A Novel Technique for Disinfection Treatment of Contaminated Dental Implant Surface Using 0.1% Riboflavin and 445 nm Diode Laser—An In Vitro Study
Abstract
1. Introduction
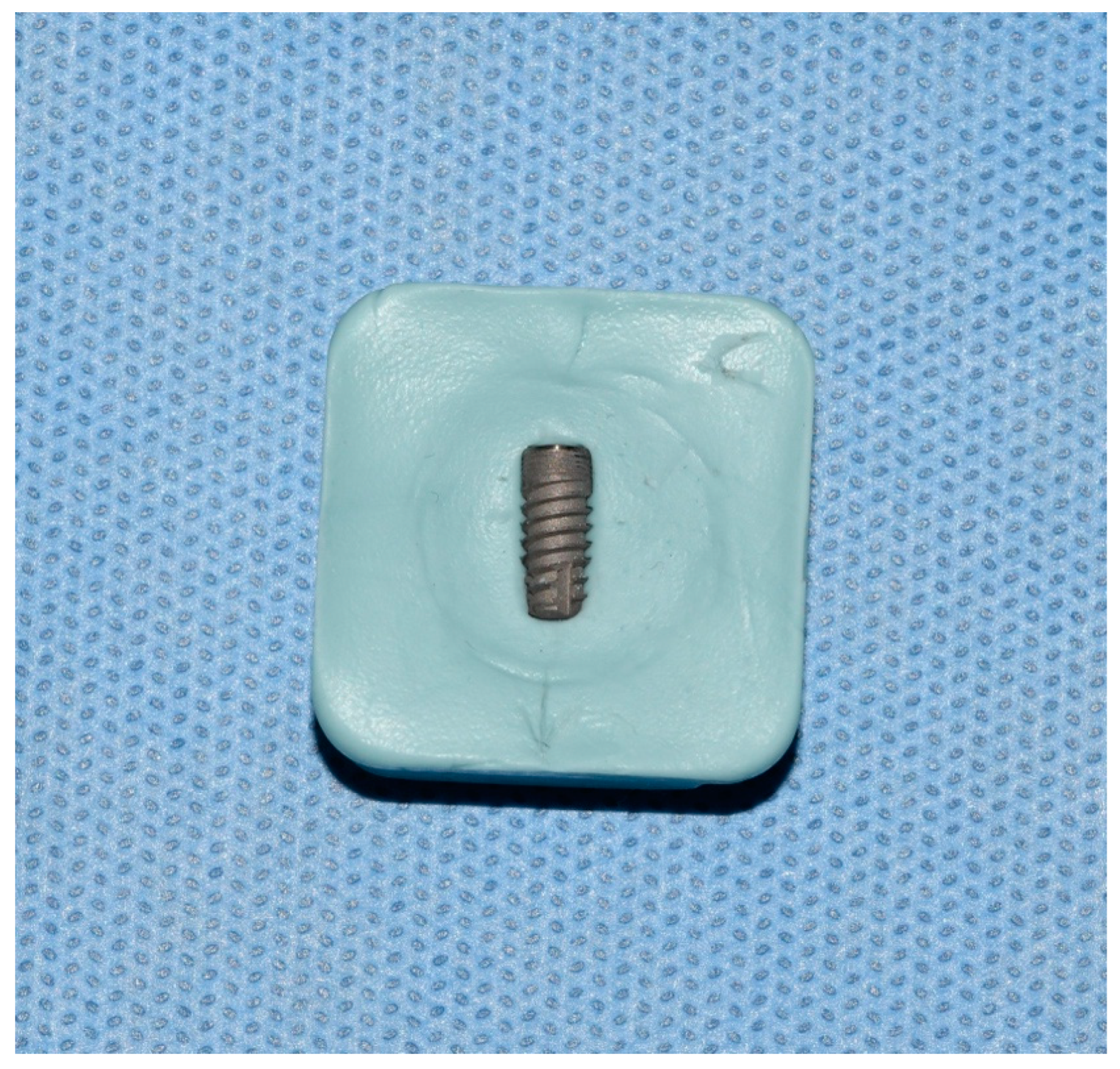
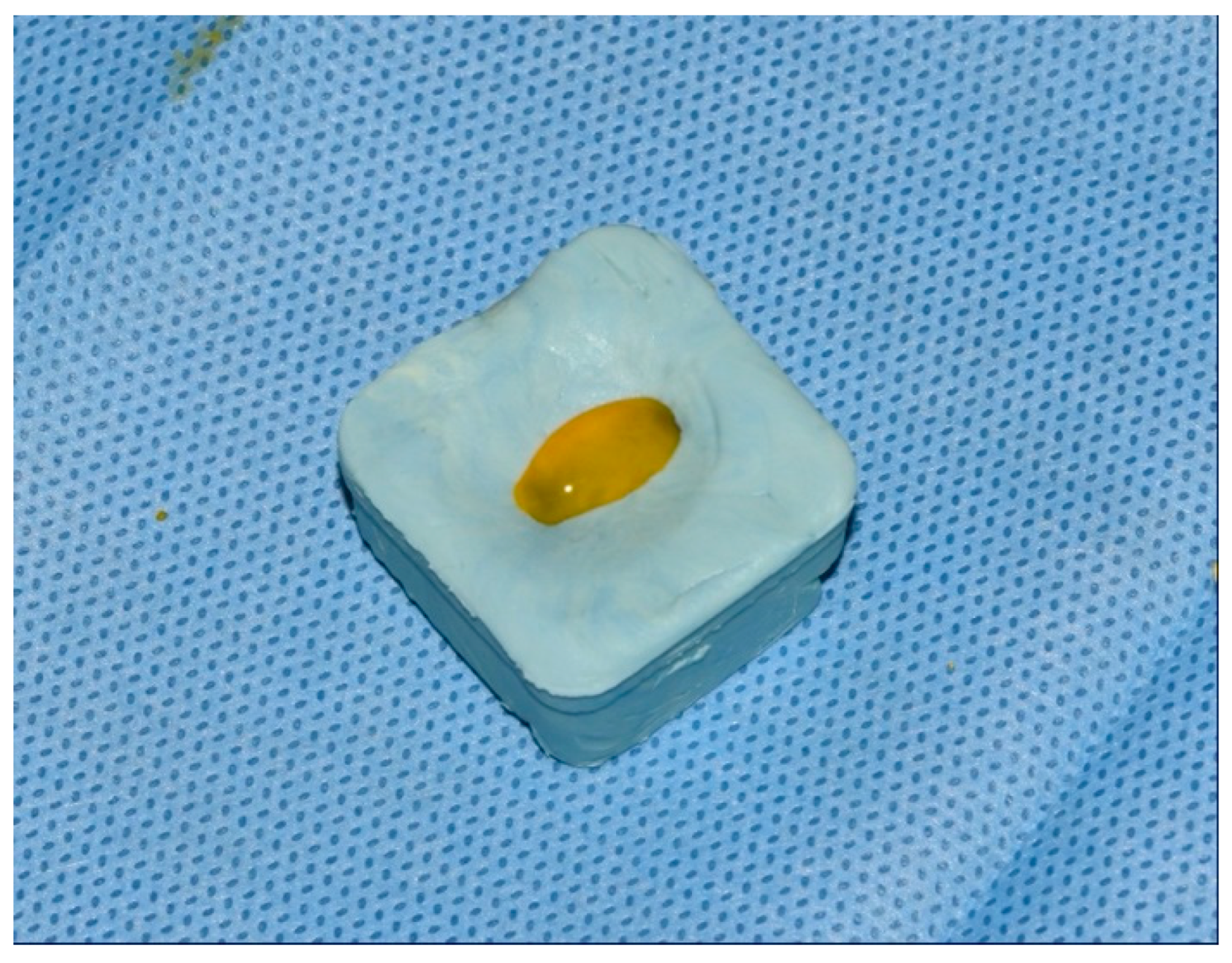
2. Material and Methods
Statistical Analysis
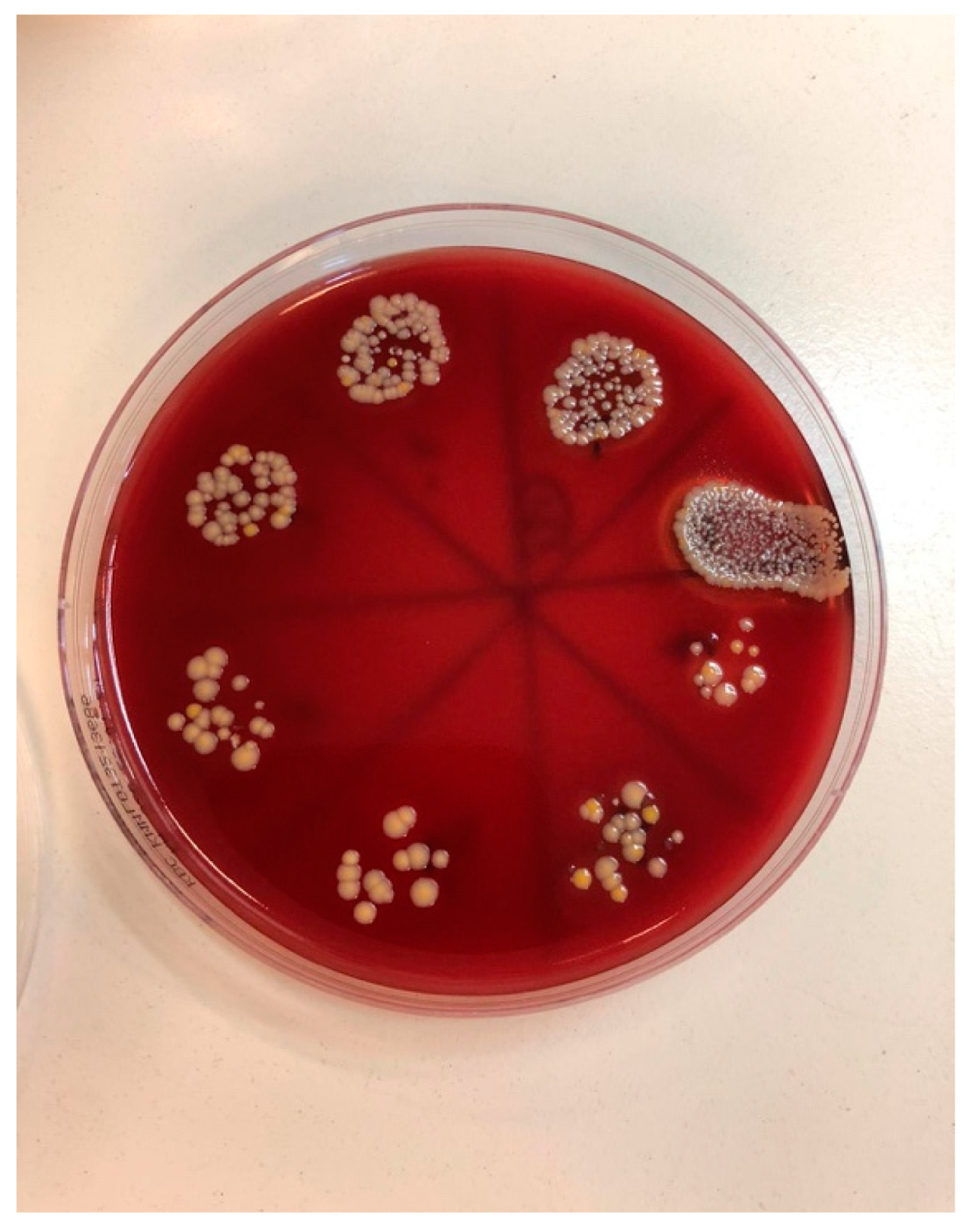
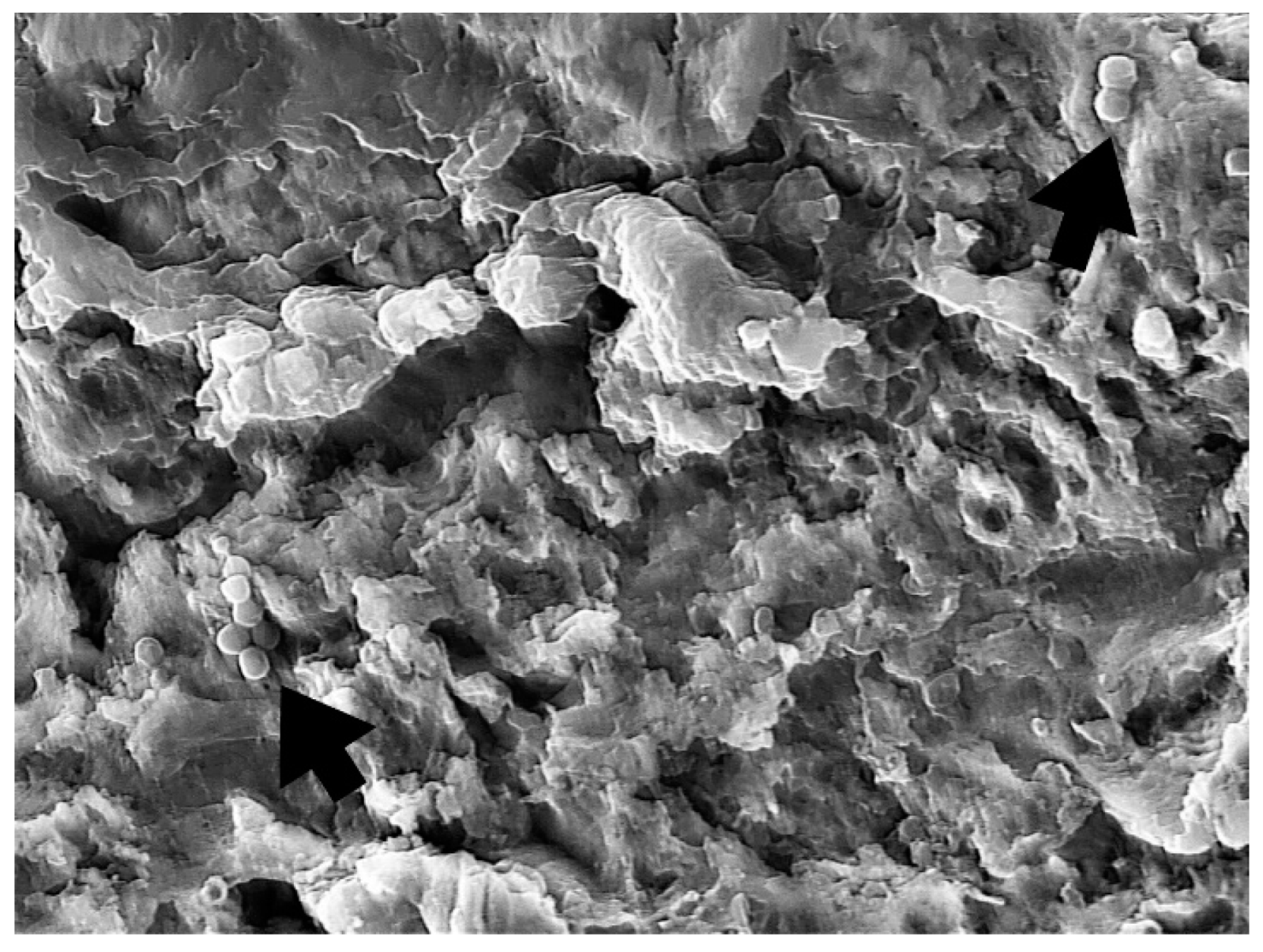
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wada, M.; Mameno, T.; Otsuki, M.; Kani, M.; Tsujioka, Y.; Ikebe, K. Prevalence and risk indicators for peri-implant diseases: A literature review. Jpn. Dent. Sci. Rev. 2021, 57, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Al-Radha, A.S.D.; Pal, A.; Pettemerides, A.P.; Jenkinson, H. Molecular analysis of microbiota associated with peri-implant diseases. J. Dent. 2012, 40, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, N.U.; Berglundh, T. Definition and prevalence of peri-implant diseases. J. Clin. Periodontol. 2008, 35, 286–291. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, C.; Monesi, N.; Ito, I.Y.; Issa, J.P.; de Albuquerque, R.F., Jr. Bacterial diversity of periodontal and implant-related sites detected by the DNA Checkerboard method. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 1607–1613. [Google Scholar] [CrossRef]
- Fürst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin. Oral Implant. Res. 2007, 18, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Fürst, M.M.; Lang, N.P.; Persson, G.R. One-year bacterial colonization patterns of Staphylococcus aureus and other bacteria at implants and adjacent teeth. Clin. Oral Implant. Res. 2008, 19, 242–248. [Google Scholar] [CrossRef]
- Tillander, J.; Hagberg, K.; Hagberg, L.; Brånemark, R. Osseointegrated titanium implants for limb prostheses attachments: Infectious complications. Clin. Orthop. Relat. Res. 2010, 468, 2781–2788. [Google Scholar] [CrossRef]
- Cai, Z.; Li, Y.; Wang, Y.; Chen, S.; Jiang, S.; Ge, H.; Lei, L.; Huang, X. Antimicrobial effects of photodynamic therapy with antiseptics on Staphylococcus aureus biofilm on titanium surface. Photodiagnosis Photodyn. Ther. 2019, 25, 382–388. [Google Scholar] [CrossRef]
- Urzúa, B.; Hermosilla, G.; Gamonal, J.; Morales-Bozo, I.; Canals, M.; Barahona, S.; Cóccola, C.; Cifuentes, V. Yeast diversity in the oral microbiota of subjects with periodontitis: Candida albicans and Candida dubliniensis colonize the periodontal pockets. Med. Mycol. 2008, 46, 783–793. [Google Scholar] [CrossRef]
- De La Torre, J.; Quindós, G.; Marcos-Arias, C.; Marichalar-Mendia, X.; Gainza, M.L.; Eraso, E.; Acha-Sagredo, A.; Aguirre-Urizar, J.M. Oral Candida colonization in patients with chronic periodontitis. Is there any relationship? Rev Iberoam Micol. 2018, 35, 134–139. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 89, S286–S291. [Google Scholar] [CrossRef] [PubMed]
- Ntrouka, V.; Hoogenkamp, M.; Zaura, E.; van der Weijden, F. The effect of chemotherapeutic agents on titanium-adherent biofilms. Clin. Oral Implant. Res. 2011, 22, 1227–3124. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Sahm, N.; Iglhaut, G.; Becker, J. Impact of the method of surface debridement and decontamination on the clinical outcome following combined surgical therapy of peri-implantitis: A randomized controlled clinical study. J. Clin. Periodontol. 2011, 38, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Mellado-Valero, A.; Buitrago-Vera, P.; Solá-Ruiz, M.F.; Ferrer-García, J.C. Decontamination of dental implant surface in peri-implantitis treatment: A literature review. Med. Oral Patol. Oral Cir. Bucal. 2013, 18, e869–e876. [Google Scholar] [CrossRef]
- Suárez-López Del Amo, F.; Yu, S.H.; Wang, H.L. Non-Surgical Therapy for Peri-Implant Diseases: A Systematic Review. J. Oral Maxillofac Res. 2016, 7, e13. [Google Scholar] [CrossRef]
- Al-Hashedi, A.A.; Laurenti, M.; Benhamou, V.; Tamimi, F. Decontamination of titanium implants using physical methods. Clin. Oral Implant. Res. 2017, 28, 1013–1021. [Google Scholar] [CrossRef]
- Louropoulou, A.; Slot, D.E.; Van der Weijden, F.A. Titanium surface alterations following the use of different mechanical instruments: A systematic review. Clin. Oral Implants Res. 2012, 23, 643–658. [Google Scholar] [CrossRef]
- Akram, Z.; Al-Shareef, S.A.; Daood, U.; Asiri, F.Y.; Shah, A.H.; AlQahtani, M.A.; Vohra, F.; Javed, F. Bactericidal Efficacy of Photodynamic Therapy Against Periodontal Pathogens in Periodontal Disease: A Systematic Review. Photomed. Laser Surg. 2016, 34, 137–149. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef]
- Hayek, R.R.; Araújo, N.S.; Gioso, M.A.; Ferreira, J.; Baptista-Sobrinho, C.A.; Yamada, A.M.; Ribeiro, M.S. Comparative Study Between the Effects of Photodynamic Therapy and Conventional Therapy on Microbial Reduction in Ligature-Induced Peri-Implantitis in Dogs. J. Periodontol. 2005, 76, 1275–1281. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy —What we know and what we don′t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta 2013, 1830, 3217–3266. [Google Scholar] [CrossRef] [PubMed]
- Kömerik, N.; Nakanishi, H.; MacRobert, A.J.; Henderson, B.; Speight, P.; Wilson, M. In Vivo Killing of Porphyromonas gingivalis by Toluidine Blue-Mediated Photosensitization in an Animal Model. Antimicrob. Agents Chemother. 2003, 47, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, A.A.; Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Wang, C.-Y.; Koshy, G.; Romanos, G.; Ishikawa, I.; Izumi, Y. Application of antimicrobial photodynamic therapy in periodontal and peri-implant diseases. Periodontology 2000 2009, 51, 109–140. [Google Scholar] [CrossRef]
- Chambrone, L.; Wang, H.L.; Romanos, G.E. Antimicrobial photodynamic therapy for the treatment of perio-dontitis and peri-implantitis: An American Academy of Periodontology best evidence review. J Periodontol. 2018, 89, 783–803. [Google Scholar] [PubMed]
- Katalinić, I.; Budimir, A.; Bošnjak, Z.; Jakovljević, S.; Anić, I. The photo-activated and photo-thermal effect of the 445/970 nm diode laser on the mixed biofilm inside root canals of human teeth in vitro: A pilot study. Photodiagnosis Photodyn. Ther. 2019, 26, 277–283. [Google Scholar] [CrossRef]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006, 17 (Suppl. 2), 68–81. [Google Scholar] [CrossRef]
- Gustumhaugen, E.; Lönn-Stensrud, J.; Scheie, A.A.; Lyngstadaas, S.P.; Ekfeldt, A.; Taxt-Lamolle, S. Effect of chemical and mechanical debridement techniques on bacterial re-growth on rough titanium surfaces: An in vitro study. Clin. Oral Implants Res. 2014, 25, 707–713. [Google Scholar] [CrossRef]
- Deppe, H.; Ahrens, M.; Behr, A.V.; Marr, C.; Sculean, A.; Mela, P.; Ritschl, L.M. Thermal effect of a 445 nm diode laser on five dental implant systems: An in vitro study. Sci. Rep. 2021, 11, 20174. [Google Scholar] [CrossRef]
- Malmqvist, S.; Liljeborg, A.; Qadri, T.; Johannsen, G.; Johannsen, A. Using 445 nm and 970 nm Lasers on Dental Im-plants-An In Vitro Study on Change in Temperature and Surface Alterations. Materials 2019, 12, 3934. [Google Scholar] [CrossRef]
- Widodo, A.; Spratt, D.; Sousa, V.; Petrie, A.; Donos, N. An in vitro study on disinfection of titanium surfaces. Clin. Oral Implants Res. 2016, 27, 1227–1232. [Google Scholar] [CrossRef]
- Ryu, H.S.; Kim, Y.I.; Lim, B.S.; Lim, Y.J.; Ahn, S.J. Chlorhexidine Uptake and Release from Modified Titanium Surfaces and Its Antimicrobial Activity. J. Periodontol. 2015, 86, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.K.; Garcia, J.; Væth, M.; Schlafer, S. Comparison of Riboflavin and Toluidine Blue O as Photosensitizers for Photoactivated Disinfection on Endodontic and Periodontal Pathogens In Vitro. PLoS ONE 2015, 10, e0140720. [Google Scholar] [CrossRef] [PubMed]
- Bärenfaller, V.; Clausen, C.; Sculean, A.; Eick, S. Effect of photoactivated disinfection using light in the blue spectrum. J. Photochem. Photobiol. B Biol. 2016, 158, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Smalley, J.W.; Silver, J.; Marsh, P.J.; Birss, A.J. The periodontopathogen Porphyromonas gingivalis binds iron protoporphyrin IX in the mu-oxo dimeric form: An oxidative buffer and possible pathogenic mechanism. Biochem. J. 1998, 331, 681–685. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Chen, J.; Wang, Y.; Sherwood, M.E.; Murray, C.K.; Vrahas, M.S.; Hooper, D.C.; Hamblin, M.R.; Dai, T. Antimicrobial blue light inactivation of Candida albicans: In vitro and in vivo studies. Virulence 2016, 7, 536–545. [Google Scholar] [CrossRef]
- Durantini, E.N. New insights into the antimicrobial blue light inactivation of Candida albicans. Virulence 2016, 7, 493–494. [Google Scholar] [CrossRef]
- Wiench, R.; Nowicka, J.; Pajączkowska, M.; Kuropka, P.; Skaba, D.; Kruczek-Kazibudzka, A.; Kuśka-Kiełbratowska, A.; Grzech-Leśniak, K. Influence of Incubation Time on Ortho-Toluidine Blue Mediated Antimicrobial Photodynamic Therapy Directed against Selected Candida Strains-An In Vitro Study. Int. J. Mol. Sci. 2021, 22, 10971. [Google Scholar] [CrossRef]
- Bouillaguet, S.; Owen, B.; Wataha, J.C.; Campo, M.A.; Lange, N.; Schrenzel, J. Intracellular reactive oxygen species in monocytes generated by photosensitive chromophores activated with blue light. Dent. Mater. 2008, 24, 1070–1076. [Google Scholar] [CrossRef]
- Bouillaguet, S.; Wataha, J.C.; Zapata, O.; Campo, M.; Lange, N.; Schrenzel, J. Production of reactive oxygen species from photosensitizers activated with visible light sources available in dental offices. Photomed Laser Surg. 2010, 28, 519–525. [Google Scholar] [CrossRef]
- Leelanarathiwat, K.; Katsuta, Y.; Katsuragi, H.; Watanabe, F. Antibacterial activity of blue high-power light-emitting diode-activated flavin mononucleotide against Staphylococcus aureus biofilm on a sandblasted and etched surface. Photodiagnosis Photodyn. Ther. 2020, 31, 101855. [Google Scholar] [CrossRef] [PubMed]
- Gambarini, G.; Plotino, G.; Grande, N.M.; Nocca, G.; Lupi, A.; Giardina, B.; De Luca, M.; Testarelli, L. In vitro evaluation of the cy-totoxicity of FotoSan™ light-activated disinfection on human fibroblasts. Med. Sci. Monit. 2011, 17, MT21–MT25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carvalho, G.G.; Felipe, M.P.; Costa, M.S. The photodynamic effect of methylene blue and toluidine blue on Candida albicans is dependent on medium conditions. J. Microbiol. 2009, 47, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Azizi, A.; Amirzadeh, Z.; Rezai, M.; Lawaf, S.; Rahimi, A. Effect of photodynamic therapy with two photosensitizers on Candida albicans. J. Photochem. Photobiol. B Biol. 2016, 158, 267–273. [Google Scholar] [CrossRef]
- Pereira, C.A.; Romeiro, R.L.; Costa, A.C.; Machado, A.K.; Junqueira, J.C.; Jorge, A.O. Susceptibility of Candida albicans, Staphylococcus aureus, and Streptococcus mutans biofilms to photodynamic inactivation: An in vitro study. Lasers Med. Sci. 2011, 26, 341–348. [Google Scholar] [CrossRef]
- Rosa, L.P.; da Silva, F.C.; Nader, S.A.; Meira, G.A.; Viana, M.S. Antimicrobial photodynamic inactivation of Staphylococcus aureus biofilms in bone specimens using methylene blue, toluidine blue ortho and malachite green: An in vitro study. Arch. Oral Biol. 2015, 60, 675–680. [Google Scholar] [CrossRef]
- Giannelli, M.; Landini, G.; Materassi, F.; Chellini, F.; Antonelli, A.; Tani, A.; Nosi, D.; Zecchi-Orlandini, S.; Rossolini, G.M.; Bani, D. Effects of photodynamic laser and violet-blue led irradiation on Staphylococcus aureus biofilm and Escherichia coli lipopolysaccharide attached to moderately rough titanium surface: In vitro study. Lasers Med. Sci. 2017, 32, 857–864. [Google Scholar] [CrossRef]
- Alasqah, M.N. Antimicrobial efficacy of photodynamic therapy on dental implant surfaces: A systematic review of in vitro studies. Photodiagnosis Photodyn. Ther. 2019, 25, 349–353. [Google Scholar] [CrossRef]
- Vohra, F.; Al-Rifaiy, M.Q.; Lillywhite, G.; Abu Hassan, M.I.; Javed, F. Efficacy of mechanical debridement with adjunct antimicrobial photodynamic therapy for the management of peri-implant diseases: A systematic review. Photochem. Photobiol. Sci. 2014, 13, 1160–1168. [Google Scholar] [CrossRef]






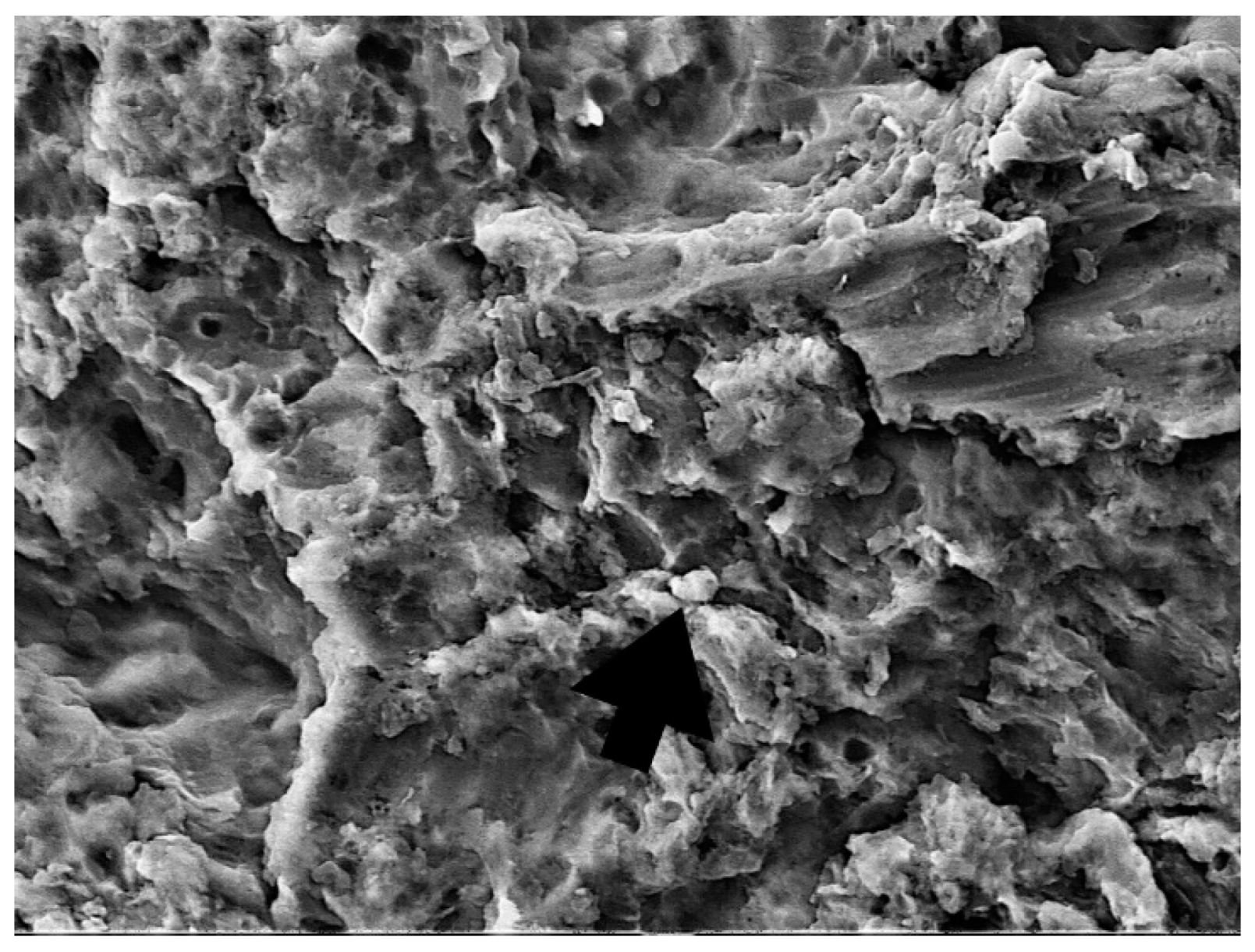


| Staphylococcus aureus | Median (Interquartile Range) | Minimum–Maximum | Difference † | 95% CI | p * |
|---|---|---|---|---|---|
| PDT1 | 0 0 (0–5.5) | 0–3 × 104 | 3.187 × 106 | 104 to 107 | <0.001 |
| NC | 3.2 × 106 (104–1.5 × 107) | 10–108 | |||
| PDT1 | 0 0 (0–0.51) | 0–1 | 0 | 0 to 0 | 0.34 |
| PC | 0 (0–0) | 0–107 | |||
| PDT2 | 0.5 (0–1) | 0–105 | 3.15 × 106 | 104 to 107 | <0.001 |
| NC | 3.2 × 106 (104–1.5 × 107) | 10–108 | |||
| PDT2 | 0.5 (0–1) | 0–105 | 0 | −1 to 0 | 0.09 |
| PC | 0 (0–0) | 0–107 | |||
| NC | 3.2 × 106 (104–1.5 × 107) | 10–108 | −4 × 105 | −1 × 107 to −1 × 104 | <0.001 |
| PC | 0 (0–0) | 0–107 |
| Candida albicans | Median (Interquartile Range) | Minimum–Maximum | Difference † | 95% CI | p * |
|---|---|---|---|---|---|
| PDT1 | 0 (0–1) | 0–104 | 103 | 20 to 104 | <0.001 |
| NC | 1.5 × 103 (20–1.5 × 104) | 0–2 × 105 | |||
| PDT1 | 0 (0–1) | 0–104 | 0 | 0 to 0 | 0.15 |
| PC | 0 (0–0) | 0–102 | |||
| PDT2 | 0(0–0) | 0–104 | 103 | 20 to 104 | 0.001 |
| NC | 1.5 × 103 (20–1.5 × 104) | 0–2 × 105 | |||
| PDT2 | 0 (0–0) | 0–104 | 0 | 0 to 0 | 0.38 |
| PC | 0 (0–0) | 0–102 | |||
| NC | 1.5 × 103 (20–1.5 × 104) | 0–2 × 105 | −1450 | −1 × 104 to −1 × 102 | <0.001 |
| PC | 0 (0–0) | 0–102 |
| Microorganism | Median (Interquartile Range) | Difference † | 95% CI | p * | |
|---|---|---|---|---|---|
| PDT1 | PDT2 | ||||
| Staphylococcus aureus | 0 (0–5.5) | 0.5 (0–1) | 0 | −1 to 0 | 0.55 |
| Candida albicans | 0 (0–1) | 0 (0–0) | 0 | 0 to 0 | 0.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morelato, L.; Budimir, A.; Smojver, I.; Katalinić, I.; Vuletić, M.; Ajanović, M.; Gabrić, D. A Novel Technique for Disinfection Treatment of Contaminated Dental Implant Surface Using 0.1% Riboflavin and 445 nm Diode Laser—An In Vitro Study. Bioengineering 2022, 9, 308. https://doi.org/10.3390/bioengineering9070308
Morelato L, Budimir A, Smojver I, Katalinić I, Vuletić M, Ajanović M, Gabrić D. A Novel Technique for Disinfection Treatment of Contaminated Dental Implant Surface Using 0.1% Riboflavin and 445 nm Diode Laser—An In Vitro Study. Bioengineering. 2022; 9(7):308. https://doi.org/10.3390/bioengineering9070308
Chicago/Turabian StyleMorelato, Luka, Ana Budimir, Igor Smojver, Ivan Katalinić, Marko Vuletić, Muhamed Ajanović, and Dragana Gabrić. 2022. "A Novel Technique for Disinfection Treatment of Contaminated Dental Implant Surface Using 0.1% Riboflavin and 445 nm Diode Laser—An In Vitro Study" Bioengineering 9, no. 7: 308. https://doi.org/10.3390/bioengineering9070308
APA StyleMorelato, L., Budimir, A., Smojver, I., Katalinić, I., Vuletić, M., Ajanović, M., & Gabrić, D. (2022). A Novel Technique for Disinfection Treatment of Contaminated Dental Implant Surface Using 0.1% Riboflavin and 445 nm Diode Laser—An In Vitro Study. Bioengineering, 9(7), 308. https://doi.org/10.3390/bioengineering9070308







