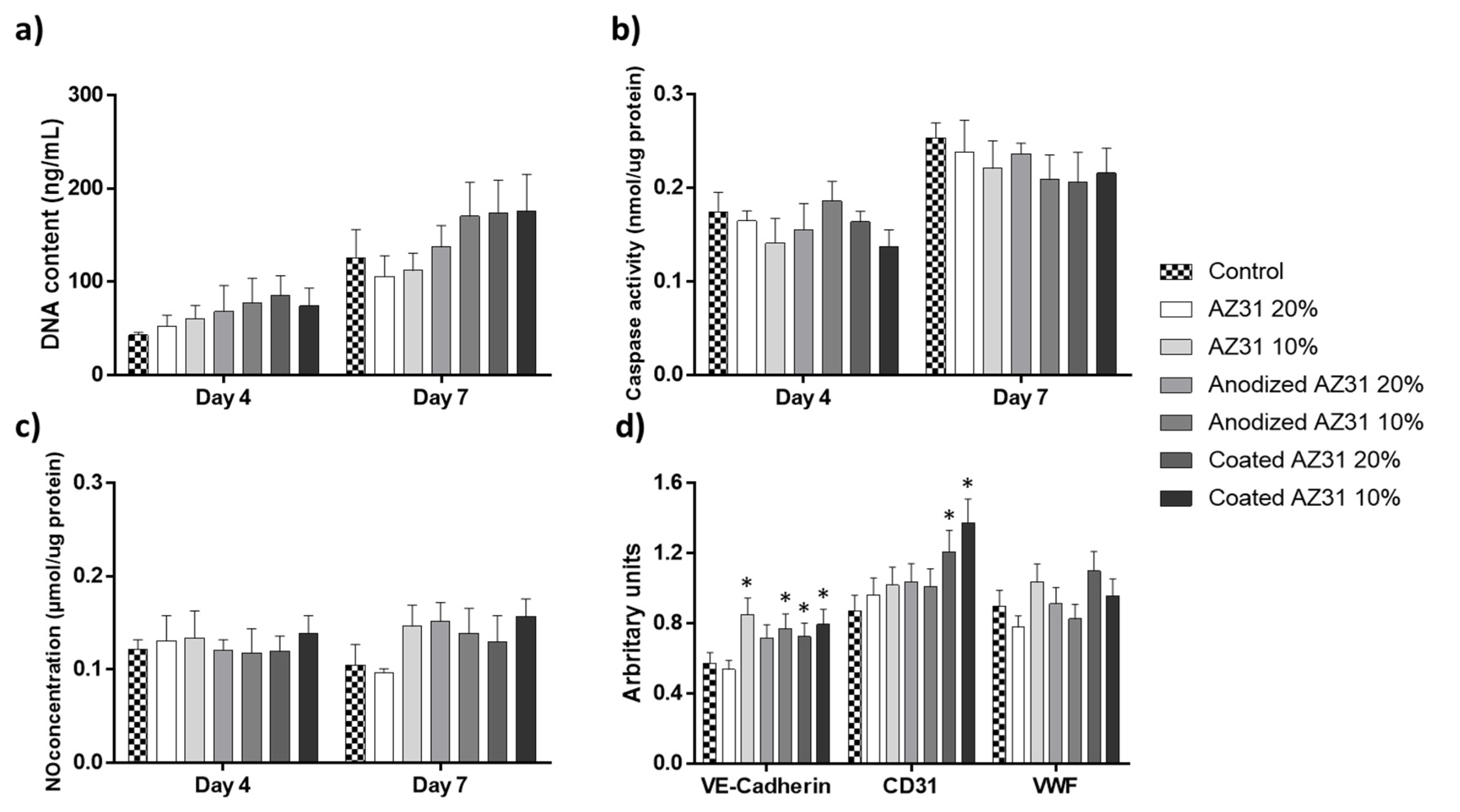
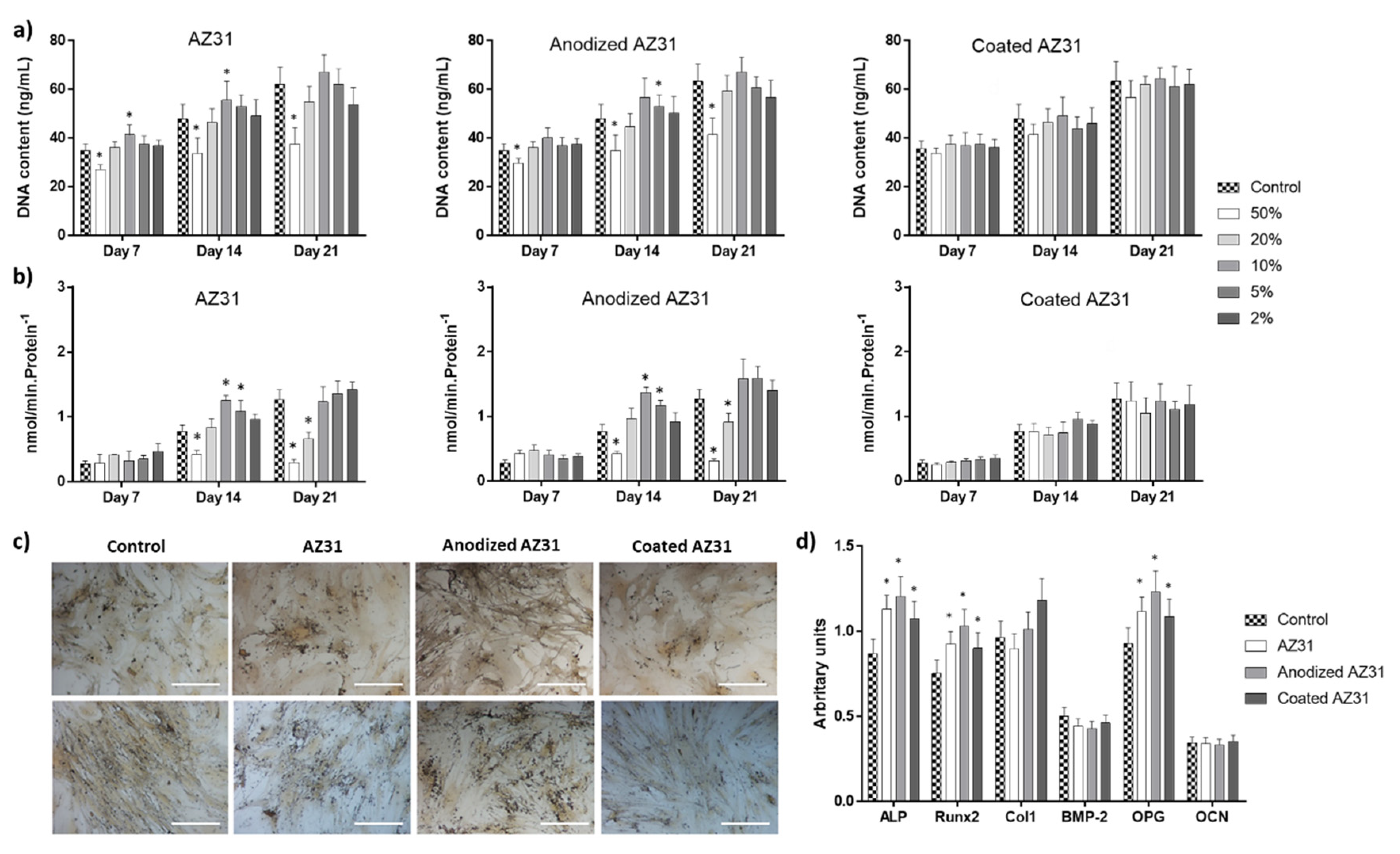
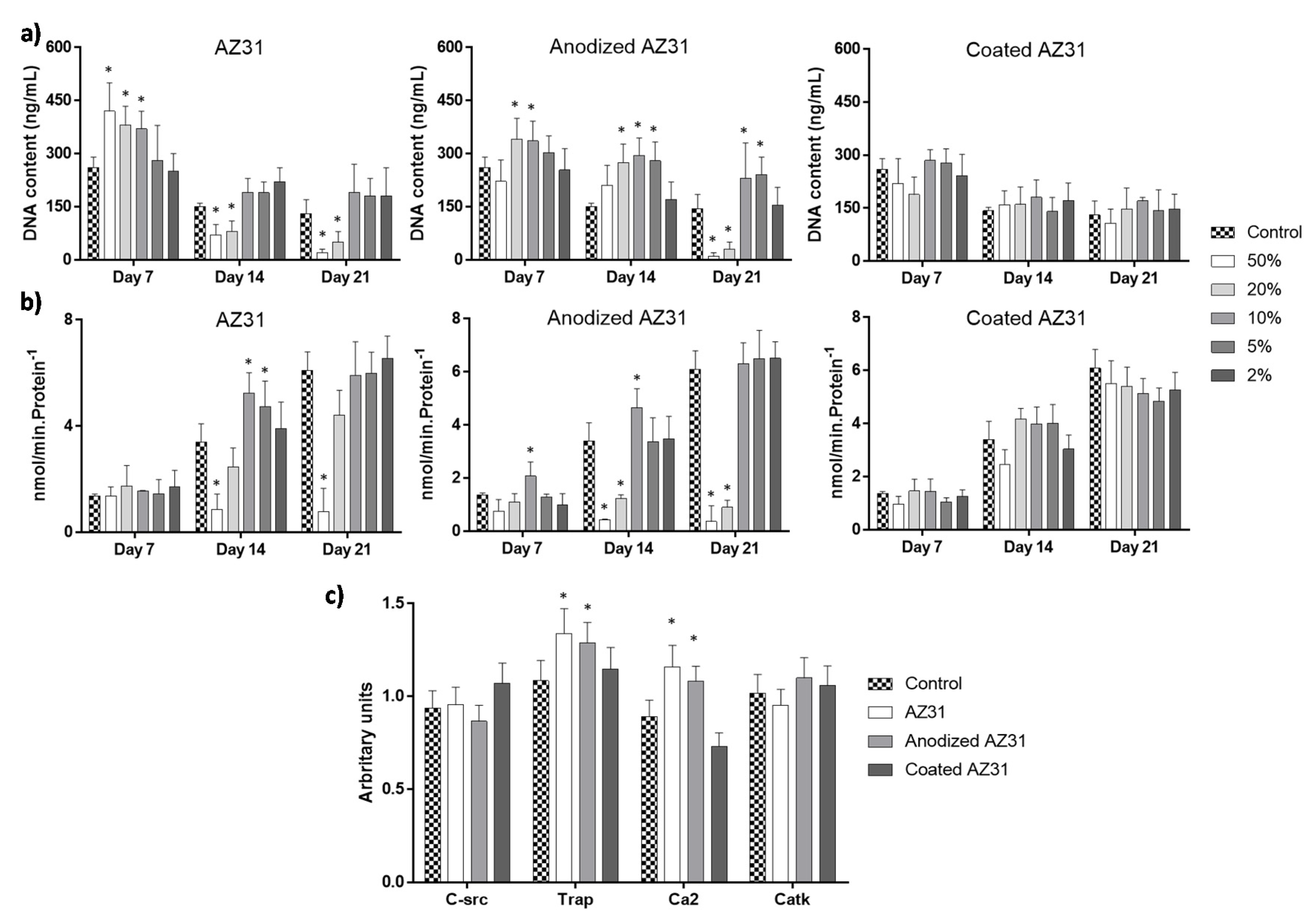
2.5.1. DNA Content Assay
2.5.2. ALP Activity
2.5.3. TRAP Activity
2.5.4. Caspase Activity
2.5.5. Histochemical Staining of Alkaline Phosphatase
2.5.6. Nitric Oxide (NO) Detection
2.5.7. Total RNA Extraction and RT-PCR Analysis
2.5.8. Tube-like Formation Assay
2.6. In Vivo Angiogenic Assay—Chick Chorioallantoic Membrane (CAM) Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. SEM Observation of the Mg-Based Samples and Levels of Mg and Ca Ions in the Extracts
3.2. Effects of the Extracts from Mg-Based Substrates in Endothelial Cells
3.3. Effects of the Extracts from Mg-Based Substrates in Bone Cells
3.3.1. Osteogenic-Differentiating Mesenchymal Stem Cells
3.3.2. Osteoclastogenic-Differentiating Mononuclear Precursor Cells
3.4. Integrating the Cell Response to the Extracts from the Mg-Based Substrates
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Goriainov, V.; Cook, R.; Latham, J.M.; Dunlop, D.G.; Oreffo, R. Bone and metal: An orthopaedic perspective on osseointegration of metals. Acta Biomater. 2014, 10, 4043–4057. [Google Scholar] [CrossRef] [PubMed]
- Tsaryk, R.; Peters, K.; Unger, R.E.; Scharnweber, D.; Kirkpatrick, C.J. The effects of metal implants on inflammatory and healing processes. Int. J. Mater. Res. 2007, 98, 622–629. [Google Scholar] [CrossRef]
- Badhe, R.V.; Akinfosile, O.; Bijukumar, D.; Barba, M.; Mathew, M.T. Systemic toxicity eliciting metal ion levels from metallic implants and orthopedic devices—A mini review. Toxicol. Lett. 2021, 350, 213–224. [Google Scholar] [CrossRef]
- Wawrose, R.A.; Urish, K.L. Diagnosis and management of adverse reactions to metal debris. Oper. Tech. Orthop. 2019, 29, 100732. [Google Scholar] [CrossRef]
- Savio, D.; Bagno, A. When the total hip replacement fails: A review on the stress-shielding effect. Processes 2022, 10, 612. [Google Scholar] [CrossRef]
- Lima, T.V.M.; Bhure, U.; Lago, M.D.S.P.; Thali, Y.; Matijasevic, S.; Roos, J.; Strobel, K. Impact of metal implants on xSPECT/CT Bone reconstruction: The “shining metal artefact”. Eur. J. Hybrid Imaging 2020, 4, 18. [Google Scholar] [CrossRef]
- Kumar, C.R.; Sood, S.; Ham, S. Complications of bioresorbable fixation systems in pediatric neurosurgery. Child’s Nerv. Syst. 2004, 21, 205–210. [Google Scholar] [CrossRef]
- Yun, Y.; Dong, Z.; Lee, N.; Liu, Y.; Xue, D.; Guo, X.; Kuhlmann, J.; Doepke, A.; Halsall, H.B.; Heineman, W.; et al. Revolutionizing biodegradable metals. Mater. Today 2009, 12, 22–32. [Google Scholar] [CrossRef]
- Chen, J.; Tan, L.; Yu, X.; Etim, I.P.; Ibrahim, M.; Yang, K. Mechanical properties of magnesium alloys for medical application: A review. J. Mech. Behav. Biomed. Mater. 2018, 87, 68–79. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.; Jun, I.; Seok, H.; Lee, K.; Lee, K.; Witte, F.; Mantovani, D.; Kim, Y.; Glyn-Jones, S.; Edwards, J.R. Biodegradable magnesium alloys promote angio-osteogenesis to enhance bone repair. Adv. Sci. 2020, 7, 2000800. [Google Scholar] [CrossRef] [PubMed]
- Song, G.L.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Sukotjo, C.; Lima-Neto, T.J.; Santiago Júnior, J.F.; Faverani, L.P.; Miloro, M. Is there a role for absorbable metals in surgery? A systematic review and meta-analysis of Mg/Mg alloy based implants. Materials 2020, 13, 3914. [Google Scholar] [CrossRef]
- Zeng, R.-C.; Dietzel, W.; Witte, F.; Hort, N.; Blawert, C. Progress and challenge for magnesium alloys as biomaterials. Adv. Eng. Mater. 2008, 10, B3–B14. [Google Scholar] [CrossRef]
- Gusieva, K.; Davies, C.; Scully, J.R.; Birbilis, N. Corrosion of magnesium alloys: The role of alloying. Int. Mater. Rev. 2014, 60, 169–194. [Google Scholar] [CrossRef]
- Rahman, M.; Dutta, N.K.; Choudhury, N.R. Magnesium alloys with tunable interfaces as bone implant materials. Front. Bioeng. Biotechnol. 2020, 8, 564. [Google Scholar] [CrossRef]
- Zomorodian, A.; Garcia, M.; e Silva, T.M.; Fernandes, J.C.S.; Fernandes, M.H.; Montemor, F. Corrosion resistance of a composite polymeric coating applied on biodegradable AZ31 magnesium alloy. Acta Biomater. 2013, 9, 8660–8670. [Google Scholar] [CrossRef]
- Zomorodian, A.; Brusciotti, F.; Fernandes, A.; Carmezim, M.; e Silva, T.M.; Fernandes, J.; Montemor, M. Anti-corrosion performance of a new silane coating for corrosion protection of AZ31 magnesium alloy in Hank’s solution. Surf. Coat. Technol. 2012, 206, 4368–4375. [Google Scholar] [CrossRef]
- Zomorodian, A.; Santos, C.; Carmezim, M.; e Silva, T.M.; Fernandes, J.C.S.; Montemor, F. “In-vitro” corrosion behaviour of the magnesium alloy with Al and Zn (AZ31) protected with a biodegradable polycaprolactone coating loaded with hydroxyapatite and cephalexin. Electrochim. Acta 2015, 179, 431–440. [Google Scholar] [CrossRef]
- Zomorodian, A.; Garcia, M.P.; Moura, E.S.T.; Fernandes, J.C.; Fernandes, M.H.; Montemor, M.F. Biofunctional composite coating architectures based on polycaprolactone and nanohydroxyapatite for controlled corrosion activity and enhanced biocompatibility of magnesium AZ31 alloy. Mater. Sci. Eng. C 2015, 48, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.S.; Zomorodian, A.; Kwiatkowski, L.; Lutze, R.; Balkowiec, A.; Colaço, B.J.A.; Pinheiro, V.; Fernandes, J.C.S.; Montemor, F.; Fernandes, M.H. In vivo assessment of a new multifunctional coating architecture for improved Mg alloy biocompatibility. Biomed. Mater. 2016, 11, 045007. [Google Scholar] [CrossRef] [PubMed]
- Zomorodian, A.; Ribeiro, I.A.; Fernandes, J.C.S.; Matos, A.C.; Santos, C.; Bettencourt, A.F.; Montemor, M.F. Biopolymeric coatings for delivery of antibiotic and controlled degradation of bioresorbable Mg AZ31 alloys. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 533–543. [Google Scholar] [CrossRef]
- Kwiatkowski, L.; Kapuścińska, A.; Bałkowiec, A.; Lutze, R. Increasing the Surface Functionality of Mg Alloys by Means of Plasma Electrolytic Oxidation. Solid State Phenom. 2015, 227, 495–498. [Google Scholar] [CrossRef]
- Anani, T.; Castillo, A.B. Mechanically-regulated bone repair. Bone 2021, 154, 116223. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, S.; Li, Y.; Crane, J.L. Type H blood vessels in bone modeling and remodeling. Theranostics 2020, 10, 426–436. [Google Scholar] [CrossRef]
- Coelho, M.J.; Fernandes, M.H. Human bone cell cultures in biocompatibility testing. Part II: Effect of ascorbic acid, β-glycerophosphate and dexamethasone on osteoblastic differentiation. Biomaterials 2000, 21, 1095–1102. [Google Scholar] [CrossRef]
- Costa-Rodrigues, J.; Fernandes, A.; Fernandes, M.H. Spontaneous and induced osteoclastogenic behaviour of human peripheral blood mononuclear cells and their CD14+ and CD14− cell fractions. Cell Prolif. 2011, 44, 410–419. [Google Scholar] [CrossRef]
- Mena-Morcillo, E.; Veleva, L. Degradation of AZ31 and AZ91 magnesium alloys in different physiological media: Effect of surface layer stability on electrochemical behaviour. J. Magnes. Alloys 2020, 8, 667–675. [Google Scholar] [CrossRef]
- Wu, L.; Feyerabend, F.; Schilling, A.F.; Willumeit, R.; Luthringer, B.J. Effects of extracellular magnesium extract on the proliferation and differentiation of human osteoblasts and osteoclasts in coculture. Acta Biomater. 2015, 27, 294–304. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2015, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, R.J. Chemokine regulation of angiogenesis during wound healing. Adv. Wound Care 2015, 4, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Villa, P.; Kaufmann, S.; Earnshaw, W.C. Caspases and caspase inhibitors. Trends Biochem. Sci. 1997, 22, 388–393. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, J.; Zhang, X.; Liang, G.; Xu, T.; Niu, W. Three-dimensional printed Mg-doped β-TCP bone tissue engineering scaffolds: Effects of magnesium ion concentration on osteogenesis and angiogenesis in vitro. Tissue Eng. Regen. Med. 2019, 16, 415–429. [Google Scholar] [CrossRef]
- Giannotta, M.; Trani, M.; Dejana, E. VE-cadherin and endothelial adherens junctions: Active guardians of vascular integrity. Dev. Cell 2013, 26, 441–454. [Google Scholar] [CrossRef] [Green Version]
- Lertkiatmongkol, P.; Liao, D.; Mei, H.; Hu, Y.; Newman, P.J. Endothelial functions of platelet/endothelial cell adhesion molecule-1 (CD31). Curr. Opin. Hematol. 2016, 23, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Randi, A.M.; Smith, K.E.; Castaman, G. von Willebrand factor regulation of blood vessel formation. Blood 2018, 132, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Willumeit-Römer, R.; Luthringer-Feyerabend, B.J. Effect of magnesium-degradation products and hypoxia on the angiogenesis of human umbilical vein endothelial cells. Acta Biomater. 2019, 98, 269–283. [Google Scholar] [CrossRef]
- Zhao, N.; Zhu, D. Endothelial responses of magnesium and other alloying elements in magnesium-based stent materials. Metallomics 2014, 7, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Sternberg, K.; Gratz, M.; Koeck, K.; Mostertz, J.; Begunk, R.; Loebler, M.; Semmling, B.; Seidlitz, A.; Hildebrandt, P.; Homuth, G.; et al. Magnesium used in bioabsorbable stents controls smooth muscle cell proliferation and stimulates endothelial cells in vitro. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 41–50. [Google Scholar] [CrossRef]
- Maier, J.A.; Bernardini, D.; Rayssiguier, Y.; Mazur, A. High concentrations of magnesium modulate vascular endothelial cell behaviour in vitro. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2004, 1689, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, A. Role of magnesium in genomic stability. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2001, 475, 113–121. [Google Scholar] [CrossRef]
- Kupetsky, E.; Uitto, J. Magnesium: Novel applications in cardiovascular disease—A review of the literature. Ann. Nutr. Metab. 2012, 61, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Mazur, A.; Maier, J.A.M.; Rock, E.; Gueux, E.; Nowacki, W.; Rayssiguier, Y. Magnesium and the inflammatory response: Potential physiopathological implications. Arch. Biochem. Biophys. 2006, 458, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Trejo, I.; Kojouharov, H.V. Understanding the fundamental molecular mechanism of osteogenic differentiation from mesenchymal stem cells. Appl. Appl. Math. Int. J. AAM 2019, 14, 687–698. [Google Scholar]
- Amukarimi, S.; Mozafari, M. Biodegradable magnesium biomaterials—Road to the clinic. Bioengineering 2022, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Willumeit-Römer, R. The interface between degradable Mg and tissue. JOM 2019, 71, 1447–1455. [Google Scholar] [CrossRef] [Green Version]
- Yoshizawa-Smith, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014, 10, 2834–2842. [Google Scholar] [CrossRef]
- Luthringer, B.J.; Willumeit-Römer, R. Effects of magnesium degradation products on mesenchymal stem cell fate and osteoblastogenesis. Gene 2016, 575, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zu, H.; Zhao, D.; Yang, K.; Tian, S.; Yu, X.; Lu, F.; Liu, B.; Wang, B.; Wang, W.; et al. Ion channel functional protein kinase TRPM7 regulates Mg ions to promote the osteoinduction of human osteoblast via PI3K pathway: In vitro simulation of the bone-repairing effect of Mg-based alloy implant. Acta Biomater. 2017, 63, 369–382. [Google Scholar] [CrossRef]
- Burmester, A.; Willumeit-Römer, R.; Feyerabend, F. Behavior of bone cells in contact with magnesium implant material. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 105, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Witte, F.; Xi, T.; Zheng, Y.; Yang, K.; Yang, Y.; Zhao, D.; Meng, J.; Li, Y.; Li, W.; et al. Recommendation for modifying current cytotoxicity testing standards for biodegradable magnesium-based materials. Acta Biomater. 2015, 21, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Nourisa, J.; Zeller-Plumhoff, B.; Helmholz, H.; Luthringer-Feyerabend, B.; Ivannikov, V.; Willumeit-Römer, R. Magnesium ions regulate mesenchymal stem cells population and osteogenic differentiation: A fuzzy agent-based modeling approach. Comput. Struct. Biotechnol. J. 2021, 19, 4110–4122. [Google Scholar] [CrossRef] [PubMed]
- Pinho, L.C.; Alves, M.M.; Colaço, B.; Fernandes, M.H.; Santos, C. Green-synthesized magnesium hydroxide nanoparticles induced osteoblastic differentiation in bone co-cultured cells. Pharmaceuticals 2021, 14, 1281. [Google Scholar] [CrossRef] [PubMed]
- Alesi, N.; Charles, J.F.; Nakamura, M.C. Basic aspects of osteoclast differentiation and function. Hypercalcemia 2020, 17–41. [Google Scholar] [CrossRef]
- Armstrong, A.P.; Tometsko, M.E.; Glaccum, M.; Sutherland, C.L.; Cosman, D.; Dougall, W.C. A RANK/TRAF6-dependent signal transduction pathway is essential for osteoclast cytoskeletal organization and resorptive function. J. Biol. Chem. 2002, 277, 44347–44356. [Google Scholar] [CrossRef] [Green Version]
- Maradze, D.; Musson, D.; Zheng, Y.; Cornish, J.; Lewis, M.; Liu, Y. High magnesium corrosion rate has an effect on osteoclast and mesenchymal stem cell role during bone remodelling. Sci. Rep. 2018, 8, 10003. [Google Scholar] [CrossRef] [Green Version]
- Notomi, T.; Kuno, M.; Hiyama, A.; Nozaki, T.; Ohura, K.; Ezura, Y.; Noda, M. Role of lysosomal channel protein TPC2 in osteoclast differentiation and bone remodeling under normal and low-magnesium conditions. J. Biol. Chem. 2017, 292, 20998–21010. [Google Scholar] [CrossRef] [Green Version]
- Mammoli, F.; Castiglioni, S.; Parenti, S.; Cappadone, C.; Farruggia, G.; Iotti, S.; Davalli, P.; Maier, J.A.; Grande, A.; Frassineti, C. Magnesium is a key regulator of the balance between osteoclast and osteoblast differentiation in the presence of vitamin D3. Int. J. Mol. Sci. 2019, 20, 385. [Google Scholar] [CrossRef] [Green Version]
- Fliefel, R.; Popov, C.; Tröltzsch, M.; Kühnisch, J.; Ehrenfeld, M.; Otto, S. Mesenchymal stem cell proliferation and mineralization but not osteogenic differentiation are strongly affected by extracellular pH. J. Cranio-Maxillofac. Surg. 2016, 44, 715–724. [Google Scholar] [CrossRef]
- Geris, L.; Gerisch, A.; Sloten, J.V.; Weiner, R.; Van Oosterwyck, H. Angiogenesis in bone fracture healing: A bioregulatory model. J. Theor. Biol. 2008, 251, 137–158. [Google Scholar] [CrossRef] [PubMed]
- Bahney, C.S.; Zondervan, R.L.; Allison, P.; Theologis, A.; Ashley, J.W.; Ahn, J.; Miclau, T.; Marcucio, R.S.; Hankenson, K.D. Cellular biology of fracture healing. J. Orthop. Res. 2019, 37, 35–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Gene | Assay ID |
|---|---|
| Housekeeping gene | |
| GAPDH | qHsaCED0038674 |
| Osteoblastic genes | |
| ALP | qHsaCED0045991 |
| Runx-2 | qHsaCED0044067 |
| Col-1 | qHsaCED0043248 |
| BMP-2 | qHsaCID0015400 |
| OPG | qHsaCED0046251 |
| OCN | qHsaCED0038437 |
| Osteoclastic genes | |
| C-src | qHsaCID0011233 |
| TRAP | qHsaCED0056724 |
| Ca2 | qHsaCID0021039 |
| Catk | qHsaCID0016934 |
| Endothelial genes | |
| VE-cadherin | qHsaCID0016288 |
| CD31 | qHsaCED0045459 |
| VWF | qHsaCED0033955 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, V.; Garcia, M.; Montemor, M.d.F.; Fernandes, J.C.S.; Gomes, P.S.; Fernandes, M.H. Simulating In Vitro the Bone Healing Potential of a Degradable and Tailored Multifunctional Mg-Based Alloy Platform. Bioengineering 2022, 9, 255. https://doi.org/10.3390/bioengineering9060255
Martin V, Garcia M, Montemor MdF, Fernandes JCS, Gomes PS, Fernandes MH. Simulating In Vitro the Bone Healing Potential of a Degradable and Tailored Multifunctional Mg-Based Alloy Platform. Bioengineering. 2022; 9(6):255. https://doi.org/10.3390/bioengineering9060255
Chicago/Turabian StyleMartin, Victor, Mónica Garcia, Maria de Fátima Montemor, João Carlos Salvador Fernandes, Pedro Sousa Gomes, and Maria Helena Fernandes. 2022. "Simulating In Vitro the Bone Healing Potential of a Degradable and Tailored Multifunctional Mg-Based Alloy Platform" Bioengineering 9, no. 6: 255. https://doi.org/10.3390/bioengineering9060255
APA StyleMartin, V., Garcia, M., Montemor, M. d. F., Fernandes, J. C. S., Gomes, P. S., & Fernandes, M. H. (2022). Simulating In Vitro the Bone Healing Potential of a Degradable and Tailored Multifunctional Mg-Based Alloy Platform. Bioengineering, 9(6), 255. https://doi.org/10.3390/bioengineering9060255










